Abstract
We recently reported that young (3 to 4 months old) mice lacking Exon 1 of the Smad7 gene (S7ΔEx1 mice) show enhanced proliferation of neural stem and progenitor cells (NPCs) in the hippocampal dentate gyrus (DG) and in the subventricular zone (SVZ) of the lateral ventricles. It remained unclear, however, whether this phenotype would persist along aging, the latter typically being associated with a profound decrease in neurogenesis. Analysis of NPCs' proliferation based on the cell cycle marker PCNA in 12 month-old S7ΔEx1 mice revealed a reversal of the phenotype. Hence, in contrast to their younger counterparts, 12 month-old S7ΔEx1 mice had a reduced number of proliferating cells, compared to wildtype (WT) mice. At the same time, the survival of newly generated cells was enhanced in the aged transgenic animals. 12 month-old S7ΔEx1 mice further displayed a reduced level of neurogenesis based on the numbers of cells expressing doublecortin (DCX), a marker for newborn neurons. The reduced neurogenesis in aged S7ΔEx1 mice was not due to a stem cell depletion, which might have occurred as a consequence of hyperproliferation in the young mice, since the number of Nestin and Sox2 positive cells was similar in WT and S7ΔEx1 mice. Instead, Nestin positive cells in the DG as well as primary neurosphere cultures derived from 12 month-old S7ΔEx1 mice had a reduced capability to proliferate. However, after passaging, when released from their age- and niche-associated proliferative block, neurospheres from aged S7ΔEx1 mice regained the hyperproliferative property. Further, pSmad2 antibody staining intensity was elevated in the DG and SVZ of 12-month old transgenic compared to WT mice, indicating increased intracellular TGF-beta signaling in the aged S7ΔEx1 mice. In summary, this points toward differential effects of S7ΔEx1 on neurogenesis: (i) a hyperproliferation in young animals caused by a cell autonomous mechanism, and (ii) a TGF-beta dependent modulation of neurogenesis in aged S7ΔEx1 animals that abrogates the cell-intrinsic hyperproliferative properties and results in reduced proliferation, increased stem cell quiescence, and enhanced survival of newly generated cells.
Keywords: Neurogenesis, Aging, Proliferation, TGF-beta signaling, Smad, Neural stem cell
1. Introduction
The existence of adult neurogenesis, i.e. the generation of new neurons, in the CNS of most mammals including human is nowadays widely recognized (Altman and Das, 1965, Eriksson et al., 1998, Spalding et al., 2013). However, the rates of neurogenesis are not static and have been shown to be dramatically diminished in aged animals. Aging typically leads to a more than 100-fold reduction of neurogenesis rates in aged compared to young adult brains (Kuhn et al., 1996). Such modulations or defects in neurogenesis might be implicated in age-related cognitive impairments, in neurodegenerative diseases or in neuropsychiatric diseases such as depression (Couillard-Despres et al., 2009, Drapeau et al., 2003, Kandasamy et al., 2010, Mirescu and Gould, 2006). Vice versa, stimulation of neurogenesis could provide additional new neurons in aged or degenerated brains and thereby maintain/restore structures and functions (Ming and Song, 2005). Thus, molecular mechanisms regulating neurogenesis in the adult healthy or diseased brain might become therapeutic targets for CNS repair.
Unfortunately, the mechanisms responsible for the age-dependent decline of neurogenesis are not fully deciphered. Reduction of neurogenesis appears to result from inactivation of neural stem cells' proliferation, which get locked into a quiescent state. Evidence supporting this hypothesis comes from preliminary studies suggesting that neural stem cell numbers remain constant within the hippocampus during aging (Bonaguidi et al., 2011, Hattiangady and Shetty, 2008). Alternatively, the stem cell pool might get depleted over time, a possibility that cannot be excluded at present. Indeed, more recent data suggest that the massive fall in neurogenesis occurring along aging might be caused by an exhaustion of the stem cell pool (Encinas et al., 2011, Yu et al., 2010). Nevertheless, progenitor cell proliferation in the aged brain can be reactivated by physical activity, by the dilution of blood-born factors, or by pharmacological treatment using for example G-CSF (Kempermann et al., 1998, Popa-Wagner et al., 2010, Villeda et al., 2011). Further, inhibition of elevated TGF-beta signaling, which is known to suppress neural stem and progenitor cell proliferation (Wachs et al., 2006), re-induces neurogenesis in the aged brain (Pineda et al., 2013).
We recently showed that young (3–4 months old) adult mice lacking Exon 1 of Smad7, an inhibitor of TGF-beta and BMP signaling, (S7ΔEx1 mice) bear enhanced proliferation of neural stem and progenitor cells (NPCs) in the dentate gyrus (DG) and the subventricular zone (SVZ) in a TGF-beta independent manner. In the present study, we investigated whether this phenotype would persist along aging, the latter being typically associated with profound decrease in neurogenesis. For that we analyzed cell proliferation, numbers of neural stem and progenitor cells, as well as cell survival and differentiation, in 12 month-old S7ΔEx1- and wildtype mice. In addition, we examined NPC proliferation ex vivo in neurosphere assays.
2. Material and methods
Four cohorts of mice were used for the study: wild type (WT) and S7ΔEx1 mice of 4 or 12 months of age (n = 8 within each group, total of 32 mice in CD1 background). The 4 month-old mice were partially characterized within a previous report (Krampert et al., 2010). All experiments were approved by the animal ethic committee (Uppsala Tingsrätt). 4 and 12 month-old male mice were injected with 50 mg/kg bodyweight BrdU (Fluka, Germany) once daily for 4 consecutive days. Four weeks later, animals were deeply anesthetized and perfused transcardially with 4% paraformaldehyde.
2.1. Immunohistochemistry
Tissue processing and immunostaining were performed as described previously (Couillard-Despres et al., 2005). Tissue was cut into 25 μm sagittal sections. The following primary antibodies were used: rat anti-BrdU 1:250 (Oxford Biotechnology, UK), goat anti-doublecortin (DCX) 1:200 (Santa Cruz, USA), rabbit anti-glial fibrillary acidic protein (GFAP) 1:500 (Dako, Germany), mouse anti-Nestin 1:300 (Abcam, UK), mouse anti-NeuN 1:500 (Chemicon, Germany), goat anti-proliferating cell nuclear antigen (PCNA) 1:300 (Santa Cruz, USA), rabbit anti-phospho Smad2 1:1000 (Millipore, USA), and goat anti-SOX2 1:500 (Santa Cruz, USA). Secondary antibodies were: donkey anti-goat, -rat or -mouse conjugated with biotin 1:500 (Jackson Immuno Research, USA). For immunofluorescence co-labeling analysis, donkey anti-goat Alexa 488, donkey anti-goat or-rat Alexa 568, and donkey anti-mouse or -rat Alexa 647 (all 1:1000, Molecular Probes, USA) were used. DAB and immunofluorescence stainings were done as described (Couillard-Despres et al., 2005). For quantification of BrdU-, PCNA-, DCX-, Nestin- and Sox2-positive cells, every 12th section of one cerebral hemisphere was selected from each mouse and processed for immunohistochemistry. The volume of the granule cell layer of the DG or the SVZ was determined by tracing the area using a semiautomatic stereology system and multiplying with the section thickness and interval (Stereoinvestigator, MicroBrightField, Colchester, USA). The total number of labeled cells for the specific marker was determined on the selected sections and multiplied by the interval. Results are depicted as labeled cell densities, i.e. the total number of labeled cells within each region divided by the regions volume.
To determine the differentiation pattern of newborn cells, every 12th section of one cerebral hemisphere from each mouse was double stained with BrdU and either Sox2, DCX, NeuN or GFAP, and every BrdU positive cell detected was analyzed using a confocal laser scanning microscope (LSM 700, Zeiss, Germany). The survival rate of the newly generated cells in the DG was obtained by calculating a relative survival index for all treatment groups. For this, the ratio between the number of BrdU+ cells (survival) and the number of PCNA+ cells (proliferation) was calculated.
2.2. In vitro analyses
Adult NPCs were isolated from the SVZ of 4 month and 12 month-old mice as described (Wachs et al., 2003) and grown in Neurobasal (NB) medium (Invitrogen) supplemented with B27 (Invitrogen), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, Germany), 2 μg/ml heparin (Sigma), 10 ng/ml FGF-2 (Peprotech, UK) and 20 ng/ml EGF (R&D Systems, UK). Cells were seeded in T-25 culture flasks, grown in suspension cultures at 37 °C in an incubator with 5% CO2 and passaged weekly. Cells of passage numbers 2 to 6 were used for the present experiments. For proliferation assays, NPCs were seeded at a density of 104 cells/ml in 24-well plates and counted after 4 days. To determine the initial number of spheres obtained from hippocampus- and SVZ tissue, we made use of the “neural colony forming cell assay”-kit (Stem Cell Technologies, Inc., US) according to the manufacturer's recommendations. Briefly, cells isolated from six 12 month-old mice were pooled in 1 ml medium; SVZ-derived cells (75 μl) or hippocampus-derived cells (200 μl) were suspended in 1.5 ml of a collagen gel and plated into 12-well plates (500 μl/well). Growth factors were supplemented weekly and spheres were counted after 3 weeks.
2.3. Statistical analysis
Statistical analyses were performed using the GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA, USA). Data were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests when necessary. For comparison of two independent groups, the Mann Whitney U-test and the Students T-test, respectively, were performed. p values < 0.05 were considered to be statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001). All values were expressed as means ± standard deviation.
3. Results and discussion
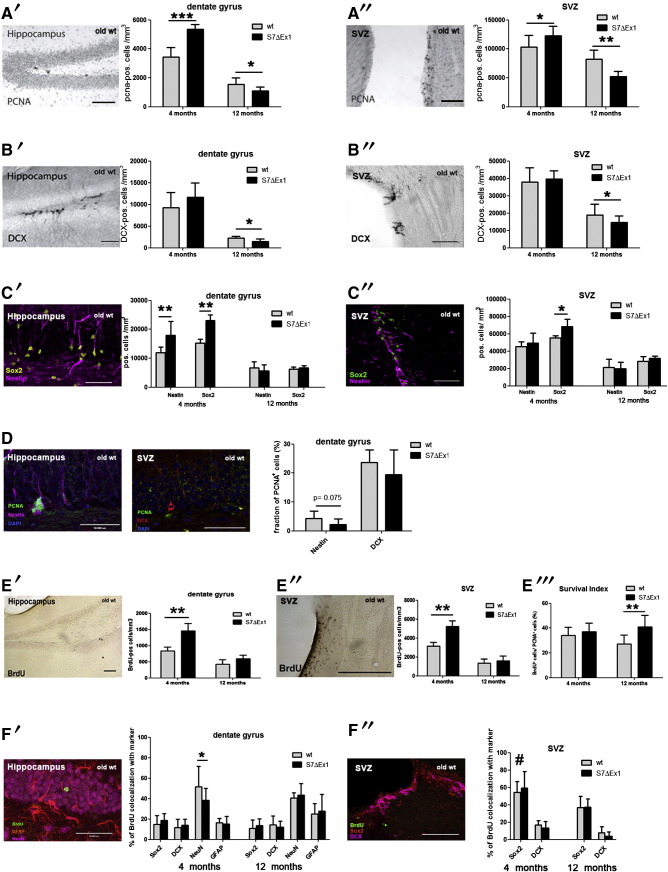
We recently reported that young S7ΔEx1 mice (3–4 month old) exhibit enhanced proliferation of NPCs in the DG and the SVZ (Krampert et al., 2010). In the present report, we made use of this observation and addressed the possibilities that either the hyperproliferative phenotype was maintained during aging or the hyperproliferation of NPCs in the young animals caused a premature depletion or inactivation of the stem cell pool. Detection of proliferating NPCs in the DG and SVZ using the cell cycle marker PCNA (Fig. 1A) revealed that the over-proliferative phenotype of S7ΔEx1 mice was actually reverted in aged mice. Hence, in contrast to the young mice, 12 month-old mutant mice showed reduced proliferation compared to their wildtype counterparts (Fig. 1A). Furthermore, compared to WT mice, old S7ΔEx1 mice also had reduced levels of neurogenesis in the DG and the SVZ, as revealed by the reduced number of DCX-positive cells, a marker for newborn neurons (Fig. 1B).
Fig. 1.
Age-dependent modulation of cell proliferation, cell differentiation, and neurogenesis in S7ΔEx1 mutant mice.
(A) Numbers of PCNA+ cells within the DG (A′) and SVZ (A″) are significantly increased in 4 month-old S7ΔEx1 mice compared to age-matched WT. In contrast, in 12 month-old animals, numbers of PCNA+ cells are significantly lower in transgenic compared to WT animals. (B) Numbers of DCX+ cells do not differ between the 4 month old S7ΔEx1 and WT mice in the DG (B′) and the SVZ (B″). At 12 months, S7ΔEx1 mice show a significant reduction of DCX+ cells. (C) In the DG of 4 month-old S7ΔEx1 mice, Nestin and Sox2 cell numbers are significantly elevated compared to WT (C′), and Sox2 cell numbers are significantly higher in the SVZ in the young transgenic mice (C″). At 12 months, the numbers of Nestin+ and Sox2+ cells are similar in WT and knockout mice in the DG and the SVZ. (D) PCNA expression within the Nestin+ cell population is decreased in 12 month-old S7ΔEx1 mice compared to WT mice (p = 0.075). In contrast, the proliferative activity (PCNA+) of DCX+ neuronal progenitor cells does not differ between 12 month-old WT and S7ΔEx1 mice. (E) Numbers of BrdU positive cells in the DG (E′) and SVZ (E″) were significantly higher in the young transgenic compared to WT mice, but did not differ in the 12 month-old mice. In the DG, the survival index of newly generated cells, based on BrdU cell number divided by PCNA cell number, was significantly higher in the 12 month-old S7ΔEx1 animals compared to the WT mice (E‴). (F) Cell fate analyses by BrdU co-labeling with specific markers revealed no differences in the percentage of BrdU double labeled cells in the DG (F′) and the SVZ (F″) between old transgenic and WT mice. In young mice, the fraction of BrdU+/NeuN+ cells was significantly lower in the S7ΔEx1 mice. In addition, independent of their genotype, young mice possessed a significant higher percentage of BrdU+/Sox2+ cells in the SVZ than aged mice (F″) Scale bars: 100 μm.
To examine if the reduced proliferation in old S7ΔEx1 animals was a result of the exhaustion of the stem cell pool due to earlier excessive proliferation, we determined the numbers of Nestin positive as well as of Sox2 positive cells (markers for neural stem/progenitor cells) in the DG and SVZ (Fig. 1C). While in the young S7ΔEx1 animals the numbers of Nestin and Sox2 positive cells were elevated compared to the WT animals (for Nestin at least in the dentate gyrus), the numbers of Nestin and Sox2 positive cells in the aged animals were unaffected by the genotype in both neurogenic regions (Fig. 1C).
This clearly points toward the presence of a similar number of neural stem cells in aged S7ΔEx1 and WT animals. However, the percentage of Nestin positive cells that were proliferating, i.e. PCNA positive, was slightly reduced in the hippocampi of S7ΔEx1 compared to WT animals (Fig. 1D, p = 0.075). DCX positive cells, in contrast, were not blocked in their capacity to proliferate (Fig. 1D).
We next analyzed if the survival and differentiation fate of newborn cells was changed in aged S7ΔEx1 compared to WT mice. Remarkably, 12 month-old mutant and WT mice showed a comparable number of BrdU positive cells (i.e. cells that had incorporated BrdU 4 weeks before) in the DG and SVZ (Fig. 1E). For the DG, considering the decreased number of newborn (PCNA+) cells in aged transgenic compared to WT mice at this site (Fig. 1A′), this indicates a significant increased survival of newly generated cells in the S7ΔEx1 animals (Fig. 1E‴). To further study the fate of newborn cells in the DG, we analyzed the amount of BrdU co-labeled neural stem cells (BrdU+/Sox2+), neuronal progenitors (BrdU+/DCX+), mature neurons (BrdU+/NeuN+), and astrocytes (BrdU+/GFAP+). No differences in the percentages of BrdU–Sox2, BrdU–DCX, BrdU–GFAP or BrdU–NeuN positive cells were found in 12 month-old transgenic compared to WT mice, indicating no changes on the fate of newborn cells in aged mice (Fig. 1F′). Within the SVZ, we concentrated on the analysis of BrdU+/Sox2+ and BrdU+/DCX+ cells (Fig. 1F″), since in both aged WT and transgenic animals, the majority of BrdU positive cells has migrated out of the neurogenic niche into the olfactory bulb (OB) during the differentiation process (WT: 13.3% BrdU+ cells in the SVZ, 4.3% rostral migratory stream (RMS), 82.4% OB; S7ΔEx1: 15.3% SVZ, 7.4% RMS, 77.3% OB; data not shown). Again, no differences in the distribution of BrdU co-labeled cells were found between 12-month transgenic and WT mice. However, independent of the genotype, the 4-month old mice possessed a significant higher number of BrdU+/Sox2+ cells compared to 12 month-old S7ΔEx1 and WT mice (Fig. 1F″), which points toward a higher proliferative activity of Sox2 positive neural stem cells in young compared to aged mice.
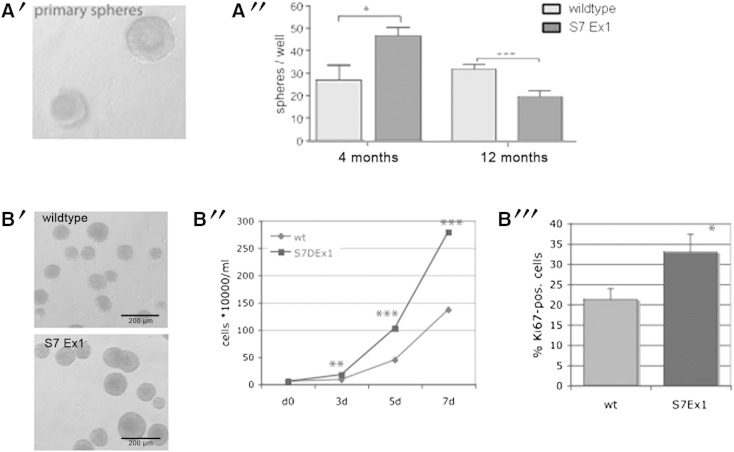
Next, we wanted to determine if the block in NPCs' proliferation in the aged S7ΔEx1 animals is of cell-autonomous nature and maintained in vitro, or whether cells can be released from the proliferation block and regain their full proliferation potential when taken out of their niche. Therefore, we performed ex vivo clonal spheres forming assays as described (Bull and Bartlett, 2005). Dissociated SVZ cells freshly isolated from WT and S7ΔEx1 mice were plated directly into a collagen gel to ensure clonal sphere formation that was then quantified 3 weeks later (Fig. 2A′). This assay revealed that, in contrast to cells derived from young animals, a lower number of spheres could be obtained from 12 month-old S7ΔEx1-mice in respect to age-matched WT mice (Fig. 2A″). This may be either because aged S7ΔEx1 mice had a smaller number of stem/progenitor cells in the neurogenic niche, or because some of the acutely isolated progenitors still suffered from the influence of a putative niche associated proliferation inhibitor and therefore did not proliferate.
Fig. 2.
NPC cultures derived from old S7ΔEx1-mice regain their hyperproliferative activity in vitro.
(A) Quantification of primary spheres forming units from the SVZ of 4 and 12 month-old mice plated directly into a collagen gel (A′). While in the 4 month age group the S7ΔEx1-mice exhibit a significant higher number of primary spheres, less spheres could be obtained from 12 month-old S7ΔEx1-mice in respect to age-matched WT mice (A″). (B) A proliferation assay on cultured NPCs derived from the SVZ of 12 month-old WT and S7ΔEx1 mice (passages 2–6) revealed increased sphere size (B′) and increased cell numbers (B″) in the S7ΔEx1-mice compared to WT mice. Further, the percentages of NPCs labeled with the cell-cycle-marker Ki67 were higher in 12 month-old S7ΔEx1-mice than in age-matched WT mice (B″). Counting was performed in triplicates (> 300 total cells per sample).
To further investigate the latter assumption, we hypothesized that after repeated passaging, NPCs from aged S7ΔEx1 mice would be released from their niche-associated proliferative block. Therefore, we analyzed growth and expansion of neurospheres after they had been passaged. Indeed, NPCs isolated from 12 month-old S7ΔEx1 mice taken in culture and passaged at least once revealed a faster proliferation compared to cells isolated from wildtype mice. This was evident based on sphere size (Fig. 2B′), cell counting (Fig. 2B″) and staining for the cell cycle marker Ki67 (Fig. 2B‴). Together, these observations argue for a cell-intrinsic mechanism in S7ΔEx1 mice that enhances the cell-extrinsic and age-associated inhibition in NPC proliferation. Alternatively, the reversal of the hyperproliferative phenotype after passaging may also be caused by the in vitro exposure to growth factors, since it has already been reported for oligodendrocyte precursors, that growth factors have the capacity to induce chromatin remodeling and to alter cellular behavior (Kondo and Raff, 2000).
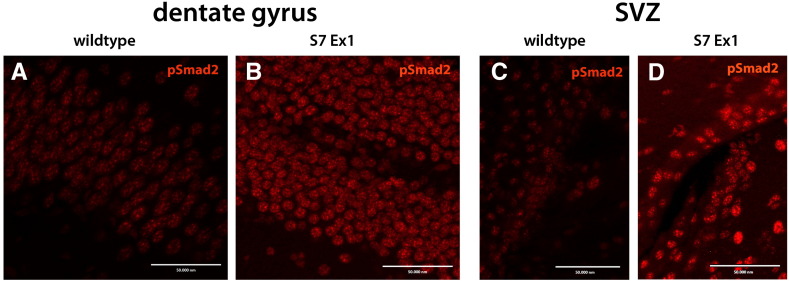
To further address the possibility of a niche-associated influence, we examined if in aged mice, in which TGF-beta levels are reported to be elevated (Pineda et al., 2013), the Smad7ΔEx1 mutation may result in increased intracellular TGF-beta signaling in the 12 month-old mice. As an immunohistological indicator for TGF-beta signaling, we made use of antibodies specific for the phospho-form of Smad2 (pSmad2) (Kandasamy et al., 2010). Typically, ligand binding triggers the phosphorylation of Smad2, and elevated TGF-beta signaling is reflected in increased intensity of pSmad2 staining. Our results showed that staining intensity for pSmad2 was indeed increased in the DG and SVZ of 12 month-old S7ΔEx1 compared to WT mice (Fig. 3).
Fig. 3.
pSmad2 staining intensity is elevated within the neurogenic regions of 12 month old S7ΔEx1 mice.
Immunohistochemical staining of pSmad2 is more intense in the dentate gyrus of 12 month old S7ΔEx1 mice (A) compared to age-matched WT mice (B). Similarly, pSmad2 staining is more apparent in the SVZ of 12 month old S7ΔEx1 mice (C) compared to WT (D).
In summary, these results point toward a TGF-beta dependent enhanced quiescence of neural stem and progenitor cells in aged Smad7ΔEx1 mice. At the same time, the survival of newborn cells is enhanced in Smad7ΔEx1 mice compared to wildtype mice. Both effects, i.e. less proliferation and stem cell quiescence as well as enhanced survival of newly generated cells are likely to involve elevated TGF-beta signaling. This is in consistence with our recent study demonstrating that TGF-beta signaling in the adult neurogenic niche promotes stem cell quiescence as well as survival of newborn cells (Kandasamy et al., accepted for publication). Nevertheless, once released from the niche- and age-associated proliferation inhibition, S7ΔEx1 NPCs regain their cell-intrinsic hyperproliferative activity. In addition, the present study illustrates that a hyperproliferation of NPCs in young age does not necessarily lead to a premature depletion of the stem cell pool.
Conflict of interest
The authors have no conflicts of interests.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grants KR 3344/2-1 to M.K. and Ai 31/3-1 to L.A.), by the Bavarian State Ministry of Sciences, Research and the Arts (ForNeuroCell grant to L.A.) and by the State of Salzburg. Moreover, the work was supported by the Austrian Science Fund, special research funding program SFB F4413-B19 “Cell Signaling in Chronic CNS Disorders” (Vienna, Austria), and by the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement nos. HEALTH-F2-2011-278850 (INMiND) and HEALTH-F2-2011-279288 (IDEA).
Section Editor: Christian Humpel
References
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G.L., Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull N.D., Bartlett P.F. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S., Winner B., Schaubeck S., Aigner R., Vroemen M., Weidner N., Bogdahn U., Winkler J., Kuhn H.G., Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S., Wuertinger C., Kandasamy M., Caioni M., Stadler K., Aigner R., Bogdahn U., Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P.V., Abrous D.N. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Hattiangady B., Shetty A.K. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M., Couillard-Despres S., Raber K.A., Stephan M., Lehner B., Winner B., Kohl Z., Rivera F.J., Nguyen H.P., Riess O., Bogdahn U., Winkler J., von Horsten S., Aigner L. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J. Neuropathol. Exp. Neurol. 2010;69:717–728. doi: 10.1097/NEN.0b013e3181e4f733. [DOI] [PubMed] [Google Scholar]
- Kandasamy M., Lehner B., Kraus S., Ramm Sander P., Marschallinger J., Rivera F.J., Trümbach D., Ueberham U., Reitsame H.A., Strauss O., Bogdahn U., Couillard-Despres S., Aigner L. TGF-beta signaling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014 doi: 10.1111/jcmm.12298. April 30 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H.G., Gage F.H. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Krampert M., Chirasani S.R., Wachs F.P., Aigner R., Bogdahn U., Yingling J.M., Heldin C.H., Aigner L., Heuchel R. Smad7 regulates the adult neural stem/progenitor cell pool in a transforming growth factor beta- and bone morphogenetic protein-independent manner. Mol. Cell. Biol. 2010;30:3685–3694. doi: 10.1128/MCB.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirescu C., Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Pineda J.R., Daynac M., Chicheportiche A., Cebrian-Silla A., Sii Felice K., Garcia-Verdugo J.M., Boussin F.D., Mouthon M.A. Vascular-derived TGF-beta increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol. Med. 2013;5:548–562. doi: 10.1002/emmm.201202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A., Stocker K., Balseanu A.T., Rogalewski A., Diederich K., Minnerup J., Margaritescu C., Schabitz W.R. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41:1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Bostrom E., Westerlund I., Vial C., Buchholz B.A., Possnert G., Mash D.C., Druid H., Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A., Lucin K.M., Czirr E., Park J.S., Couillard-Despres S., Aigner L., Li G., Peskind E.R., Kaye J.A., Quinn J.F., Galasko D.R., Xie X.S., Rando T.A., Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs F.P., Couillard-Despres S., Engelhardt M., Wilhelm D., Ploetz S., Vroemen M., Kaesbauer J., Uyanik G., Klucken J., Karl C., Tebbing J., Svendsen C., Weidner N., Kuhn H.G., Winkler J., Aigner L. High efficacy of clonal growth and expansion of adult neural stem cells. Lab. Invest. 2003;83:949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]
- Wachs F.P., Winner B., Couillard-Despres S., Schiller T., Aigner R., Winkler J., Bogdahn U., Aigner L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 2006;65:358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- Yu S., Patchev A.V., Wu Y., Lu J., Holsboer F., Zhang J.Z., Sousa N., Almeida O.F. Depletion of the neural precursor cell pool by glucocorticoids. Ann. Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]