Abstract
The antiestrogen tamoxifen is a well-tolerated, effective treatment for estrogen receptor-α-positive (ER+) breast cancer, but development of resistance eventually limits its use. Here we show that expression of MAGEA2, and related members of this cancer-testis antigen family, is upregulated in tamoxifen-resistant tumor cells. Expression of MAGEA2 in tumor lines grown in vitro or as xenografts led to continued proliferation in the presence of tamoxifen. At the molecular level, we demonstrate that MAGEA2 protein localizes to the nucleus and forms complexes with p53 and ERα, resulting in repression of the p53 pathway but increased ER-dependent signaling. In a series of ER+, tamoxifen-treated breast cancer patients, we show a highly significant (P=0.006) association between MAGEA (melanoma-associated antigen) expression and reduced overall survival, confirming the clinical significance of our observations.
Introduction
The use of the selective estrogen receptor modulator, tamoxifen, to treat hormone receptor-positive early breast cancer has had significant positive impact on the risk of recurrence and on long-term patient survival rates.1 Tumor expressions of estrogen receptor-α (ER) and progesterone receptor are currently the best predictors of response to antiestrogen therapy. However, all patients with metastatic disease and 30–40% of early disease patients will relapse while on tamoxifen treatment owing to de novo or acquired drug resistance.2
In an attempt to circumvent resistance, additional selective estrogen receptor modulators and the total estrogen antagonist, fulvestrant, plus a series of aromatase inhibitors (AIs), which act to block the synthesis of estrogens in postmenopausal women, have been developed. However, these newer compounds also eventually elicit tumor resistance.3,4 Several large clinical trials have compared tamoxifen treatment with newer endocrine therapies, and, in particular, the use of AIs as first-line treatment has been reported to show improvements in disease-free survival in postmenopausal women (reviewed in Hughes-Davies et al.5). However, meta-analyses have suggested that significant improvements in overall survival (OS) may only be obtained by using switching or sequencing protocols whereby patients receive tamoxifen for 2–3 years before moving to an AI.6 This suggests that, far from being surpassed by newer therapies, tamoxifen remains a vital drug in the treatment of breast cancer, and therefore studies to further biological understanding of tamoxifen resistance prolong its clinical effectiveness remain highly relevant.
In tumor cells resistant to tamoxifen, ER expression is usually retained and continues to regulate breast cancer cell proliferation and survival through cross-talk with other signaling pathways, leading to ligand-independent ER activation (reviewed in Ali and Coombes3 and Musgrove and Sutherland4). Here we have examined gene expression profiles in tamoxifen-resistant (TR) breast tumor-derived lines and report the identification and functional validation of MAGEA2 as a novel TR-associated gene. MAGEA2 is a member of the class 1 melanoma-associated antigen (MAGEA) family of cancer-testis antigens encoded by 12 highly homologous genes located on the X chromosome. They are defined by their lack of expression in somatic adult tissues, but frequent upregulation in a range of solid tumors.7 These proteins are also highly immunogenic, and their ability to induce spontaneous cytotoxic T-lymphocyte-dependent immune responses in cancer patients particularly marks them out as useful targets for immunotherapy.8 Cancer vaccine phase II trials based on recombinant MAGEA3 antigen have shown promise for the treatment of lung cancer and melanoma,9,10 and this has led to increased interest in the expression profile and activity of these proteins in other tumor types.
MAGE proteins are characterized by a ~170-amino-acid MAGE homology domain, which forms a tandem winged-helix structure that can act as a scaffold for protein–protein interactions.7,11 Their precise biological role is still emerging; however, they have been reported to interact with key cellular proteins, notably p53. In a study examining MAGEA2 expression in the acquisition of resistance to the chemotherapy drug, etoposide, MAGEA2 was shown to complex directly with p53 and recruit the histone deactylase, HDAC3, to repress p53 transcriptional activity, thereby protecting cells from apoptosis.2 In a separate study, a number of class I MAGE antigens (from the A, B and C subfamilies) were found to interact indirectly with p53 via another scaffolding protein, the transcriptional corepressor, KAP1 (also termed TRIM28; TIF14), again resulting in apoptosis suppression.13 In multiple myeloma, the interaction of MAGEA proteins with p53 was shown to inhibit apoptosis through repression of Bax and stabilization of survivin.14 All of these studies have suggested that tumor expression of MAGE antigens is linked to treatment failure, prompting us to determine if overexpression of MAGEA2 can contribute to TR in breast tumors.
Results
MAGEA2 expression in TR breast tumor lines
The ER+ human breast tumor lines T47D and ZR75-1 were maintained in media containing 10−7 M tamoxifen until they re-entered the cell cycle and could be expanded. At this point, they were considered as separate, TR lines. In addition, estrogen-deprived (mimicking resistance to an AI), TR (ODTR) lines were also established (see Materials and methods). Gene expression profiles for these derived lines were compared with wild-type (WT) cells using Affymetrix arrays, and the data were analyzed to identify up- or downregulated genes common to the derived lines.
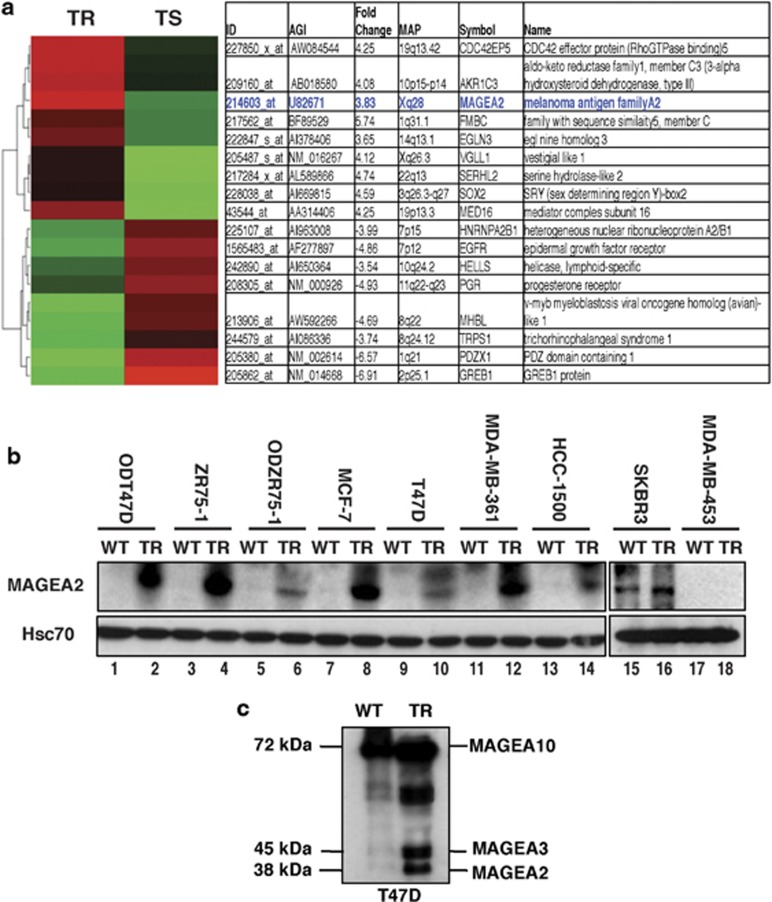
Several known ER target genes, including PGR, GREB1 and PDZK1, were among the downmodulated genes (Figure 1a), but additional genes with no previous link to ER signaling were also significantly regulated. Of these, MAGEA2 showed a consistent fourfold upregulation on the arrays, which was validated by quantitative real-time polymerase chain reaction analysis. On immunoblotting a panel of TR lines and their WT, tamoxifen-sensitive counterparts, WT ER+ cells showed little or no MAGEA2 protein expression, but all had significant induction in their TR derivatives (Figure 1b, lanes 1–14). ER− lines are inherently TR, but most of these lines assayed were also negative for MAGEA2 expression, even when cultured in the presence of tamoxifen (exemplified by MDA-MB-453 cells; Figure 1b, lanes 17 and 18); however, the SKBR3 line did express elevated levels of MAGEA2, which were maintained when the cells were grown in tamoxifen media (Figure 1b, lanes 15 ad 16). As MAGEA genes are often coinduced in tumor cells,7 we also probed blots with a ‘pan-MAGEA' antibody able to recognize several members of the MAGEA family. As illustrated for the T47DTR line (Figure 1c), upregulation of additional MAGEA-related proteins (including MAGEA10, A3 and A1) was observed in MCF-7 and ZR75-1TR lines (data not shown).
Figure 1.
MAGEA2 is upregulated in TR breast cancer cell lines. (a) Heat map of gene expression analysis of TR derivatives of the human breast tumor lines ZR75-1 and T47D. The most significantly up- (red) and downregulated (green) genes compared with WT, sensitive (TS) lines are shown (false discovery rate-corrected P-value <0.05) after unsupervised hierarchical clustering. Information includes Affymetrix probe ID, fold change, cytoband location, gene symbols and SWISSPROT keywords; data for MAGEA2 highlighted in blue color. (b) Whole-cell extracts (20 μg) from WT and TR breast cell lines were western blotted for MAGEA2 expression, as indicated. For T47D and ZR75-1 lines, estrogen-deprived (OD and ODTR) sublines were also available. Blots were reprobed for Hsc70 (loading control). (c) T47D (WT and TR) cell lysates (20 μg) were blotted using the pan-MAGEA antibody 6C1. Identity of bands marked was inferred from size (A10 and A1) or comparison with lysates from cells expressing exogenous genes (A2 and A3).
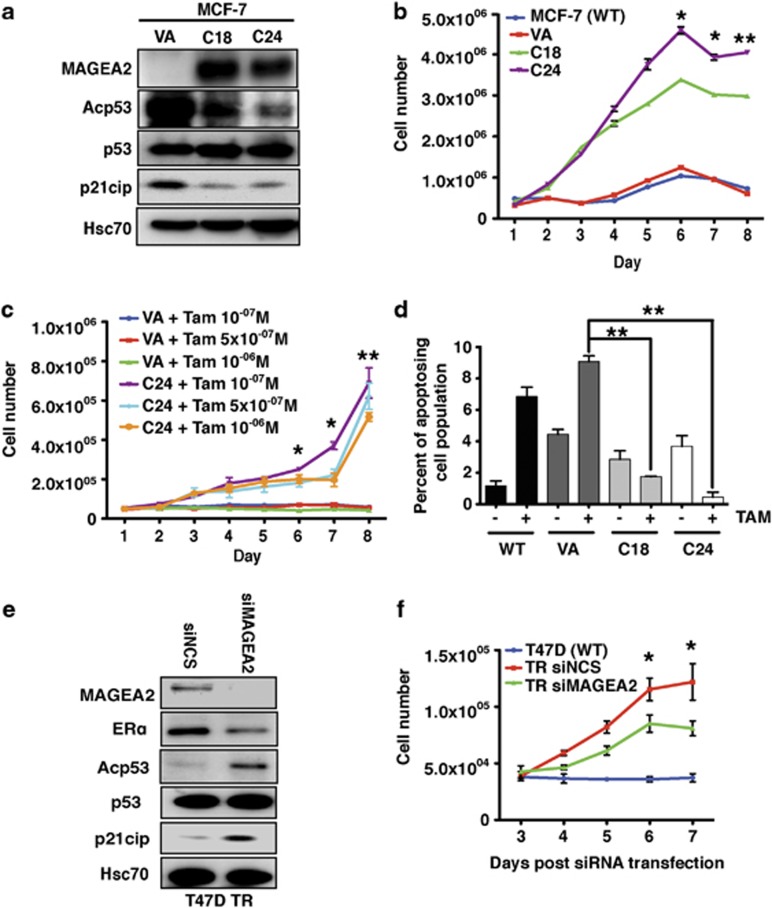
As induction of MAGEA2 overexpression was a characteristic of all of our resistant cell lines, we tested if overexpression of this gene alone could have a functional role in resistance to tamoxifen. Stable MAGEA2-overexpressing lines were generated in WT, tamoxifen-sensitive MCF-7 and T47D breast tumor cells. Several individual colonies were expanded and checked for MAGEA2 expression (see Figure 2a and Supplementary Figure S1A); vector alone (VA)-transfected, non-expressing control lines were also established. Figure 2b illustrates changes in cell number for two individual MAGEA2-expressing MCF-7 derived lines when cultured with tamoxifen, compared with WT and VA control cells. There was a highly significant (P<0.01) difference between growth of the MAGEA2-expressing lines, which continued to proliferate in tamoxifen-containing media, and the controls, which underwent growth arrest. Similar results were also obtained when T47D-derived lines were analyzed: the MAGEA2-expressing clones were again able to sustain their growth in tamoxifen-containing media (P<0.001; Supplementary Figure S1B). In addition, we generated MAGEA3-expressing lines, as this family member was also found to be upregulated in several TR lines, and these also continued to proliferate in the presence of tamoxifen (P<0.001; Supplementary Figure S2). Similar growth assays were used to compare the proliferation capacity of control and MAGEA2-expressing cells over a range of tamoxifen concentrations or in the presence of the total estrogen antagonist, fulvestrant. Expression of MAGEA2 allowed cells to continue to proliferate in 10−6 M tamoxifen (P<0.01; Figure 2c), the highest dose tested; however, neither these cells nor the controls grew in media containing fulvestrant, even at 10−8 M, the lowest dose tested (Supplementary Figure S3).
Figure 2.
MAGEA2 overexpression is functionally linked to TR. (a) Western blot analysis of lysates (20 μg) from MCF-7-stable clones (C18 and C24) expressing exogenous MAGEA2, plus the VA control line, was probed for MAGEA2, p53, acetylated p53 and p21cip, as indicated. Blots were reprobed for Hsc70 (loading control). (b) MCF-7 (WT and VA) and clones C18 and C24 were split into six-well plates (5 × 105 cells per well) and treated with 10−7M tamoxifen (TAM) 24 h later for 8 days. Triplicate wells were harvested and counted daily. (c) MCF-7 VA and C24 lines were split into 12-well plates (5 × 104 cells per well) and treated with a range of tamoxifen concentrations, as indicated, over 8 days. For statistical analysis, data from all the C24 samples were averaged and compared with the averaged VA data. (d) MCF-7 control (WT and VA) and clones C18 and C24 were plated in triplicate on six-well plates (105 cells per well), grown with or without 10−7M tamoxifen for 144 h, and then assayed for Annexin V binding and PI uptake. The graph shows the percentage of early apoptotic (AnnV+PI−) cells for each line and the condition (see Supplementary Figure S4A for full analysis). (a–d) Error bars indicate the s.e. Student's t-test was used to compare data from the indicated sample with VA control; *P<0.05, **P<0.01. (e) Western blot analysis of lysates (20 μg) from T47DTR cells transiently transfected with siRNA targeting MAGEA2 or non-silencing control (NSC) for 48 h probed for the proteins indicated. (f) T47DTR cells were transfected with NSC or siRNA targeting MAGEA2. After 48 h, transfected cells and WT T47D were split into 24-well plates (3 × 104 cells per well in triplicate) and treated with 10−6M tamoxifen 24 h later for 5 days. Error bars indicate the s.e. Student's t-test was used to compare data from NSC- and siMAGEA2-transfected cells; *P<0.05.
Proliferation assays in normal media suggested that MAGEA2 expression did not confer an inherent proliferation advantage (data not shown). We therefore examined if MAGEA2 expression can protect cells from apoptosis, particularly in the presence of tamoxifen, which has been found to induce both cytostasis and programmed cell death in ER+ cells.15 MCF-7-derived control (WT and VA) and two independent MAGEA2-expressing lines were grown in the presence and in the absence of tamoxifen-containing media for 6 days and then assayed for surface expression of Annexin V (AnnV), a recognized hallmark of early apoptotic cells. The samples were stained additionally with propidium iodide (PI) to differentiate between intact cells (AnnV−PI−), and early apoptotic (AnnV+PI−) and late apoptotic/necrotic cells (AnnV+PI+), using fluorescence-activated cell sorting analysis (full cell cycle profile; Supplementary Figure S4a). When the percentage of apoptosing cells in each population was examined (Figure 2d), both MAGEA2-expressing clones showed a significantly (P<0.01) lower proportion of dead and dying cells in the presence of tamoxifen compared with the controls. Again, similar results were also found for the T47D-derived MAGEA2-expressing clones (Supplementary Figure S4B).
As a further functional test, we transiently transfected one of the derived TR cell lines with control or MAGEA2-targeted small interfering RNA (siRNA), and then monitored growth in tamoxifen-containing media over 5 days. Silencing MAGEA2 in T47DTR cells occurred with reasonable efficiency (Figure 2e) and these cells showed a significantly (P<0.05) reduced ability to proliferate in 10−6 M tamoxifen, compared with cells transfected with control siRNA (Figure 2f). In our gene expression profiling of the T47DTR line, nearly 2000 genes were identified as differentially regulated compared with WT cells. Therefore, our finding that growth of these cells in tamoxifen is compromised by silencing MAGEA2 alone strongly implicates it as a causal gene in the TR phenotype.
MAGEA2 regulates the transcriptional activity of p53 and ER
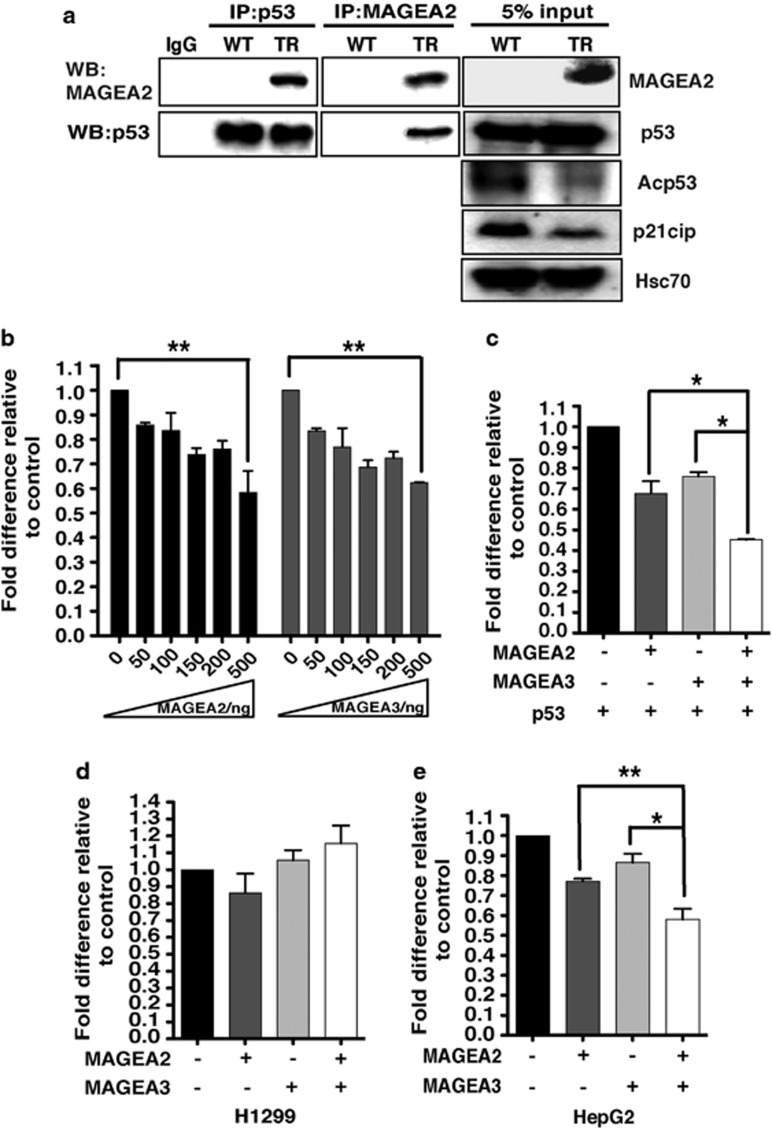
Published data12 have suggested that, in chemoresistant cells, MAGEA proteins can interact with, and thereby inhibit the activity of, p53. We therefore used co-immunoprecipitation (CoIP) to examine if MAGEA2 can complex with p53 in breast tumor cells, and whether this impacts on p53 signaling, initially analyzing a T47D-derived line (C30 cells) expressing exogenous MAGEA2. MAGEA2 was detected in p53 complexes in C30 cells but not in control cells (Supplementary Figure S5A, upper left panel). Similarly, p53 was found in MAGEA immunoprecipitates (Supplementary Figure S5A, middle lower panel), thus confirming formation of a complex between p53 and exogenous MAGEA2. Additional western blots demonstrated equal expression of total p53, but markedly reduced levels of both acetylated p53 and its downstream target gene; the cell cycle inhibitor, p21cip/CDKN1A, was observed in the MAGEA2-expressing cells (Supplementary Figures S5A (right panels), S1A and S5B). The constant p53 level, but reduction in acetylated p53 and p21cip levels, was also observed in the MCF-7 MAGEA2-expressing cell lines compared with controls (see Figure 2a).
A CoIP experiment was also performed to study complex formation by endogenous MAGEA2 expressed by the T47DTR-derived line. This confirmed complex formation between endogenous p53 and MAGEA2 in the TR cells, which again showed reduced levels of p21cip and acetylated p53, compared with cell lysates from WT cells, which lack MAGEA2 expression (see Figure 3a and Supplementary Figure S5B). Consistent with these findings, silencing MAGEA2 in T47DTR cells caused levels of acetylated p53 to rise compared with cells treated with control siRNA (Figure 2e). Of note here, although T47D cells carry mutant p53, this mutation (L194F) retains significant ability to induce p53 target genes, and this cell line can still undergo p53-dependent apoptosis.16, 17, 18, 19
Figure 3.
MAGEA2 interacts with the p53 pathway in breast cancer lines. (a) Whole-cell extract (WCE) lysates from WT and TR T47D cells were immunoprecipitated (IP) for either p53 (left panels) or MAGEA2 (middle panels), and subsequent western blots (WBs) were probed for the same proteins, as indicated. Separate WBs (loaded with 5% of the protein levels used for the IP experiments; right panels) were probed for the indicated proteins. (b) H1299 (p53-null) cells were transfected with the pG1PyLuc reporter plasmid (150 ng), a p53 expression vector (10 ng) and increasing amounts of MAGEA2 or MAGEA3 expression vectors, as indicated. (c) As in (b) but with MAGEA2 and/or MAGEA3 expression vectors (100 ng), as indicated. (d and e). As in (c) but with the p21cip-Luc reporter plasmid (150 ng) without the addition of p53 expression plasmid using p53-null H1299 cells (d) or p53 WT HepG2 cells (e). (b–e) Cells were harvested after 48 h. Luciferase values were corrected for transfection efficiency and expressed as fold activity compared with the reporter alone control (set at 1) using data from three independent experiments, each performed in triplicate; error bars indicated the s.e. Student's t-test was used to compare data from the indicated samples, *P<0.05; **P<0.01.
As acetylation of p53 is a hallmark of transcriptional activity,20,21 the reduction in its acetylation level in MAGEA2-expressing lines, coupled with decreased expression of the p53 target gene p21cip/CDKN1A in tamoxifen media, strongly suggests that MAGEA2 protein is able to protect cells from growth arrest by interfering with the p53 pathway, possibly by altering p53 transcriptional activity directly. To examine this, p53-null H1299 cells were transfected with the p53-dependent pG13PyLuc synthetic reporter construct, an optimized amount of p53 expression vector and a range of concentrations of either an MAGEA2 or an MAGEA3 expression vector. In each case, a dose-responsive repression of reporter activity was observed as MAGEA expression increased (Figure 3b). We also compared the activity of the natural p53 target gene, p21cip, in H1299 and HepG2 (p53 WT) cells; expression of MAGEA2 or MAGEA3 inhibited p21cip reporter activity only in HepG2 cells, confirming the p53-dependent nature of the repression (compare Figures 3d and e). Moreover, co-transfection of MAGEA2 and MAGEA3 expression vectors further repressed the p53-dependent transcriptional activity of both reporter constructs (Figures 3c and e), suggesting that MAGEA family members can act cooperatively to regulate p53 activity and hence p21cip expression.
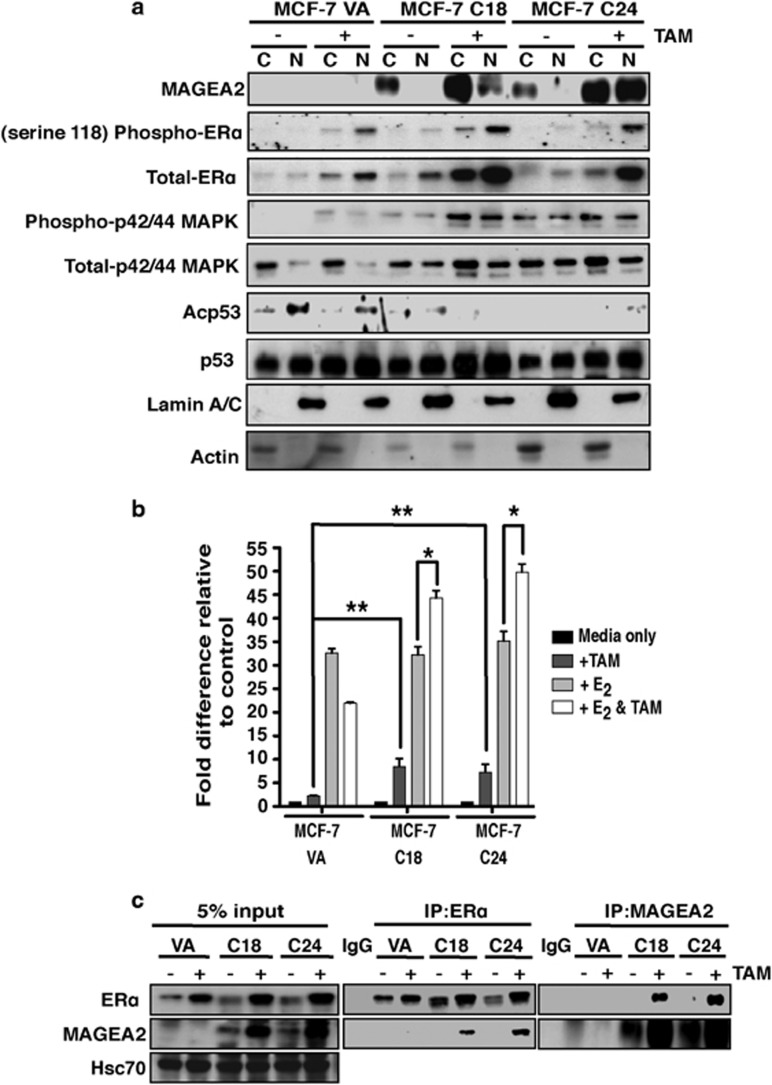
To complex with p53 and repress its activity, MAGEA2 must either localize to the nucleus or cause the relocalization of p53. Control and MAGEA2-expressing MCF-7 cells were grown with and without tamoxifen and subfractionated to isolate cytoplasmic and nuclear extracts for analysis by western blotting. As illustrated in Figure 4a, MAGEA2 protein was detected chiefly in the cytoplasm of overexpressing cells grown in normal media, but was increased in the nucleus in cells exposed to tamoxifen. This correlated with loss of acetylated p53, whereas the levels of total p53 remained constant. Similar observations concerning MAGEA2 subcellular localization were also made in fractionated T47DTR cells (Supplementary Figure S5B) and on MCF-7 C24 cells using confocal microscopy (Supplementary Figure S5C).
Figure 4.
MAGEA2 interacts with the ER. (a) MCF-7-derived MAGEA2-expressing (C18 and C24) and VA lines were incubated with and without 10−7 M tamoxifen (TAM) for 8 days. Nuclear (N) and cytoplasmic (C) extracts (20 μg) were analyzed by western blotting for the indicated antigens. Blotting for lamin A/C and actin confirmed extract integrity. (b) MCF-7VA and C18/C24 cells were transiently transfected with an ERE-luciferase reporter construct (100 ng) and left untreated or given 10−8 M E2 and/or 10−6 M TAM, as indicated, 24 h later. Luciferase values were corrected for transfection efficiency and expressed as fold activity compared with untreated cells (set at 1) using data from three independent experiments, each performed in triplicate; error bars indicate the s.e. Student's t-test was used to compare data from the indicated samples, *P<0.05; **P<0.01. (c) WCE lysates from MCF-7VA and C18/C24 cells treated as in (a) were immunoprecipitated (IP) for ER (middle panels) or MAGEA2 (right panels), and then blots were probed for ER (top row) or MAGEA2 (middle row), as indicated. Control blots (left panels) carrying 5% of the protein used for the IP were additionally probed for Hsc70 as a loading control.
Taken together, these data suggest that in tamoxifen-treated cells MAGEA2 is increased and localizes to the nucleus, which in turn prevents p53-dependent cell cycle arrest. Another protein known to be increased by tamoxifen treatment is ERα itself (Laios et al.,22 and this effect was also observed in our VA control cells; Figure 4a, first 4 lanes). Total ERα and its active form, phospho-ERα (serine 118) levels were even further enhanced, however, in the MAGEA2-overexpressing lines, particularly upon tamoxifen treatment (Figure 4a, lanes 5–8), whereas silencing MAGEA2 in T47DTR cells had the reverse effect, reducing total ERα load (Figure 2e). Furthermore, our data also indicate that phospho-p42/44 mitogen-activated protein kinase (MAPK) activity is enhanced in MAGEA2-overexpressing cells after tamoxifen treatment (Figure 4a, lanes 5–8). As MAPK also regulates ERα transcriptional activity,3,4 these observations together suggest strongly that MAGEA2 can also impact on ER-mediated pathways. To determine whether MAGEA2 affects ERα transcriptional activity, MCF-7 VA control and MAGEA2-overexpressing clones were pre-treated with estrogen-free media for 48 h, and then transiently transfected with an ERE-dependent luciferase reporter construct in the presence or in the absence of β-estradiol (E2) or tamoxifen. Compared with observations in VA cells, overexpression of MAGEA2 significantly enhanced the transcriptional activity of ERα in the presence of tamoxifen alone and reversed the suppression of E2-mediated activation in cells treated with both reagents (Figure 4b). Further CoIP assays were then performed to investigate if MAGEA2 and ERα are able to form a complex in these cells. As illustrated in Figure 4c, ERα was found in MAGEA2 immunoprecipitates and MAGEA2 was found in ERα precipitates, confirming that these two proteins can associate, in particular, with tamoxifen-treated cells. Again, this interaction was verified using CoIP with endogenous proteins expressed in the T47DTR line (see Supplementary Figure S6).
MAGEA2-expressing xenografts are resistant to tamoxifen treatment
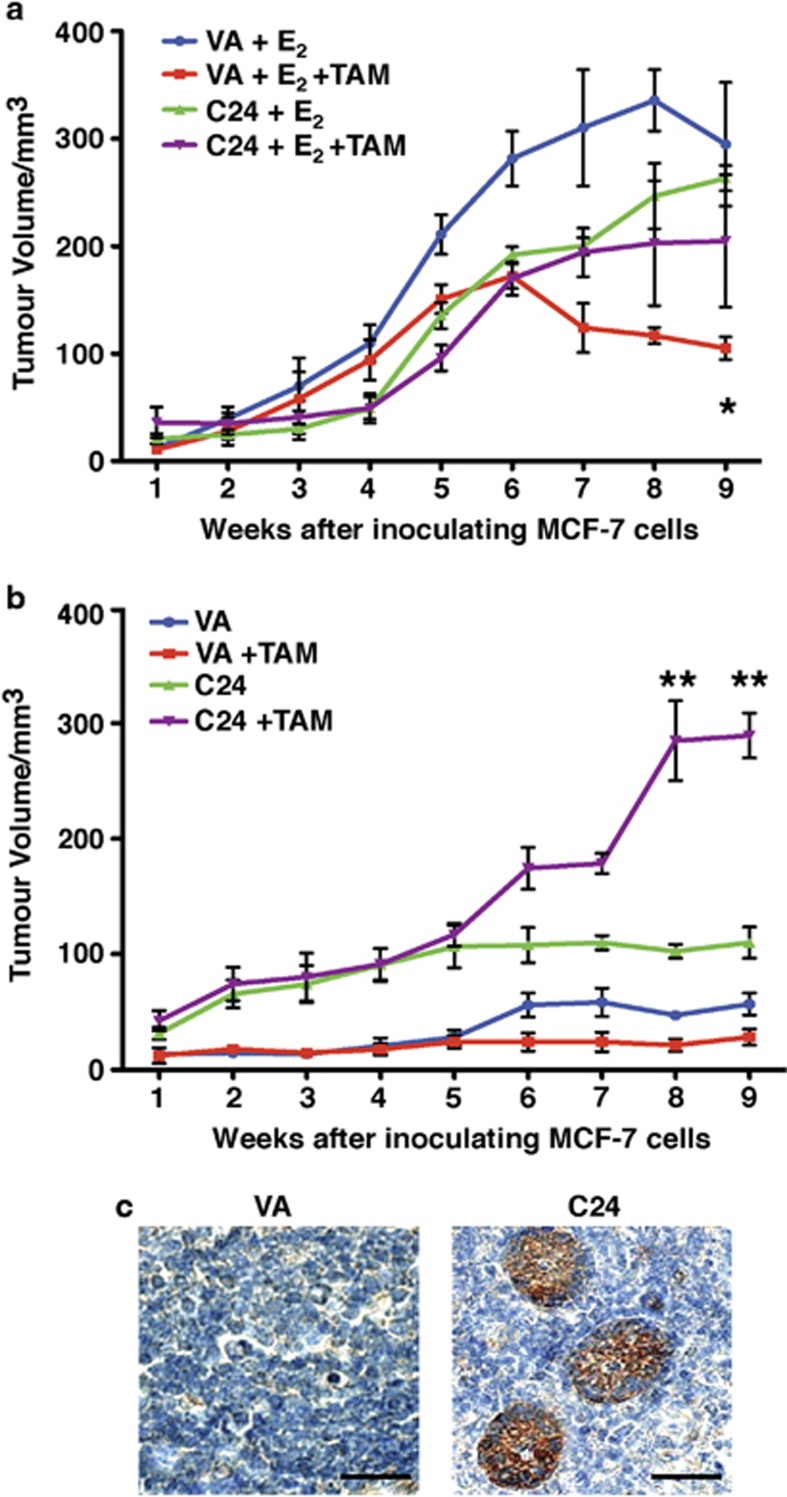
We next tested the ability of MAGEA2 to confer resistance to tamoxifen in an in vivo model of breast cancer. MAGEA2-expressing MCF-7 C24 and VA control cells were injected subcutaneously into opposite flanks of ovariectomized nude mice in the presence or in the absence of E2 pellets. In the presence of E2, both control and MAGEA2-overexpressing cells established tumors that grew at a similar rate (Figure 5a). In contrast, in the absence of E2 supplementation, VA control cells were unable to form tumors, whereas MAGEA2-expressing cells formed small tumors (Figure 5b).
Figure 5.
MAGEA2-expressing lines are also resistant to tamoxifen when grown as xenografts. MCF-7-derived VA and C24 cells (5 × 106) were inoculated into opposite flanks of female ovariectomized nude mice and tumor growth was monitored over 9 weeks. (a) In vivo tumor growth of C24 and VA xenografts in 20 mice carrying E2 pellets at the time of inoculation. After 3 weeks, half the mice were additionally implanted with a tamoxifen pellet as indicated. Growth of VA tumors in mice with tamoxifen pellets (red line) was significantly impaired compared with VA tumors in mice with just E2 pellets (blue line; *P<0.05); there was no significant difference in the growth of C24 tumors±tamoxifen (green and purple lines; NS). (b) In vivo tumor growth of C24 and VA xenografts in mice with either no hormone supplement or carrying a tamoxifen pellet at the time of inoculation (10 mice each group), as indicated. Growth of C24 tumors was significantly accelerated in the presence of tamoxifen at 8 and 9 weeks compared with tumors grown without supplementation (purple and green lines; **P<0.01). (c) Representative tumors (from a) were recovered at the time of being killed, fixed and stained for MAGEA. Scale bar, 20 μm.
To evaluate the effect of antiestrogens on xenograft growth, we implanted the mice with tamoxifen pellets after establishing E2-induced tumors. In agreement with the cell culture assays, the growth of MAGEA2-overexpressing tumors was not inhibited by tamoxifen, whereas the control tumors regressed upon tamoxifen treatment (P<0.05; Figure 5a). One cohort of mice was implanted with tamoxifen pellets alone. Surprisingly, tamoxifen supplementation accelerated growth of MAGEA2-overexpressing xenografts threefold relative to the same cells grown without tamoxifen pellets (P<0.01; Figure 5b). This response may reflect the increased MAGEA2 nuclear expression observed in tamoxifen-treated cells noted above (Figure 4a) coupled with activation of ER-dependent proliferation and survival pathways. Immunohistochemical analysis confirmed the persistence of MAGEA2 overexpression in the C24 xenografts (Figure 5c). Overall, our data demonstrate that overexpression of MAGEA2 lowers the E2 requirement for MCF-7 cells to grow as xenografts and renders the tumors insensitive to antiestrogen therapy.
MAGEA expression is associated with reduced OS in patients with ER+ primary breast cancer treated with tamoxifen
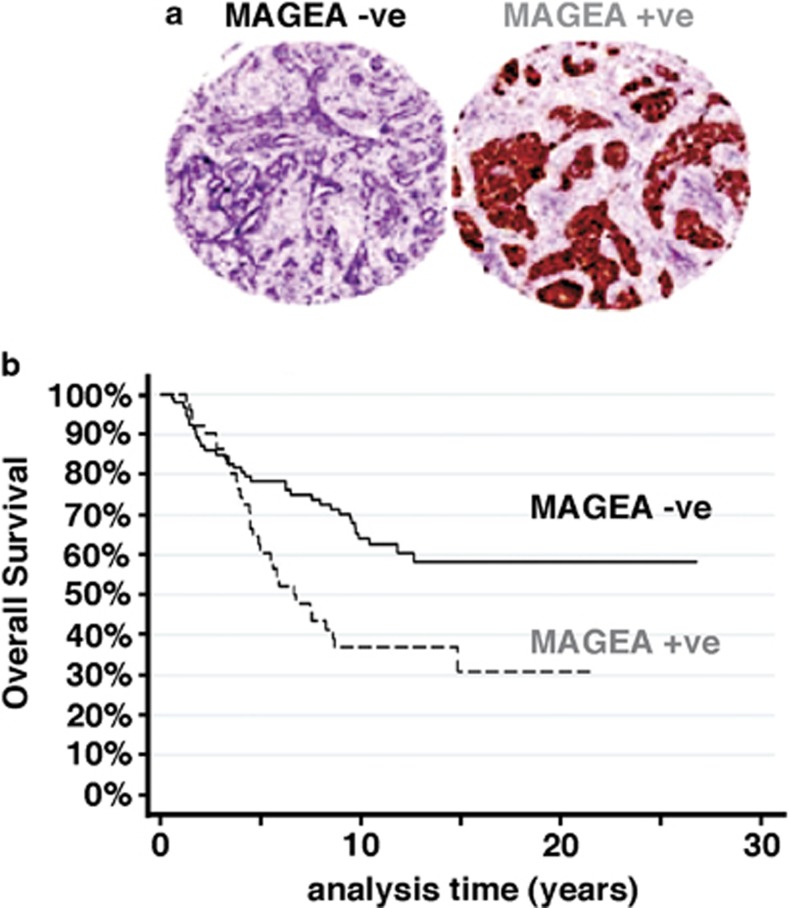
To determine if these observations have clinical significance, pan-MAGEA immunostaining was assessed on sections from formalin-fixed, paraffin-embedded material from 144 cases of ER+ primary breast carcinoma collected from three separate centers (see Materials and methods). All the patients had been treated with adjuvant tamoxifen alone until relapse. Tumors positive for MAGEA (35%) all showed moderate or strong intensity staining with both nuclear and cytoplasmic distribution (Figure 6a). As noted previously,23 there was significant intertumor variation as to which compartment predominated.
Figure 6.
MAGEA expression in ER+ tumors correlates with reduced OS. Expression of pan-MAGEA antigens was assessed in 144 cases of ER+, tamoxifen-treated primary breast cancer by immunohistochemical analysis on whole sections or TMA, as available. (a) Representative patterns of positive and negative staining on TMA (x5 magnification). (b) Kaplan–Meier survival curves showing the relationship between positive and negative staining for MAGEA on OS. The two-sided P-value (P=0.006) was calculated using log-rank testing.
Positive MAGEA staining was associated with a highly significant reduction in OS (P=0.006) as shown in Figure 6b, where a marked separation in survival curves is evident after 5 years of follow-up. This therefore suggests a link between MAGEA expression in the primary tumor and clinical resistance to tamoxifen therapy, consistent with our in vitro and xenograft data.
Discussion
Although initially identified over 20 years ago, the functional role and mode of action of MAGE antigens is still highly controversial. Here we demonstrate, for the first time, the pleiotropic effects of MAGEA2 antigen on ER+ breast tumor cells, leading to their continued proliferation and survival in the presence of physiological levels of the therapeutic antihormone, tamoxifen.
In TR ER+ breast cancer cells, and lines engineered to overexpress MAGEA2, we were able to detect a complex between MAGEA2 protein and p53, which was consistent with the observed inhibition of the p53 pathway in these cells. Previous studies have also noted a functional interplay between p53 and several MAGEA antigens including MAGEA2; however, the nature of this interaction at the molecular level is disputed. Two studies suggested that MAGEA and p53 proteins do not interact directly but via binding to different regions of RING (Really Interesting New Gene) domain-containing proteins, particularly in the transcriptional corepressor, KAP1 (also termed TRIM28, TIF-1β). The association with KAP1 has been suggested variously to recruit histone deactylases to p53,13 or to lead to p53 ubiquitination and degradation.11 In contrast, others have used p53-derived peptides or bacterially made proteins to map a direct interaction between MAGEA antigens and the DNA-binding surface of p53.12,24 Using published reagents, we have not been able to detect an interaction between MAGEA2 and KAP1 or histone deactylases in the breast tumor lines studied here (unpublished data), nor did we see any reduction in either WT or mutant p53 levels in a range of MAGEA2-expressing breast lines; however, we did observe a clear diminution in the level of p53 acetylation both in MAGEA2-overexpressing cells and in lines selected for TR (Figures 2a, e and 4a and Supplementary Figures S1A and S4A), which was completely consistent with the reduced expression of the cell cycle inhibitor, p21cip/CDNK1A, and the continued growth in tamoxifen exhibited by these lines. Our demonstration that MAGEA2 represses the transcriptional activity of p53 (Figures 3b and d) agrees with work by Meek and co-workers24 who linked MAGEA binding to the p53 DNA-binding domain to a lack of p53 interaction at its endogenous target genes, including p21cip/CDKN1A, as monitored using chromatin immunoprecipitation. As found here, p53 stability was not altered by coexpression with MAGEA antigens. This suggests therefore that MAGEA2 expression in resistant cells reduces the ability of tamoxifen-induced p53 to bind to its target genes, resulting in less efficient G1/S arrest and apoptosis and continued proliferation in the presence of the antihormone, as observed using cell culture (Figures 2b and d) and xenografts (Figure 5b). Reduced p53 association with chromatin will also limit its access to histone acetyl transferases, thus accounting for the lower levels of acetylated p53 in our TR- and MAGEA2-expressing cells. Consistent with this proposed molecular interplay between MAGEA2 and the p53 pathway, we have also confirmed that MAGEA2 protein is increased in the nucleus, and, in particular, in tamoxifen-treated cells (Figure 4a).
Although MAGEA2-expressing cells were found to be resistant to a range of tamoxifen concentrations (Figure 2c), they were not cross-resistant to the total estrogen antagonist, fulvestrant. As fulvestrant also acts as a selective estrogen receptor degrader, stimulating cellular turnover of ERα,25 this demonstrates that intact ER signaling is also essential for MAGEA2 activity in TR breast cancer cells. Using reporter assays, we show that ERα transcriptional activity is no longer inhibited by tamoxifen in MAGEA2-expressing lines (Figure 4b); instead, tamoxifen acts as an ER agonist in these cells. This change in cellular response to tamoxifen is a hallmark of resistant breast cancer and results from alterations in the activity, abundance or stoichiometric balance of ER corepressors and coactivators in TR cells (reviewed in McDonnell and Wardell26 and Green and Carroll27). Significantly, we were able to show that MAGEA2 and ERα can form complexes in tamoxifen-treated MAGEA2-expressing lines and TR cells (Figure 4c and Supplementary Figure S6). Given the fact that the tamoxifen treatment induces nuclear expression of MAGEA2 (Figure 4a), it is tempting to speculate that the interaction of ERα with MAGEA2 is predominantly in the nucleus, as it is not observed in non-tamoxifen-treated cells (Figure 4c). At this stage, the interaction of ERα with MAGEA2 could be either direct or could involve ER cofactors. Given that our data show an increase in MAPK activity, which correlates with increased ER phosphorylation (Figure 4a), this pathway may act to aid downstream ERE promoter activity via MAGEA2 (Figure 4b).
We examined MAGEA expression in a series of ER+ breast cancers with an antibody validated for use on formalin-fixed, paraffin-embedded material,23,28 which detects several members of the MAGEA family. In our panel of derived resistant lines, although MAGEA2 was the most consistently overexpressed, we also readily detected the expression of MAGEA3 and A10 and to a lesser extent MAGEA1 (Figure 1c). Furthermore, silencing MAGEA2 alone in these cells led to a significant but incomplete reversal of the TR phenotype (Figure 2f). We have also shown that MAGEA3 can cooperate with MAGEA2 in the transcriptional repression of p53 (Figures 3b and c), and lines selected to overexpress MAGEA3 were also resistant to tamoxifen (Supplementary Figure S2). Taken together, these observations suggest that several members of this highly homologous family can functionally contribute to TR, in agreement with the overlap in MAGEA activity reported in other studies.12,24
Analysis of our tumor study revealed a highly significant association (P=0.006) between positive MAGEA staining and reduced OS (Figure 6b). Previous studies have shown that a low percentage of ER-positive breast cancers are MAGEA positive.23,28 However, these studies were restricted to cancers that were not TR. In contrast, although all the patients in our study were treated with tamoxifen only, 66% of them were TR substantiating our results. This strongly implies a link between expression of members of the MAGEA family and failure of tamoxifen therapy. When logistic regression was used to impute the missing values (see Supplementary Methods) as an exploratory analysis, both grade (P=0.001) and MAGEA expression (P=0.006) could be shown to be significant covariates with an increased hazard ratio of 2.027 (95% confidence interval: 1.228–3.345) for MAGEA expression, implying that patients with MAGEA-positive primary tumors were two times as likely to progress. Relating these findings to the clinic suggests that MAGEA-positive cases are unlikely to respond to tamoxifen; indeed, from our observations on MAGEA2-expressing xenografts (Figure 5b), treatment may even accelerate tumor growth. However, patients with MAGEA-negative tumors could be treated and then monitored for MAGEA upregulation as a marker of TR onset. In this regard, it is significant that the reliable detection of MAGEA3 expression in circulating breast tumor cells has recently been described.29 Patients with de novo, or acquired MAGEA expression could be switched to treatment with an AI; however, we show here that a selective estrogen receptor degrader such as fulvestrant may be more effective (Supplementary Figure S3). Unfortunately, the poor bioavailability of fulvestrant has meant that its effectiveness in vitro has, to date, not been replicated in the clinic (reviewed McDonnell and Wardell26). As an alternative, current MAGEA immunotherapy regimens,9,10 given either as cotherapy with tamoxifen or at the onset of MAGEA expression, may be a viable approach to treating hormone-resistant breast cancer.
Materials and methods
Cell lines
HepG2, H1299 and all breast tumor-derived lines (apart from H3396) were obtained from the ATCC (Manassas, VA, USA) and grown according to their recommendations. H3396 cells30 were cultured in RPMI plus 10% fetal calf serum. TR sublines were derived by continuous culture in media supplemented with 10−7M 4-hydroxy tamoxifen (Sigma, Dorset, UK) for 3–6 months until the cells could be expanded. Estrogen-deprived (OD) lines were maintained in low estrogen conditions for 6 months as described previously,31 using phenol red-free media supplemented with charcoal-stripped serum (Sigma), and ODTR versions were derived by subsequent culture in tamoxifen (10−7M). The following lines have been authenticated (May 2011) by STR profiling (LGC Standards): MCF-7 (WT, TR, C18, C24, VA), T47D (WT, TR) and H3396 (WT, TR). All proliferation assays were repeated at least two times with the repeats showing similar results; representative experiments are presented. Media were supplemented with vehicle, tamoxifen or fulvestrant (Sigma) where indicated. Proliferation and apoptosis assays were carried out as detailed previously.32
Plasmids, siRNA and transfection conditions
The insert from a MAGEA2 cDNA clone (IMAGE Consortium, clone ID: 8327628) was subcloned into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). Plasmid DNA (with and without MAGEA2 insert) was introduced into T47D and MCF-7 cells using nucleofection (Amaxa, Basel, Switzerland). Stable clones were selected in media containing 500 μg/ml G418, expanded and characterized for MAGEA2 expression by western blot and quantitative polymerase chain reaction analyses. The p21cip/CDKN1A promoter luciferase reporter vector and pCMV p53 expression plasmid were generous gifts from Prof Dennis McCance (Queen's University, Belfast, UK). The p53 reporter PG13PyLUC construct was purchased from Addgene (Cambridge, MA, USA) and the pGL3-ERE-luciferase vector was kindly provided by Professor Simak Ali (Imperial College London, UK). For reporter assays, cells were transfected using GeneJuice (Merck Bioscience, Darmstadt, Germany) and efficiency was monitored using the pRL-TK Renilla control vector (Promega, Madison, MI, USA). All samples in a series were transfected with an equal amount of DNA with an appropriate amount of pcDNA3.1 vector added as makeweight. Cell extracts were harvested at 48 h and assayed by Dual Luciferase assays following the manufacturer's protocol (Promega). For siRNA transfection, subconfluent T47DTR cells were transiently transfected using INTERFERin (Polyplus, Illkirch-Graffenstaden, France) with 40 ng of non-silencing control siRNA (Qiagen, Valencia, CA, USA; no.1022076) or On Target Plus Smartpool MAGEA2 targeting siRNA (Dharmacon, Lafayette, CO, USA; no. 00635001).
Gene expression profiling and data analysis
RNA was extracted from log-phase T47DTR, ZR75-1TR, ODT47DTR and ODZR75-1TR cells using TRIZOL reagent (Invitrogen) and purified over an RNeasy Mini column (Qiagen), including the DNAse I digestion step. Total RNA (5 μg) was prepared for hybridization to Affymetrix GeneChip Human Genome U133 Plus 2.0 oligonucleotide arrays following the manufacturer's recommendations. The data can be viewed at http://www.ncbi.nlm.nih.gov/geo using accession number GSE22664. Details regarding data handling and analysis of differential gene expression compared with WT lines are given in the Supplementary information.
Western blotting
Cells were washed with phosphate-buffered saline and scraped into chilled lysis buffer (8 M urea, 1 M thiourea, 0.5% CHAPS (3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate), 50 μM dithiothreitol and 24 μM spermine). Lysates were assayed for protein using Bradford reagent (Pierce, Rockford, IL, USA), resolved by denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then electroblotted onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Proteins were detected with antibodies to: pan-MAGEA (clone 6C1; Santa Cruz, Dallas, TX, USA; sc-20034), MAGEA2 (sc-130164), MAGEA3 (sc-130809), p53 (DO1, sc-126), acetylated(Ac)-p53 (Cell Signaling, Beverly, MA, USA; no. 2525), lamin A/C (Cell Signaling; no. 4477), p21cip (Cell Signaling; no. 2946), ERα (sc-56833), phospho-ERα (Ser 118) (Cell Signaling, no. 2511), p42/44 MAPK (Cell Signaling; no. 9102), phospho-p42/44 MAPK (Cell Signaling; no. 4370), actin (sc-130301) or Hsc 70 (sc-65521), and visualized with ECL chemiluminescence reagent (Amersham, Buckinghamshire, UK).
CoIP assays
Immunoprecipitation was carried out using the Immunoprecipitation Kit (Invtirogen) according to the manufacturer's protocol. Briefly, cells were lysed in buffer (20 mM Tris-HCl (pH7.6), 170 mM NaCI, 1 mM ethylenediaminetetraacetic acid, 0.5% NP40, 1 mM dithiothreitol) supplemented with 5 μM trichostatin A and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Cell lysate (1 mg) was incubated with 50 μl of Dynabeads (Invitrogen) and either 2 μg anti-p53 (DO1), 4 μg anti-pan-MAGEA, 4 μg anti-ERα or control immunoglobulin G (Santa Cruz; sc-2027) overnight at 4 °C. The next day, immunoprecipitates were washed three times with the wash buffer (Invitrogen) for 5 min at 4 °C, and resuspended in 45 μl of 2 × sodium dodecyl sulfate western loading buffer. The samples were resolved via sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes and protein detection was achieved by western blotting.
Xenograft studies
Control (VA) or MAGEA2-expressing (C24) MCF-7 cells were suspended in matrigel and injected subcutaneously (5 × 106 cells per mouse) in opposite flanks of 6–8-week-old ovariectomized athymic mice (Harlan, Oxfordshire, UK). Subcutaneous implantation with E2 pellets (17E2 0.25 mg, 60-day release; Innovative Research, Novi, MI, USA; 20 mice) or tamoxifen pellets (25 mg, 21-day release pellet, 10 mice) occurred a day before cell inoculation. A further 10 mice were not implanted with any pellets. After 3 weeks, 10 of the estrogen-treated mice were additionally implanted with tamoxifen pellets. Tamoxifen pellets were replaced by reinsertion on day 21. Tumor size was measured in three dimensions in millimeters weekly and tumor volumes were calculated according to the formula: volume=π/6 × (length × width × height) and expressed in mm3. For histological analysis, dissected tumors were fixed overnight in formaldehyde, sectioned and stained with the pan-MAGE antibody C61.
Clinical material
Paraffin sections from formalin-fixed primary tumors from 144 patients with a confirmed diagnosis of ER+ breast cancer were analyzed from three separate cohorts as follows: 74 cases from the EORTC 10850/1 trials33,34 at Guys and St Thomas' Hospital (London, UK); 32 cases identified from clinical records (by CCY and JLJ) of patients from Bart's and the London Hospital (BLT); 38 cases representing a subset of ER+ cases from a series collected at St James' University Hospital (Leeds, UK).35 All patients were operated on for their primary tumor and received adjuvant tamoxifen treatment for an average of 62 months (BLT and Leeds cases; Guys and St Thomas' Hospital cases received tamoxifen for life) or until relapse when patients were switched to a range of chemotherapeutic regimens. Clinicopathological details are given in Supplementary Table S1.
Immunohistochemistry
For the BLT cases, a tissue microarray (TMA) was constructed from routinely fixed, paraffin-embedded tumor blocks taking 3 × 0.5 mm2 cores selected from representative tumor areas. A TMA for the Leeds cases was already available;35 the Guys and St Thomas' Hospital cases were examined on whole sections. Sections from MCF-7 WT and C24 cell pellets embedded in paraffin were used as negative and positive controls, respectively. Sections were dewaxed and rehydrated using standard methods and antigen retrieval was performed by pressure cooking with antigen unmasking solution (Vector Labs, Peterborough, UK; H-3300). The pan-MAGEA mouse monoclonal antibody 6C1 was applied at 1:100 dilution for 40 min at room temperature. Slides were then processed using a DAKO autostainer and Vectastain Elite (Vector Labs, PK-6200) with DAB (3,3′-diaminobenzidine tetrahydrochloride) as chromogen and then counterstained. MAGEA staining was scored by two pathologists. Staining was either homogeneously positive throughout the tumor, both cytoplasmic and nuclear, or absent (see Figure 6a).
Statistical analysis
Survival curves were calculated by Kaplan–Meier method for the clinical study. Student's t-test was used for growth curves. All P-values were two-sided; P<0.05 was considered significant.
Research ethics
All studies in mice were performed under license and followed UK Home Office regulations. Access to patient samples for immunohistochemistry was via ethical approval granted by the East London and City Research Ethics Committee (LREC Ref: 06/Q0603/25; Guys and St Thomas' Hospital samples) or (LREC Ref: 05/Q403/199; BLT samples) or from the Leeds (East) Local Research Ethics committee(LREC Ref: 06/Q1206/180; Leeds samples).
Acknowledgments
We are grateful to our colleagues Andrew Clear, Keyur Trivedi and Abeer Shaaban for technical assistance with the TMA for the BLT and Leeds cases, respectively, Dr Yaohe Wang for help with scoring the Immunohistochemistry study, Ian Hart and Steven Robinson for invaluable advice and help with the xenograft study and to Richard Grose for useful comments on the manuscript. We thank Tracey Chapman for expert handling of the Affymetrix arrays and Simak Ali, Dennis McCance and Hinrich Gronemeyer for the generous provision of reagents. This work was funded by Cancer Research UK and Barts and the London Charity.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- (EBCTCG) EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Loi S, Haibe-Kains B, Desmedt C, Wirapati P, Lallemand F, Tutt AM, et al. Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genom. 2008;9:239. doi: 10.1186/1471-2164-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–115. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- Hughes-Davies L, Caldas C, Wishart GC. Tamoxifen: the drug that came in from the cold. Br J Cancer. 2009;101:875–878. doi: 10.1038/sj.bjc.6605231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, et al. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JM, Gao J, Wang J, Yang M, Potts PR. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–974. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte M, Simonatto M, Peche LY, Bublik DR, Gobessi S, Pierotti MA, et al. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 2006;103:11160–11165. doi: 10.1073/pnas.0510834103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, O'Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM, et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007;67:9954–9962. doi: 10.1158/0008-5472.CAN-07-1478. [DOI] [PubMed] [Google Scholar]
- Nardiello T, Jungbluth AA, Mei A, Diliberto M, Huang X, Dabrowski A, et al. MAGE-A inhibits apoptosis in proliferating myeloma cells through repression of Bax and maintenance of survivin. Clin Cancer Res. 2011;17:4309–4319. doi: 10.1158/1078-0432.CCR-10-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AJ, Sutherland RL, Musgrove EA. Live or let die: oestrogen regulation of survival signalling in endocrine response. Breast Cancer Res. 2007;9:306. doi: 10.1186/bcr1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toillon RA, Chopin V, Jouy N, Fauquette W, Boilly B, Le Bourhis X. Normal breast epithelial cells induce p53-dependent apoptosis and p53-independent cell cycle arrest of breast cancer cells. Breast Cancer Res Treat. 2002;71:269–280. doi: 10.1023/a:1014422101452. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Li XS, Chen JC, Shao ZM, Ordonez JV, Fontana JA. Mechanisms of regulation of WAF1/Cip1 gene expression in human breast carcinoma: role of p53-dependent and independent signal transduction pathways. Oncogene. 1994;9:3407–3415. [PubMed] [Google Scholar]
- Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, et al. Understanding the function–structure and function–mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA. 2003;100:8424–8429. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas RA, Bruning PF, Top B, Breedijk AJ, Peterse HL. Growth arrest associated changes of mRNA levels in breast cancer cells measured by semi-quantitative RT-PCR: potential early indicators of treatment response. Cancer Lett. 1995;97:107–116. doi: 10.1016/0304-3835(95)03959-z. [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W. Ubiquitination phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13:454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laios I, Journe F, Laurent G, Nonclercq D, Toillon RA, Seo HS, et al. Mechanisms governing the accumulation of estrogen receptor alpha in MCF-7 breast cancer cells treated with hydroxytamoxifen and related antiestrogens. J Steroid Biochem Mol Biol. 2003;87:207–221. doi: 10.1016/j.jsbmb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, et al. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One. 2011;6:e17876. doi: 10.1371/journal.pone.0017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcar L, Maclaine NJ, Hupp TR, Meek DW. Mage-A cancer/testis antigens inhibit p53 function by blocking its interaction with chromatin. Cancer Res. 2010;70:10362–10370. doi: 10.1158/0008-5472.CAN-10-1341. [DOI] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10:620–628. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7:713–722. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A, Caballero OL, Hoek KS, da Silva L, Chen YT, Shin SJ, et al. CT-X antigen expression in human breast cancer. Proc Natl Acad Sci USA. 2009;106:13493–13498. doi: 10.1073/pnas.0906840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem. 2011;57:421–430. doi: 10.1373/clinchem.2010.154328. [DOI] [PubMed] [Google Scholar]
- Clarke N, Jimenez-Lara AM, Voltz E, Gronemeyer H. Tumor suppressor IRF-1 mediates retinoid and interferon anticancer signaling to death ligand TRAIL. EMBO J. 2004;23:3051–3060. doi: 10.1038/sj.emboj.7600302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- Williams CM, Scibetta AG, Friedrich JK, Canosa M, Berlato C, Moss CH, et al. AP-2gamma promotes proliferation in breast tumour cells by direct repression of the CDKN1A gene. EMBO J. 2009;28:3591–3601. doi: 10.1038/emboj.2009.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman IS, Christiaens MR, Paridaens R, Van Geel A, Rutgers E, Berner J, et al. Treatment of operable breast cancer in the elderly: a randomised clinical trial EORTC 10851 comparing tamoxifen alone with modified radical mastectomy. Eur J Cancer. 2003;39:309–316. doi: 10.1016/s0959-8049(02)00673-1. [DOI] [PubMed] [Google Scholar]
- Fentiman IS, van Zijl J, Karydas I, Chaudary MA, Margreiter R, Legrand C, et al. Treatment of operable breast cancer in the elderly: a randomised clinical trial EORTC 10850 comparing modified radical mastectomy with tumorectomy plus tamoxifen. Eur J Cancer. 2003;39:300–308. doi: 10.1016/s0959-8049(02)00672-x. [DOI] [PubMed] [Google Scholar]
- Maraqa L, Cummings M, Peter MB, Shaaban AM, Horgan K, Hanby AM, et al. Carcinoembryonic antigen cell adhesion molecule 6 predicts breast cancer recurrence following adjuvant tamoxifen. Clin Cancer Res. 2008;14:405–411. doi: 10.1158/1078-0432.CCR-07-1363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.