Abstract
Although many strong risk factors for osteoporosis—such as family history, fracture history and age—are not modifiable, a number of important risk factors are potential targets for intervention. Thus, simple, non-pharmacological intervention in patients at increased risk of osteoporotic fractures could include reduction of excessive alcohol intake, smoking cessation, adequate nutrition, patient education, daily physical activity and a careful review of medications that could increase the risk of falls and fractures. There remains, however, an unmet need for high-quality intervention studies in most of these areas.
Introduction and aim
With the increasing availability of effective osteoporosis drugs in the past 40 years, much effort has been focused on identifying patients at high risk of osteoporotic fractures.1 However, many risk factors for osteoporosis and osteoporotic fractures are in themselves modifiable and such risk factors are potential targets for intervention.2 However, the strongest risk factors such as age, family history and prior fracture are essentially non-modifiable; therefore, osteoporosis drugs of course remain essential in managing patients with high fracture risk due to osteoporosis. In the following overview of modifiable risk factors, findings are generally based on observational studies with only a few areas having been the focus of large high-quality intervention trials.
The review is based on a larger number of references than what could be accommodated in the reference list; a full list of references is available from the authors.
Physical activity and the bone
Physical activity transmits loads to the skeleton, improves muscle strength and balance and may thereby reduce the risk of falls and fractures.3 It is generally assumed that a high level of activity corresponds to a high level of mechanical loading, with the exception of a few non-weight-bearing activities, such as swimming. We shall review the evidence in the following.
Regular weight-bearing physical activity is likely of particular importance in childhood and youth as half of adult peak bone mass is accrued during the teenage years.4 Several studies in childhood and young adults support a beneficial effect in this age group.5,6,7 In the adult, long-term data are somewhat more limited. To our knowledge, there are only few long-term prospective studies. In a 25-year prospective study, physical activity reduces forearm bone loss in post-menopausal women.8 However, there is growing evidence from especially meta-analyses that physical activity in adulthood can preserve bone mineral density (BMD) and decrease the risk of fractures. Kelley et al.9 recently performed a meta-analysis on this in pre-menopausal women and showed a significant inverse association with the overall risk of total fractures. This confirms and extends the findings from several earlier meta-analyses in both men and women.10
Physical activity and the transmission of the load to the bone might be highly related to the bone–muscle interface. An aggregate data meta-analytic approach recently examined the effects of ground and/or joint reaction force on BMD at the femoral neck and lumbar spine in post-menopausal women not participating in exercise currently recommended for bone health.11 Twenty-five studies were included, representing 63 groups (35 exercise and 28 controls), and they assessed femoral neck and lumbar spine BMD in 1775 participants. Overall, a significant benefit of ground and/or joint reaction force exercise on femoral neck and lumbar spine BMD was found.
Sarcopenia and osteoporosis are major health issues in post-menopausal women. There is evidence that these two conditions regularly co-exist and have largely overlapping risk factors.12 The clinical importance of physical inactivity and sarcopenia in relation to osteoporosis has increased in the ageing society. Sarcopenia, defined as decreased muscle mass and impaired muscle function, is directly related to the risk of osteoporosis, falls and fracture. The prevalence of sarcopenia has been reported to range from 10 to 40% in post-menopausal populations depending on which method is used and also the choice of reference population.13 There is growing and consistent evidence from the literature that lean body mass is positively related to bone mass.14 Adults who are skinny, that is, body mass index (BMI) <18, is well established as risk factor for osteoporotic fractures.15 On the other hand, being obese, that is, BMI >30, may increase fracture risk as well as discussed in more detail later in this review.
Sjöblom et al.16 found that sarcopenia was significantly associated with osteoporosis, fractures and falls. Skeletal muscle index, that is, appendicular muscle mass (kg) divided by the square of height (m2), grip strength and physical performance of sarcopenia combined, exhibited a stronger relationship with osteoporosis, fracture and falls than any of these individual components alone. Sarcopenia does not seem to be only loss of muscle mass but also loss of muscle strength and performance. Even though muscle power can be evaluated in several ways, grip strength seems to be the one component that revealed the strongest association with osteoporosis, fractures and falls. It is challenging to separate the components sarcopenia and falls as risk parameters of osteoporotic fractures, as sarcopenia also increases the risk of falling.
Fractures in the elderly arise predominantly from the combination of falls and osteoporosis. The odds of a fracture are seven to nine times higher among community-dwelling post-menopausal women, with both a fall and osteoporosis/osteopenia, compared with women having a fall or osteoporosis/osteopenia only.17 Morrison et al.18 have shown that in Western cohorts of older women and men, annual prevalence rates of low-impact falls were within the range of 0.217–0.625. Fall prevalence rates were 20% lower in men than in women. A median of 4.1% of low-impact falls resulted in fractures in cohorts of Western women and men. The percentages of all low-trauma fractures attributable to low-impact falls and all fractures that were osteoporotic were similar, ranging from 86.0 to 95.0% and 71.6 to 92.4%, respectively.
An abundance of risk factors for falls among the elderly and old has been identified in cohort studies.18 With an increasing number of risk factors accrued over time, the risk of falls increases accordingly. During the last decades, different strategies to prevent falls have been tested in trials and different results have been reported. Trials of fall prevention among different groups of patients or citizens have been performed. The trials have included single to interventions or multifactorial fall prevention. A recent Cochrane study included 159 trials conducted with a total of 79 193 participants. Group and home-based exercise programs, and home safety interventions reduced the rate of falls and risk of falling.24 Multifactorial assessment and intervention programs reduced the rate of falls but not risk of falling, whereas Tai Chi reduced the risk of falling.
Smoking
Smoking is one of the most important lifestyle factors that reduce bone mass. It is of course a modifiable risk factor and should be appraised when assessing individual fracture risk.19 The negative effects of smoking on cardiovascular and pulmonary function are well described, and smoking cessation remains the single most effective intervention to reduce the risk of tobacco-induced pulmonary disease such as chronic obstructive pulmonary disease (COPD) and to slow its progression.20 Determining the independent role of smoking in decreasing bone mass is more complicated, as smoking may be correlated to other risk factors for osteoporosis such as low BMI, decreased physical activity and poor diet.19
Pathophysiological mechanisms of tobacco smoking on development of osteoporosis
Several underlying pathophysiological mechanisms predispose smokers to bone loss. Such mechanisms include direct and indirect cellular effects on bone cells involving all skeletal sites.21
Direct effects on bone cells
Nicotine is thought to affect cell proliferation in a biphasic manner, with toxic and antiproliferative effects at high levels of nicotine and stimulatory effects at low levels.21 The actions may be receptor-mediated via a nicotinic acetylcholine receptor. Low levels of nicotine upregulate osteocalcin, type I collagen and alkaline phosphatase gene expression.22
In human osteoblasts, nicotine induces an increase in tumor necrosis factor-α secretion leading to reduced bone formation by osteoblasts and increased osteoclastic resorption. tumor necrosis factor-α has particularly potent effect on osteoclastogenesis, as it not only promotes RANKL production but synergizes with RANKL to amplify osteoclastogenesis.23 Strong evidence supporting the role of the RANK/RANKL/OPG system in osteoclast formation and the regulation of bone resorption exists.24
In clinical studies of patients with COPD, increased levels of cytokines such as interleukin-6, and tumor necrosis factor-α are present in the peripheral blood.25 Although smoking in itself induces the release of pro-inflammatory markers, the production and release are even higher in patients with COPD compared with asymptomatic smokers, indicating an additional role of COPD per se on the local and systemic inflammatory response negatively affecting bone remodeling.
Studies on effects of smoking on the RANK-RANKL-OPG system are few; clinical studies are limited to subjects with periodontitis showing lower levels of OPG in smokers versus non-smokers.26
Indirect effects on bone cells
Smoking has been shown to induce alterations in the calciotropic and adrenal cortical hormone metabolism, leading to further increased bone resorption.26 In clinical studies, smoking has been associated with increased cortisol levels.27 Excess glucocorticoid affects bone remodeling both direct and indirectly, inducing alterations in osteoblastic and osteoclastic activities.28
Part of the negative effect of smoking on bone metabolism is mediated by effects on sex steroid metabolism. This most important hormonal effect seems to be the lowering of estradiol levels,29 which may in itself contribute to increased fracture risk.30
Intestinal calcium absorption is lower in smokers as compared with non-smokers. This impairment of calcium absorption could lead to accelerated bone loss and limit the usefulness of dietary calcium supplementation.31In addition to the effects of nicotine on bone metabolism, the vast amount of other toxic compounds may contribute to bone degradation. A recent study on the effects of two toxic agents benzo(a)pyrene (BaP) and 2,3,7,8-Tetrachlorodibenzo-p-dioxin revealed excessive formation of osteoclasts in mice.32 To what degree the individual compounds contribute to bone degradation, and if their effects are mutually synergistic, remains unclear.
Smoking and smoking cessation
Smoking is almost universally recognized as a risk factor for low BMD and increased fracture risk.33,34 Smoking has been found in meta-analyses to increase the lifetime risk of developing a vertebral fracture by 13% in women and 32% in men. Smoking may increase lifetime fracture risk of hip fracture by 31% in women and 40% in men.35 There is an independent, dose-dependent effect on bone loss that may be partially reversed by smoking cessation. Reduced BMD in smokers is supported by a large meta-analysis including 29 cross-sectional studies reporting the difference in bone density in smokers and non-smokers. The estimated cumulative risk of hip fracture in women was 19% in smokers and 12% in non-smokers to age 85; 37 and 22% in smokers and non-smokers, respectively, to age 90. In post-menopausal women, it is estimated that one hip fracture in eight is attributable to smoking. This association could not explained by smokers being thinner, younger at menopause and exercising less nor by actions of smoking on estrogen, indicating an independent negative effect of smoking on bone where current smokers lose bone at faster rates.36
Results from the population-based Gothenburg Osteoporosis and Obesity Determinants study also indicate that smoking is associated with a lower (a)BMD and reduced cortical thickness in young men.37 The Swedish Mr OS study suggests that smokers are at elevated risk for vertebral fractures as well as nonvertebral osteoporotic fractures, defined as humerus, radius, pelvis and hip fractures. However, after adjustment for BMD and other covariates, no significant association between smoking and incident fractures was found.38 Previously, in a large prospective cohort study of middle-aged women, smoking was not associated with elevated forearm and hip fracture risk.39 Overall, although some studies provide conflicting results, based on the largest meta-analysis the central negative role of smoking on bone health seems unarguable.
The comprehensive analyses by Kanis et al.33 found that current smoking was associated with a significantly increased risk of any fracture compared with non-smokers. As a consequence, current smoking was included as a risk factor in the World Health Organization methodology for calculating the absolute risk of fracture (FRAX).
Quantification of risk as a function of dose is currently not possible. However, the prevalence of osteoporosis in patients with a long-term smoking history, leading to COPD, has been reported as high as 69% depending on the clinical setting and methodology.40 Patients often present with additional risk factors of osteoporosis such as the use of corticosteroids, as opposed to smokers with no COPD diagnosis. As severity of COPD progresses, the proportion of patients with osteoporosis increases.41 Further, survival after hip fracture is much poorer in patients with COPD.42,43
On the basis of observational studies, the effects of smoking on the skeleton are at least partly reversible. In a large Danish population study, tobacco smoking was also found to be an independent risk factor for hip fracture in both men and women. No gender differences were found in the impact of smoking on risk. The observations indicated a reduced risk of hip fracture in men after 5 years following smoking cessation, whereas the deleterious effect of smoking seems long-lasting in female ex-smokers.44
Results from other comprehensive longitudinal observational studies indicate that smoking cessation is associated with BMD levels between that of never-smokers and current smokers,45,46 pointing to a beneficial effect of addressing this modifiable risk factor.
The negative impact of smoking on bone health reaches beyond increased risk of osteoporosis and fracture. Smoking has been suggested to worsen the prognosis of surgical fracture procedures and to prolong healing time. Indeed, in a systematic review including 19 cohort studies, it was concluded that smoking significantly increased the risk of nonunion of fractures overall. Increased time to union in all fractures and increased postoperative rates of superficial and deep infections in smokers compared with nonsmokers have been observed.47
Alcohol
The effects of alcohol consumption on the risk of osteoporotic fractures are mediated through both direct, endocrine, metabolic and nutritional effects that converge on the bone.48 In a subset of patients, the consequences of skeletal fragility are further exacerbated by an increased risk of falling due to intoxication and/or neuropathy.49 The effects of alcohol on sex steroids are complex and dose-dependent. Although chronic, heavy consumption of alcohol can decrease free testosterone and estradiol levels, light or moderate alcohol intake can be associated with a later menopause50 and with higher serum-free testosterone after menopause.51 Hypercortisolism and a lowering of serum leptin can also be a consequence of chronic large intake.52,53 Alcoholism may be accompanied by poor nutrition and exocrine pancreas insufficiency with malabsorption and vitamin D deficiency. Whereas BMD effects of low to moderate alcohol intake appear modestly positive,54 studies have consistently found an increased risk of fractures in men and women with moderate or high alcohol consumption.55,56,57,58,59,60 A patient level meta-analysis of three prospective cohorts found no increased fracture risk with intake of two units of alcohol daily but an increased risk of (relative risk) 1.23 for fracture in general and 1.68 for major osteoporotic fractures with three units of alcohol or more. The study found no effect modification by baseline BMD, age or sex.54 Osteopenia in alcoholism may be reversible as suggested by a small observational cohort study;61 however, there is a lack of longitudinal studies to properly inform the evidence base regarding the reversibility of fracture risk upon reduction of excessive alcohol intake.
Nutrition
The nutrients that have received the bulk of attention are vitamin D and calcium, two pivotal contributors to bone mineralization. Although the optimum serum vitamin D level is the focus of keen scientific debate,62,63 there is certainly evidence that fracture risk is increased with vitamin D levels below 50 nmol l−1.64,65 Interestingly, randomized intervention studies have shown that neither vitamin D supplementation nor calcium supplementation on its own reduce the risk of osteoporotic fractures, whereas the risk is modestly decreased—by 20% or less—by supplementation with these two nutrients in small amounts (10 μg of vitamin D and 400–800 mg of calcium) in combination. There is some evidence suggesting that daily but not semi-annual or annual supplementation confers a benefit; however, the combination with calcium supplementation is usually performed on a daily basis as only vitamin D itself is fat-soluble and can be given at longer intervals. The existing trials cannot properly separate the effect of daily dosing from the effect of calcium. The role of vitamin K, another fat-soluble vitamin, in bone health is interesting and has a potential for development of new therapeutics, although high-quality trials are lacking. Readers are referred to the recent review by Hamidi et al.66 Both vitamin A and vitamin E have complex and dose-dependent actions on the bone. In the case of vitamin E, gamma-tocopherol may be associated with increased bone formation, whereas alpha-tocopherol may antagonize this effect.67 Within the water-soluble vitamins, B1, B6 and B12 have all been linked to bone health. The relationship is complex, with potential effect modification by MTHFR genotype.68 A comprehensive review of other aspects of nutrition, including magnesium and protein intake and the risk of osteoporosis, is available elsewhere.69,70
Drug exposures
Osteoporosis is a familiar adverse effect of a few classes of drugs, including glucocorticoids71,72 and aromatase inhibitors,73 and a strongly suspected adverse effect with others, such as proton pump inhibitors (PPIs) or SSRIs. Although the drugs may have been prescribed for the best of clinical reasons, there may be a potential for risk modification by successfully controlling diseases with drugs that are not harmful to the skeleton (for example, using H2 blockers instead of PPIs where possible) or by modifying risk by co-administration of osteoporosis drugs. Prevention of glucocorticoid-induced osteoporosis can be accomplished with a significant risk reduction by co-prescription of antiresorptives.74 Although based on limited evidence, the use of calcium and vitamin D supplementation as a first step in prevention of GIO is universally endorsed;75 therefore, it is unlikely that controlled trials will be undertaken. The mechanisms linking PPIs, controversially, to fracture risk are poorly understood but may involve effects on calcium absorption, hypomagnesemia, increased gastric histamine release, ulcer disease and Helicobacter colonization.76,77 In the case of risedronate, there is evidence supporting the efficacy in patients who are also treated with PPIs; however, this is not at present clear for alendronate.78 Taken together, it is important for the clinician or pharmacist to be aware of the importance of doing a full review of the medications used by osteoporosis patients in order to identify opportunities for reducing fracture risk by simple modifications to treatment.
Body mass index
As alluded to earlier in the review, a low BMI is a well-established risk factor for osteoporotic fracture.15,79,80 A BMI of <21 kg m−2 or a body weight of <127 pounds is associated with increased risk of low BMD and increased fracture risk in women.15 There is a strong correlation between BMD and BMI, and several studies have shown that a decrease in body weight leads to bone loss.81 A recent meta-analysis investigated the association between BMI and future fracture risk at different skeletal sites and concluded that low BMI remains a risk factor for hip and all osteoporotic fractures.80 Even when adjusted for BMD, low BMI remains a risk factor for hip fracture.15,80 BMI is more predictive of osteoporosis than is weight alone.79 The GLOW study recently showed that the relationship between fracture and weight, BMI and height is surprisingly site-specific.82 Thus, whereas BMI showed a significant inverse association with hip, clinical spine and wrist fractures, weight was ignificantly associated with the risk of malleolar fractures. For upper arm/shoulder and clavicle fractures, only linear height was significantly associated. A number of mechanisms whereby weight and BMI may influence fracture risk have been proposed. These include, for instance, the effects on BMD and muscle weakness. Further suggestions are nutritional deficiencies of protein or vitamin D, decreased padding over the greater trochanter or a greater liability to fall.15,82 The association between BMI and fracture risk is complex, differs across skeletal sites and is modified by the interaction between BMI and BMD.
A recent study in post-menopausal women (369 healthy women aged 40–88 years) found that the optimal value of BMI, with the lowest risk of low femoral neck BMD (osteopenia or osteoporosis), was 26.9 kg m−2. A further increase in BMI was not favorable in terms of BMD.83
Educational intervention
Modifiable risk factors are potential targets for intervention to decrease the risk of osteoporosis or osteoporotic fractures. Beaudion et al.2 have recently investigated the impacts of educational interventions on modifiable risk factors for osteoporosis after a fragility fracture. The study did not find a significant difference in the proportion of women improving their behaviors on modifiable risk factors such as smoking and alcohol consumption or level of physical activity between the usual care group and intervention group. The education approaches only had a significant impact on Ca and vitamin D supplement consumption. Few other studies have also assessed the utility of simple educational interventions on preventive behaviors for osteoporosis among women who sustained a fragility fracture,84,85,86 all reached similar results.
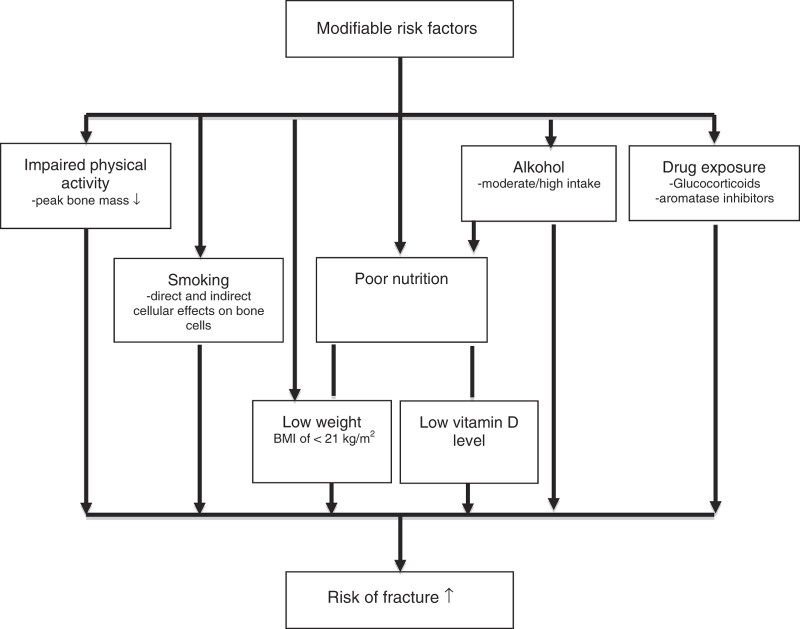
Another approach in the attempt to get patients to change the habits of the modifiable risk factors could be the use of risk assessment tools in communicating risk of osteoporosis or fracture. Explanation of risk and its implication is often challenging and is not always understood by the patient and the health-care personnel. Moreover, pathophysiological pathways are entangled so that modifying one risk factor may have direct effects on other risk factors or modify their impact as illustrated in simplified form in Figure 1.
Figure 1.
Simplified chart of major modifiable risk factors for osteoporotic fractures.
Risk assessment tools and reversibility of risk
Numerous risk assessment tools have been developed to integrate risk factors into a single estimate of fracture risk for individuals. There are great differences in the number of the risk factors included in the algorithms and the weighting of each risk factor in the calculation of risk. Many of these risk factors are modifiable and weight/BMI is, for instance, present as a risk factor in all risk assessment tools. Recently developed prediction tools, such as the FRAX algorithm, Qfracture and Garvan Fracture Risk Calculator, are aimed to assist clinicians in the management of their patients through the calculation of the patient's 5- or 10-year risk of fracture based on a combination of known risk factors.1 Although relying on observational data rather than intervention studies, the tools may also be useful to some extent in patient consultations to assist discussions on the impact of changing modifiable risk factors.87 The difficulty in adequately predicting fracture risk after intervention is reflected in the current debate surrounding the accuracy of risk estimation once intervention with anti-osteoporotic drugs has taken place.88 As reviewed above, smoking is one of the several modifiable risk factors for osteoporosis and it may be that ‘risk' illustrated by risk assessment tool could be used when advising and assisting patients to stop smoking. We are not aware of any studies investigating such an approach, but it could be an area for future research.
Conclusions
Our understanding of the potential for modifying fracture risk by lifestyle intervention is gradually improving through a large number of observational cohort studies and clinical database studies, albeit with a fairly slim evidence base when it comes to intervention studies. Unlike most pharmaceutical interventions to reduce fracture risk, modifying lifestyle through physical activity, by stopping smoking and by reducing excessive alcohol intake, is associated with a host of added health benefits and no perceivable long-term harms. This same is true for weight gain in the underweight patient. Because of this, it makes good clinical sense to encourage such lifestyle interventions, despite the limited amount and moderate quality of evidence that intervention cuts risk. However, several questions remain. It is tempting to recommend weight-bearing physical activity but how much and how often is still essentially unknown because of the difficulties in performing adequately powered good-quality trials. For calcium and vitamin D supplements, the issue is even much more difficult and controversial with considerable disagreement between scientific bodies about the benefit: harm relationship, the appropriate population in which to intervene and even the vitamin D serum level to aim at. In conclusion, simple intervention in patients with osteoporosis should include reduction of excessive alcohol intake, smoking cessation, adequate nutrition, patient education, daily physical activity and a careful review of medications that could increase the risk of falls and fractures. There remains, however, an unmet need for high-quality intervention studies in most of these areas.
Footnotes
BA has received grants from or conducted trials for Novartis, Nycomed/Takeda and Amgen. Advisory board member Nycomed/Takeda, Merck and Amgen. Speakers fees from Eli-Lilly, Merck, Amgen and Nycomed. PS has served as an investigator in clinical trials for Amgen, Novartis and Merck. The remaining authors declare no conflict of interest.
References
- Rubin KH, Friis-Holmberg T, Hermann AP, Abrahamsen B, Brixen K. Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res 2013;28:1701–1717. [DOI] [PubMed] [Google Scholar]
- Beaudoin C, Bessette L, Jean S, Ste-Marie L-G, Brown JP. The impact of educational interventions on modifiable risk factors for osteoporosis after a fragility fracture. Osteoporos Int 2014;25:1821–1830. [DOI] [PubMed] [Google Scholar]
- Schwarz P. Physical activity and bone strength. Scand J Med Sci Sports 2004;14:1. [DOI] [PubMed] [Google Scholar]
- Ma NS, Gordon CM. Pediatric osteoporosis: where are we now? J Pediatr 2012;161:983–990. [DOI] [PubMed] [Google Scholar]
- Detter F, Rosengren BE, Dencker M, Lorentzon M, Nilsson J-Å, Karlsson MK. A six-year exercise program improves skeletal traits without affecting fracture risk—a prospective controlled study in 2621 children. J Bone Miner Res 2014;29:1325–1336. [DOI] [PubMed] [Google Scholar]
- Linden C, Ahlborg HG, Besjakov J, Gardsell P, Karlsson MK. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: two-year data from the pediatric osteoporosis prevention (POP) study. J Bone Miner Res 2006;21:829–835. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Sundh D, Ohlsson C, Karlsson M, Mellström D, Lorentzon M. Exercise during growth and young adulthood is independently associated with cortical bone size and strength in old Swedish men. J Bone Miner Res 2014;29:1795–1804. [DOI] [PubMed] [Google Scholar]
- Svejme O, Ahlborg HG, Karlsson MK. Physical activity reduces bone loss in the distal forearm in post-menopausal women-a 25-year prospective study. Scand J Med Sci Sports 2014;24:159–165. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol 2013;2013:741639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 2013;53:103–111. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS, Kohrt WM. Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 2012;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol 2003;95:1717–1727. [DOI] [PubMed] [Google Scholar]
- Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging 2009;13:708–712. [DOI] [PubMed] [Google Scholar]
- Gourlay ML, Hammett-Stabler CA, Renner JB, Rubin JE. Associations between body composition, hormonal and lifestyle factors, bone turnover, and BMD. J Bone Metab 2014;21:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 2005;16:1330–1338. [DOI] [PubMed] [Google Scholar]
- Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 2013;75:175–180. [DOI] [PubMed] [Google Scholar]
- Geusens P, Autier P, Boonen S, Vanhoof J, Declerck K, Raus J. The relationship among history of falls, osteoporosis, and fractures in postmenopausal women. Arch Phys Med Rehabil 2002;83:903–906. [DOI] [PubMed] [Google Scholar]
- Morrison A, Fan T, Sen SS, Weisenfluh L. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res 2013;5:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PKK, Christie JJ, Wark JD. The effects of smoking on bone health. Clin Sci (Lond) 2007;113:233–241. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- Kallala R, Barrow J, Graham SM, Kanakaris N, Giannoudis PV. The in vitro and in vivo effects of nicotine on bone, bone cells and fracture repair. Expert Opin Drug Saf 2013;12:209–233. [DOI] [PubMed] [Google Scholar]
- Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab 2009;27:555–561. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 2004;15:457–475. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int 2012;23:2081–2092. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol 2006;59:228–235. [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007;18:1319–1328. [DOI] [PubMed] [Google Scholar]
- Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause 11:104–109. [DOI] [PubMed] [Google Scholar]
- Mosekilde L, Vestergaard P, Rejnmark L. The pathogenesis, treatment and prevention of osteoporosis in men. Drugs 2013;73:15–29. [DOI] [PubMed] [Google Scholar]
- Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin BEC. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int 2002;13:83–88. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Sun L, Cao J, Yuen T, Lu P, Bab I et al. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci USA 2013;110:11115–11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int 2005;16:155–162. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Mosekilde L. Fracture risk associated with smoking: a meta-analysis. J Intern Med 2003;254:572–583. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 2001;68:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 1997;315:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab 2007;92:497–503. [DOI] [PubMed] [Google Scholar]
- Hemenway D, Colditz GA, Willett WC, Stampfer MJ, Speizer FE. Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 1988;78:1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemenway D, Colditz GA, Willett WC, Stampfer MJ, Speizer FE. Fractures and lifestyle: effect of cigarette smoking, alcohol intake, and relative weight on the risk of hip and forearm fractures in middle-aged women. Am J Public Health 1988;78:1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graat-Verboom L, Wouters EFM, Smeenk FWJM, van den Borne BEEM, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 2009;34:209–218. [DOI] [PubMed] [Google Scholar]
- Vrieze A, de Greef MHG, Wijkstra PJ, Wýkstra PJ, Wempe JB. Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int 2007;18:1197–1202. [DOI] [PubMed] [Google Scholar]
- De Luise C, Brimacombe M, Pedersen L, Sørensen HT. Chronic obstructive pulmonary disease and mortality following hip fracture: a population-based cohort study. Eur J Epidemiol 2008;23:115–122. [DOI] [PubMed] [Google Scholar]
- Regan EA, Radcliff TA, Henderson WG, Cowper Ripley DC, Maciejewski ML, Vogel WB et al. Improving hip fractures outcomes for COPD patients. COPD 2013;10:11–19. [DOI] [PubMed] [Google Scholar]
- Høidrup S, Prescott E, Sørensen TI, Gottschau A, Lauritzen JB, Schroll M et al. Tobacco smoking and risk of hip fracture in men and women. Int J Epidemiol 2000;29:253–259. [DOI] [PubMed] [Google Scholar]
- Hollenbach KA, Barrett-Connor E, Edelstein SL, Holbrook T. Cigarette smoking and bone mineral density in older men and women. Am J Public Health 1993;83:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Kelly PJ, Sambrook PN, Gilbert C, Pocock NA, Eisman JA. Lifestyle factors and bone density in the elderly: implications for osteoporosis prevention. J Bone Miner Res 1994;9:1339–1346. [DOI] [PubMed] [Google Scholar]
- Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am 2014;96:674–681. [DOI] [PubMed] [Google Scholar]
- Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 2012;23:1–16. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Harrison SL, Barrett-Connor E, Fink HA, Cauley JA, Lewis CE et al. Alcohol intake and its relationship with bone mineral density, falls, and fracture risk in older men. J Am Geriatr Soc 2006;54:1649–1657. [DOI] [PubMed] [Google Scholar]
- Sapre S, Thakur R. Lifestyle and dietary factors determine age at natural menopause. J Midlife Health 2014;5:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S, Peeters PHM, Bezemer ID, Dossus L, Biessy C, Sacerdote C et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control 2006;17:1033–1043. [DOI] [PubMed] [Google Scholar]
- Besemer F, Pereira AM, Smit JWA. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med 69:318–323. [PubMed] [Google Scholar]
- Cardoso Fernandes Toffolo M, Aparecida Marliére C, Nascimento de Freitas S, Silva de Aguiar Nemer A. Increasing leptin level in abstaining alcohol-dependent women. Nutr Hosp 27:781–788. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johansson H, Johnell O, Oden A, de Laet C, Eisman JA et al. Alcohol intake as a risk factor for fracture. Osteoporos Int 2005;16:737–742. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kristenson H, Redlund-Johnell I. Lower limb fractures and registration for alcoholism. Scand J Soc Med 1985;13:95–97. [DOI] [PubMed] [Google Scholar]
- Høidrup S, Grønbaek M, Gottschau A, Lauritzen JB, Schroll M. Alcohol intake, beverage preference, and risk of hip fracture in men and women. Copenhagen Centre for Prospective Population Studies. Am J Epidemiol 1999;149:993–1001. [DOI] [PubMed] [Google Scholar]
- Felson DT, Kiel DP, Anderson JJ, Kannel WB. Alcohol consumption and hip fractures: the Framingham Study. Am J Epidemiol 1988;128:1102–1110. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Kröger H, Honkanen R, Puntila E, Huopio J, Saarikoski S et al. Risks of perimenopausal fractures--a prospective population-based study. Acta Obstet Gynecol Scand 1995;74:624–628. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Kasagi F, Yamada M, Kodama K. Risk factors for hip fracture in a Japanese cohort. J Bone Miner Res 1997;12:998–1004. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila M, Colditz GA, Stampfer MJ, Rosner B, Speizer FE, Willett WC. Caffeine, moderate alcohol intake, and risk of fractures of the hip and forearm in middle-aged women. Am J Clin Nutr 1991;54:157–163. [DOI] [PubMed] [Google Scholar]
- Alvisa-Negrín J, González-Reimers E, Santolaria-Fernández F, García-Valdecasas-Campelo E, Valls MRA, Pelazas-González R et al. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol 44:468–475. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Abrams Sa, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu Ra et al. IOM committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab 2012;97:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- Gerdhem P, Ringsberg KaM, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int 2005;16:1425–1431. [DOI] [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, Del HB. IOM Dietary Reference Intakes for Calcium and Vitamin D The National Academies Press: Washington DC, 2011;. [PubMed] [Google Scholar]
- Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom 16:409–413. [DOI] [PubMed] [Google Scholar]
- Hamidi MS, Corey PN, Cheung AM. Effects of vitamin E on bone turnover markers among US postmenopausal women. J Bone Miner Res 2012;416:1–31. [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR et al. Are effects of mthfr (C677T) genotype on BMD confined to women with low folate and riboflavin intake? Analysis of food records from the Danish Osteoporosis Prevention Study. Bone 2005;36:577–578. [DOI] [PubMed] [Google Scholar]
- Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr 2009;28:131–141. [DOI] [PubMed] [Google Scholar]
- Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep 2009;7:111–117. [DOI] [PubMed] [Google Scholar]
- Van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 2005;98:191–198. [DOI] [PubMed] [Google Scholar]
- De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 2007;56:208–214. [DOI] [PubMed] [Google Scholar]
- Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011;22:2546–2555. [DOI] [PubMed] [Google Scholar]
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 1998;339:292–299. [DOI] [PubMed] [Google Scholar]
- Hansen KE, Wilson HA, Zapalowski C, Fink Ha, Minisola S, Adler Ra. Uncertainties in the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Res 2011;26:1989–1996. [DOI] [PubMed] [Google Scholar]
- Yang Y-X. Chronic proton pump inihibitor therapy and calcium metabolism. Curr Gastroenterol Rep 2012;14:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk—effect modification by histamine H1 receptor blockade. Observational case-control study using National Prescription Data. Bone 2013;57:269–271. [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. ArchInternMed 2011;171:998–1004. [DOI] [PubMed] [Google Scholar]
- Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15:1028–1034. [DOI] [PubMed] [Google Scholar]
- Johansson H, Kanis JA, Odén A, McCloskey E, Chapurlat RD, Christiansen C et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223–233. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV et al. Determinants of bone mineral density in obese premenopausal women. Bone 2011;48:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston JE, Flahive J, Hosmer DW, Watts NB, Siris ES, Silverman S et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res 2014;29:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzek A, Kozieł S, Ignasiak Z. The optimal value of BMI for the lowest risk of osteoporosis in postmenopausal women aged 40-88 years. Homo 2014;65:232–239. [DOI] [PubMed] [Google Scholar]
- Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc 2006;54:450–457. [DOI] [PubMed] [Google Scholar]
- Kulp JL, Rane S, Bachmann G. Impact of preventive osteoporosis education on patient behavior: immediate and 3-month follow-up. Menopause 2004;11:116–119. [DOI] [PubMed] [Google Scholar]
- Cranney A, Lam M, Ruhland L, Brison R, Godwin M, Harrison MM et al. A multifaceted intervention to improve treatment of osteoporosis in postmenopausal women with wrist fractures: a cluster randomized trial. Osteoporos Int 2008;19:1733–1740. [DOI] [PubMed] [Google Scholar]
- Shams J, Spitzer AB, Kennelly AM, Tosi LL. Bone quality: educational tools for patients, physicians, and educators. Clin Orthop Relat Res 2011;469:2248–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewiecki EM, Cummings SR, Cosman F. Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab 2013;98:946–953. [DOI] [PubMed] [Google Scholar]