Abstract
Modifiable lifestyle changes, including dietary changes, could translate into a great reduction in the global burden of cognitive impairment and dementia. Few studies evaluated the benefits of fish intake for delaying cognitive decline, and no studies were conducted in a Chinese population, which may differ with respect to types, amounts, and correlates of fish consumption compared with Western populations. We hypothesized that higher consumption of fish would predict slower decline in cognitive function, independent of a wide range of potential confounders. This prospective cohort study comprised 1566 community-dwelling adults aged ≥55 y who completed a cognitive screening test at ≥2 waves of the China Health and Nutrition Survey in 1997, 2000, or 2004, with a mean follow-up of 5.3 y [age at entry (mean ± SD): 63 ± 6 y]. Diet was measured by 3-d 24-h recalls at baseline. Outcomes included repeated measures of global cognitive scores (baseline mean ± SD: 19 ± 6 points), composite cognitive Z-scores (standardized units), and standardized verbal memory scores (standardized units). Multivariable-adjusted linear mixed-effects models were used to evaluate the relation of fish intake with changes in cognitive scores. Age was found to significantly modify the association between fish consumption and cognitive change (P = 0.007). Among adults aged ≥65 y, compared with individuals who consumed <1 serving/wk (i.e., 100 g) fish, the mean annual rate of global cognitive decline was reduced by 0.35 point (95% CI: 0.13, 0.58) among those consuming ≥1 serving/wk, equivalent to the disparity associated with 1.6 y of age. Fish consumption was also associated with a slower decline in composite and verbal memory scores. No associations were observed among adults aged 55–64 y. Our findings suggest a potential role of fish consumption as a modifiable dietary factor to reduce the rate of cognitive decline in later life.
Introduction
With the population aging, the global burden of cognitive impairment and dementia is expected to increase substantially in coming decades (1). One strategy for reducing long-term risk of these disorders may be the promotion of dietary changes, such as increasing intake of fish, which was hypothesized to reduce the rate of cognitive decline. Although evidence remains limited, several mechanisms linking nutrients such as ω-3 PUFAs in fish to cognitive health were proposed (2). However, because fish is rich in other nutrients that may benefit cognitive function, such as magnesium, selenium, vitamin D, and several B vitamins (3–5), the interplay of a number of nutrients in fish may be responsible for the health benefits of fish consumption (6, 7).
Few studies, all conducted in Western populations in which fish intake was strongly related to socioeconomic status and other health behaviors, evaluated the benefits of fish intake for delaying cognitive decline; some inconsistencies were found. In 2 prospective studies with moderate-to-high proportions of nonconsumers (43% and 24%, respectively), fish consumption was associated with a slower rate of cognitive decline among adults aged ≥65 or ≥70 y (8, 9). Another study, also in a population with moderate nonconsumption (20%), found higher intakes of fatty fish to be beneficially associated with changes in verbal memory (10). However, a prospective study in a more sociodemographically homogeneous population with few nonconsumers (mean intakes of 0.2 serving/wk in the lowest quartile) found no association between fish intake and cognitive decline among men aged 50–85 y at baseline (11). These findings suggest that any benefits of fish consumption for delayed cognitive decline may be limited to specific population subgroups or specific types of fish, might be confounded by sociodemographic correlates of fish consumption, or may be accrued even at relatively low amounts of intake.
As a result of inconsistencies in the literature, it remains uncertain to what extent benefits of fish consumption for cognition can be generalized to all older adults and to populations consuming different types of fish. Few studies examined whether beneficial effects may be observed at relatively young ages, in which cognitive decline may not be as pronounced (12). It also remains uncertain whether effects may vary depending on seafood subtypes analyzed or whether apparent benefits of fish may be attributable to confounding by other dietary factors or health behaviors. Moreover, no studies on fish consumption and cognitive decline were conducted in a general Chinese population, in which population aging and dementia burden are projected to increase dramatically in the next 4 decades (13).
The current study examined prospectively whether fish consumption is associated with reduced cognitive decline in community-dwelling Chinese older adults. We specifically examined associations in subgroups characterized by age, assessed whether effects may be stronger after excluding selected types of seafood, and addressed confounding by dietary patterns.
Materials and Methods
Study population.
We used longitudinal data from the China Health and Nutrition Survey (CHNS) (14). The CHNS was conducted in 9 provinces of China, which are substantially different geographically and in terms of economic development. This household-based survey collected demographic, socioeconomic, physical activity, and diet and health information at the individual, household, and community levels. In 1997, 2000, and 2004, the CHNS administered a brief cognitive status measure to adults aged ≥55 y, which included items assessing immediate and delayed memory, attention, calculation, and orientation.
This study included adults aged ≥55 y who were administered the cognitive measure on ≥2 measurement occasions from 1997 to 2004. Among the 2408 adults with ≥1 wave of measurement (participation rate for a baseline cognitive test among eligible CHNS participants of 73%), 1677 (70%) had ≥2 measurement occasions of cognitive function. Selectivity of the sample based on a wide array of characteristics was evaluated before additional data analysis. After eliminating 27 individuals with missing dietary information and 84 with missing important covariates, a total of 1566 were included in this study. The number of observations per person ranged from 2 to 3, with a mean of 2.3.
All participants in the CHNS provided written informed consent. The institutional review committees of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, approved this study.
Dietary assessment.
The baseline dietary intake information in the CHNS was collected using 3-d, 24-h dietary recalls obtained during the same period as the first cognitive test. Individual, in-person 24-h recalls were conducted over 3 consecutive days using food pictures to aid quantification. Recalls were complemented by measured changes in a weighed household inventory of all foods conducted over the same 3 d, adjusted based on measured or estimated waste related to preparation, edible portion, spoilage, uneaten items, or discarded items (15).
The health benefits of fish consumption may vary depending on the type of fish and culinary methods. Compared with fatty fish, shellfish may contain somewhat lower concentrations of certain ω-3 PUFAs while also containing chemicals that may offset cognitive and other health benefits (16–18). Preserved fish, a common culinary method in southern China, is high in salt and may also contain high concentrations of chemicals hazardous to health (19, 20). Therefore, consumption of fish was evaluated in several ways in our study: 1) all fish and shellfish, fresh and preserved, referred to as “total fish” 2) fish and shellfish, fresh only; 3) fish only, fresh or preserved; and 4) fresh fish only. We were not able to analyze intakes of shellfish or preserved fish separately because they were infrequent in these data, with 5% and 7% consumers, respectively. Two liang (the Chinese unit for weight) or 100 g is a typical serving for meat and fish in China. Based on traditional Chinese consumption and the distribution of fish intake, we categorized participants into 2 groups: 1) <1 100-g serving/wk fish (referent group); 2) ≥1 serving/wk fish.
Cognitive function assessment.
The cognitive screening items administered in CHNS comprised a subset of the items from the Telephone Interview for Cognitive Status–modified (21, 22) and have been used in population-based studies in the United States (23) and non-English speaking populations, including Chinese (24, 25). The cognitive screening included immediate and delayed recall of a 10-word list, counting backward from 20, serial 7 subtraction, and orientation. Higher scores on all items indicate better performance. Scores for both immediate and delayed recall ranged from 0 to 10. Counting backward and serial 7’s were used to evaluate attention and calculation ability, with scores ranging from 0 to 7. Orientation was assessed by asking the respondent the current date (1 point each for year, month, and date) and to name the tool usually used to cut paper (1 point).
The primary outcome was a global cognitive score, which was the sum of the scores of all test items, ranging from 0 to 31 points. Because the global score emphasizes memory more than other domains, we constructed a composite score (measured in standardized units) as an alternative global cognitive measure. This score was constructed by averaging Z-scores for memory items and the other items assessing attention, calculation, and orientation. We also constructed a verbal memory score (in standardized units) that combined the immediate and delayed 10-word recall.
Covariates.
All covariates were collected at the time of the first cognitive measure. Demographic and socioeconomic covariates included age, gender, region (south/north), urbanization index (a multicomponent continuous scale) (26), education (highest level of education attained, primary school vs. less), and annual household income per capita (inflated to 2011, ≥5000 yuan vs. less). Physical activity was self-reported based on hours per week spent in different occupational, household, transportation, and leisure-time activities (27), which were converted into metabolic equivalent-hours per week and included in the model as tertiles. Other lifestyle factors included current alcohol use (yes/no) and current smoking (yes/no). Nondietary variables included in the sensitivity analysis included BMI (calculated by measured weight and height), measured waist circumference, hypertension (measured mean systolic/diastolic blood pressure ≥ 140/90 mm Hg or self-report of antihypertension medications), history of diabetes (self-report), history of stroke (self-report), and history of myocardial infarction (MI) (self-report).
Statistical analysis.
Characteristics of participants by frequency of total fish intake were compared using t tests for continuous variables and χ2 tests for categorical variables. These tests were also used in comparisons of participants completing 1 vs. 2 or more waves of cognitive tests to evaluate selectivity of the sample eligible for analysis. We performed linear mixed-effects models using the XTMIXED procedure in STATA (version 11.2; StataCorp) (28) to assess the relation between fish consumption and rate of cognitive change over time, in which the continuous outcome measure was modeled as a function of fixed effects, whereas individual participant variables were modeled as random effects. Both the intercept and slope were fitted with random-effects components to account for interindividual differences in baseline cognitive function and rate of change (29). Two-sided P values <0.05 were used to define statistical significance for main effects.
The primary outcome was the global cognitive score. Our basic multivariable-adjusted model (model 1) included the following: mean-centered age; gender; region; urbanization index; education; annual household income per capita; total energy intake; time; and interaction between time and all covariates. A positive coefficient for the fish intake × time interaction suggests that more consumption was associated with a slower rate of decline in cognitive function. In model 2, we additionally adjusted for lifestyle and dietary confounders including the following: physical activity; current alcohol use; current smoking; consumption of fresh vegetables; fruit consumer; fresh legumes consumer; high-fat meat; and time interactions with the above covariates. To alleviate the concern that the benefit of fish on cognitive function was due to correlations between fish consumption and other components of an overall healthy diet, we further adjusted in model 3 for 2-factor analysis-derived dietary patterns, which reflect how foods are eaten in combination in this population, a wheat-based diverse diet characterized by high intakes of foods such as wheat, fruit, nuts, and plant oils, and a much less diverse rice/pork pattern.
Based on results from stratified analyses using 5-y age group categories (55–59, 60–64, 65–69, and ≥70 y), we also evaluated whether the association between fish intake and cognitive decline was modified by 1 key factor, age, defining statistical significance as P < 0.1. The stratified model indicated results similar to inconsistencies in previous studies on fish and cognition, some of which found weaker results when populations included relatively younger vs. older age groups (11, 30).
Multiple sensitivity analyses were conducted. First, based on model 2 of the primary analysis, we additionally adjusted for dietary antioxidant intake, including vitamin E and C intakes. Second, we repeated our primary analysis adjusting for BMI, waist circumference, and hypertension, all of which may be considered to be either confounders or mediators on the causal pathway between fish intake and cognitive decline. Separately, we tested whether the association between fish intake and cognitive decline was modified by hypertension or weight status. Although diabetes was self-reported, which underestimated the presence of diabetes in our sample (31), we estimated the association by excluding adults with a reported history of diabetes. In addition, we excluded adults with a self-reported history of stroke or MI.
We also repeated the primary analyses removing those with the lowest 10% baseline cognitive scores to assess whether the finding remained the same when those who may be categorized as having cognitive impairment at baseline were excluded. Furthermore, we accounted for the potential clustering effect of individuals within the same household because 28% of participants shared a residence. Finally, we conducted propensity score nearest-neighbor matching to further ensure the comparability between fish consumers eating ≥1 serving/wk and those who did not and repeated the primary analysis among matched samples (32–34).
In a secondary analysis, we evaluated the association between fish consumption and composite Z-scores or verbal memory scores over time. Outcomes in the secondary analysis were all standardized (Z-scores) to allow direct comparisons both within this study and with other studies.
Results
Characteristics of eligible participants who completed the cognitive tests at least twice (n = 1677) and those with 1 wave of cognitive testing (n = 731) were compared (Supplemental Table 1). No significant differences were found regarding gender, education, household income, BMI, or fish consumption, but those who did not complete ≥2 assessments were 2.8 y older and were more likely to live in northern China.
Among 1566 participants in the analysis sample, compared with individuals who consumed <1 serving/wk fish, those who consumed ≥1 serving/wk did not appreciably differ in age, gender, smoking, or alcohol use. They were more likely to reside in southern China and to have completed education beyond primary school, a higher household income, a higher urbanization index, lower levels of physical activity, and a higher BMI. They also had higher total caloric intakes, a higher prevalence of fruit consumption, and more high-fat meat consumption (Table 1). Because age significantly modified the association between fish intake and cognitive decline (P = 0.007), we stratified all subsequent analysis by age 55–64 y at the first cognitive measure and ≥65 y based on results from analyses stratified using 5-y age group categories. Characteristics of more vs. less frequent fish consumers within each age subgroup were similar to those for the overall sample, with the exception of a difference in legume consumption limited to those aged 55–64 y, and there was no difference in total caloric intake associated with fish intake among those aged ≥65 y.
TABLE 1.
Baseline characteristics of Chinese older adults in the CHNS by frequency of total fish consumption1
| Among all (n = 1566) |
Age at entry <65 y (n = 968) |
Age at entry ≥65 y (n = 598) |
|||||||
| Characteristics | <1 serving/wk | ≥1 servings/wk | P | <1 serving/wk | ≥1 serving/wk | P | <1 serving/wk | ≥1 serving/wk | P |
| Participants, n (%) | 1003 (64.0) | 563 (36.0) | 625 (64.6) | 343 (35.4) | 378 (63.2) | 220 (36.8) | |||
| Fish, servings/wk | 0.0 ± 0.12 | 4.2 ± 2.7 | 0.0 ± 0.1 | 4.3 ± 2.9 | 0.0 ± 0.1 | 4.1 ± 2.5 | |||
| Baseline global cognitive score (max = 31) | 19.0 ± 5.9 | 19.9 ± 5.4 | 0.002 | 19.8 ± 5.6 | 21.1 ± 4.9 | 0.001 | 17.5 ± 6.1 | 18.1 ± 5.9 | 0.29 |
| Age, y | 63.2 ± 6.5 | 63.7 ± 7.0 | 0.19 | 59.0 ± 2.9 | 59.1 ± 2.9 | 0.68 | 70.2 ± 4.4 | 70.9 ± 5.0 | 0.10 |
| Women, % | 49.9 | 50.1 | 0.93 | 50.2 | 50.4 | 0.95 | 49.2 | 49.5 | 0.94 |
| North region, % | 38.1 | 46.2 | <0.001 | 38.6 | 24.2 | <0.001 | 38.4 | 22.7 | <0.001 |
| Urbanization index | 54.5 ± 18.5 | 65.2 ± 16.1 | <0.001 | 53.0 ± 18.6 | 63.9 ± 16.6 | <0.001 | 57.3 ± 18.1 | 67.2 ± 15.2 | <0.001 |
| Graduated from primary school or higher, % | 42.6 | 53.8 | <0.001 | 50.1 | 62.4 | <0.001 | 30.2 | 40.5 | 0.01 |
| Household income per capita ≥5000 yuan/y, % | 30.8 | 46.2 | <0.001 | 34.9 | 49.6 | <0.001 | 24.1 | 40.9 | <0.001 |
| Current smoking, % | 32.5 | 28.9 | 0.13 | 35.5 | 30.6 | 0.12 | 27.5 | 26.0 | 0.67 |
| Current alcohol use, % | 35.2 | 32.0 | 0.18 | 38.1 | 32.9 | 0.11 | 30.7 | 30.5 | 0.95 |
| Physical activity, % | <0.001 | <0.001 | 0.002 | ||||||
| Low | 31.1 | 37.3 | 20.3 | 27.4 | 48.9 | 52.7 | |||
| Medium | 29.5 | 40.1 | 31.3 | 44.0 | 26.5 | 34.1 | |||
| High | 39.4 | 22.6 | 48.3 | 28.6 | 24.6 | 13.2 | |||
| Hypertension, % | 38.8 | 42.8 | 0.13 | 32.2 | 36.4 | 0.20 | 49.4 | 52.9 | 0.43 |
| BMI, kg/m2 | 22.5 ± 3.5 | 23.4 ± 3.6 | <0.001 | 22.6 ± 3.3 | 23.5 ± 3.3 | <0.001 | 22.4 ± 3.7 | 23.2 ± 4.1 | 0.01 |
| Waist circumference, cm | 80.5 ± 10.5 | 82.0 ± 10.3 | 0.01 | 80.2 ± 9.8 | 81.9 ± 9.8 | 0.02 | 81.0 ± 11.6 | 82.1 ± 11.1 | 0.25 |
| Total caloric intake, kcal/d | 1432 ± 516 | 1510 ± 519 | 0.004 | 1483 ± 507 | 1575 ± 537 | 0.008 | 1348 ± 520 | 1409 ± 473 | 0.15 |
| Vegetable consumption, g/d | 178 ± 108 | 185 ± 96 | 0.23 | 187 ± 106 | 199 ± 102 | 0.06 | 164 ± 109 | 161 ± 81 | 0.69 |
| Fruit consumer, % | 12.5 | 19.5 | <0.001 | 12.6 | 20.4 | 0.001 | 12.2 | 18.2 | 0.04 |
| Fresh legume consumer, % | 37.9 | 41.0 | 0.22 | 36.5 | 44.0 | 0.02 | 40.2 | 36.4 | 0.35 |
| High-fat meat intake >2 servings/wk, % | 43.6 | 61.5 | <0.001 | 42.6 | 60.6 | <0.001 | 45.2 | 62.7 | <0.001 |
Values are means ± SDs unless otherwise noted. Independent-sample t tests were used for continuous variables and χ2 tests for categorical variables. Total fish includes all fish and shellfish, either fresh or preserved (1 serving = 100 g). For hypertension and BMI, n = 1468; for age ≥65 y, n = 564. CHNS, China Health and Nutrition Survey.
Among those eating <1 serving/wk, 968 or 96.5% were nonconsumers.
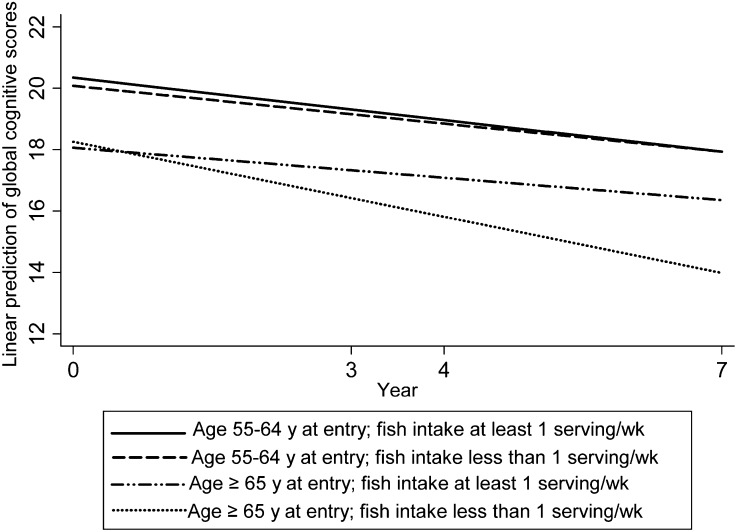
The mean follow-up among the 1566 older adults in the analysis sample was 5.3 y. At baseline, the mean ± SD global cognitive score was 19.0 ± 5.9 points. The mean annual rate of cognitive change was a decline of 0.40 point. After stratifying by age, among participants aged ≥65 y, the rate of cognitive decline was consistently slower for those who consumed ≥1 serving/wk fish (Table 2). In model 1, which adjusted for demographic and socioeconomic covariates, total fish intake of ≥1 serving/wk was associated with a lower rate of cognitive decline by 0.37 point (95% CI: 0.15, 0.59; P = 0.001). The rate difference was slightly attenuated in model 2 after further adjusting for multiple lifestyle and dietary components hypothesized to influence cognitive health: comparing those consuming ≥1 vs. <1 serving/wk fish, the mean rate of global cognitive decline was reduced by 0.35 point/y (95% CI: 0.13, 0.58; P = 0.002) (Fig. 1), which is equivalent to the disparity associated with being 1.6 y younger in age. This observation was not materially altered after adjusting for dietary patterns in model 3.
TABLE 2.
Adjusted mean difference in rate of change in global cognitive score comparing fish intake of ≥1 vs. <1 serving/wk among Chinese older adults1
| All participants (n = 1566) |
Age at entry <65 y (n = 968) |
Age at entry ≥65 y (n = 598) |
||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Fish and shellfish, fresh and preserved (“total fish”) | ||||||
| Model 1 | 0.12 (−0.01, 0.26) | 0.07 | −0.04 (−0.20, 0.13) | 0.67 | 0.37 (0.15, 0.59) | 0.001 |
| Model 2 | 0.12 (−0.01, 0.25) | 0.07 | −0.04 (−0.20, 0.13) | 0.67 | 0.35 (0.13, 0.58) | 0.002 |
| Model 3 | 0.11 (−0.03, 0.24) | 0.11 | −0.06 (−0.22, 0.11) | 0.52 | 0.34 (0.11, 0.56) | 0.004 |
| Fish, fresh only (“fresh fish”) | ||||||
| Model 1 | 0.15 (0.01, 0.28) | 0.03 | 0.01 (−0.16, 0.18) | 0.90 | 0.35 (0.12, 0.57) | 0.002 |
| Model 2 | 0.15 (0.01, 0.28) | 0.03 | 0.01 (−0.16, 0.18) | 0.89 | 0.32 (0.10, 0.55) | 0.005 |
| Model 3 | 0.14 (0.00, 0.27) | 0.05 | 0.00 (−0.17, 0.17) | 0.99 | 0.30 (0.07, 0.53) | 0.009 |
The mean annual change (points/y) in the participants was as follows: all participants, −0.40; age at entry <65 y, −0.34; age at entry ≥65 y, −0.49. Age significantly modified the association between fish intake and change in global cognitive score (total fish in model 2, P = 0.007) (1 serving = 100 g). The global score combined the results of immediate recall of a 10-word list, delayed recall of a 10-word list, counting backward from 20, serial 7 subtraction, and orientation. Model 1 adjusted for mean-centered age, gender, region (south/north), urbanization index, education (graduated from primary school/less), annual household income per capita (≥5000 yuan/less), total energy intake, time (year since baseline), and time interactions with each covariate. Model 2 adjusted for model 1 covariates, as well as for lifestyle factors and multiple dietary confounders, including physical activity (tertile), current alcohol use (yes/no), current smoking (yes/no), fresh vegetable intake (tertiles), fruit consumer (yes/no), fresh legume consumer (yes/no), high-fat meat intake (>2 servings/wk or less), and time interactions with each covariate. Model 3 adjusted for model 2 covariates and for 2 empirical dietary patterns with their time interactions. A positive β coefficient indicates slower rate of decline in cognitive performance.
FIGURE 1.
Linear trajectories of global cognitive scores among Chinese older adults aged 55–64 and ≥65 y at entry comparing total fish intake of ≥1 vs. <1 serving/wk (1 serving = 100 g). The global cognitive score combined the results of immediate recall of a 10-word list, delayed recall of a 10-word list, counting backward from 20, serial 7 subtraction, and orientation. Results held constant were gender, region (south/north), urbanization index, education (graduated from primary school/less), annual household income per capita (≥5000 yuan/less), total energy intake, time, physical activity, current alcohol use (yes/no), current smoking (yes/no), consumption of fresh vegetables (tertiles), fruit consumer (yes/no), fresh legume consumer (yes/no), high-fat meat intake (>2 servings/wk or less), and time interactions with each covariate. Age significantly modified the association between fish intake and change in the global cognitive score (total fish, P = 0.007).
Removing shellfish and/or preserved fish from the total fish did not appreciably alter the results (Supplemental Table 2). For example, for individuals who consumed fresh fish at least weekly, the rate of cognitive decline among adults aged ≥65 y was slower by 0.32 point/y (95% CI: 0.10, 0.55; P = 0.005). The fish–cognitive decline association was not significant among adults aged <65 y.
Supplementary analyses using a number of alternative approaches did not change the observed association, including the following: 1) adjusting for vitamin E and C intakes; 2) adjusting for waist circumference, BMI, and hypertension; 3) removing those with the lowest 10% baseline cognitive scores; 4) accounting for the potential non-independence of data for participants who shared a residence; or 5) using propensity score matching (Supplemental Table 3). Excluding those with self-reported diabetes, stroke and MI strengthened the association of total fish and change of global cognitive scores (0.41 point; 95% CI: 0.17, 0.64; P = 0.001).
Because memory tests contribute disproportionately to points in the cognitive global score, in our secondary analysis, we constructed an alternative score based on composite Z-scores for tests. Results were very similar to the primary analysis: that is, slower cognitive decline among adults aged ≥65 y who consumed fish at least weekly (Table 3). The rate of verbal memory score decline among infrequent consumers was 2.5 times faster than those consuming ≥1 serving/wk fish. Similar results applied for immediate and delayed recall. No rate difference was found for any cognitive test items for adults younger than age 65 y.
TABLE 3.
Adjusted mean difference in rate of change in composite cognitive score and memory test scores comparing total fish intake of ≥1 vs. <1 serving/wk among Chinese older adults1
| All (n = 1566) |
Age at entry <65 y (n = 968) |
Age at entry ≥65 y (n = 598) |
|||||||
| Test score in standardized units | Mean annual change | β (95% CI) | P | Mean annual change | β (95% CI) | P | Mean annual change | β (95% CI) | P |
| SU/y | SU/y | SU/y | |||||||
| Composite scores2 | −0.061 | 0.018 (−0.001, 0.038) | 0.06 | −0.052 | −0.005 (−0.029, 0.019) | 0.67 | −0.077 | 0.053 (0.020, 0.087) | 0.002 |
| Verbal memory scores3 | −0.054 | 0.017 (−0.006, 0.040) | 0.15 | −0.049 | −0.006 (−0.035, 0.024) | 0.71 | −0.063 | 0.051 (0.013, 0.088) | 0.008 |
| Immediate 10-word recall | −0.049 | 0.020 (−0.003, 0.043) | 0.10 | −0.044 | 0.001 (−0.029, 0.030) | 0.97 | −0.058 | 0.046 (0.007, 0.085) | 0.020 |
| Delayed 10-word recall | −0.053 | 0.013 (−0.010, 0.036) | 0.28 | −0.049 | −0.011 (−0.041, 0.020) | 0.49 | −0.060 | 0.050 (0.013, 0.087) | 0.008 |
Age significantly modified the association between fish intake and change in global cognitive score (total fish in model 2, P = 0.007) (1 serving = 100 g). Model was adjusted for model 2 covariates as in primary analysis, which include mean-centered age, gender, region (south/north), urbanization index, education (graduated from primary school/less), annual household income per capita (≥5000 yuan/less), total energy intake, time, physical activity (tertile), current alcohol use (yes/no), current smoking (yes/no), fresh vegetable intake (tertiles), fruit consumer (yes/no), fresh legume consumer (yes/no), high-fat meat intake (>2 servings/wk or less), and time interactions with each covariate. A positive β coefficient indicates a slower rate of decline in cognitive performance. SU, standard unit.
Computed by averaging baseline Z-scores of memory scores and the rest of the cognitive scores.
Combined results of immediate and delayed recall of a 10-word list.
Discussion
In this large prospective cohort study, we showed in a Chinese population that consuming ≥1 servings/wk fish may reduce the rate of cognitive decline for individuals aged ≥65 y, independent of a wide array of nondietary and dietary factors. For adults aged 55–64 y, we did not find such an association.
Comparisons of our results with previous literature suggest that some of the heterogeneity in literature on this topic may be related to disparities in population characteristics, such as the proportion of nonconsumers and the age range over which cognitive changes are being assessed. As with our findings, both the Zutphen study and the Chicago Health and Aging Project reported associations between consuming fish ≥1 times/wk over ∼5 y of follow-up in populations with adults aged ≥65 y at enrollment compared with nonconsumers (8, 9). In contrast, higher fish consumption was not associated with cognitive change in the Veterans Affairs Normative Aging Study, a study with few nonconsumers (0.2 serving/wk in the lowest quartile), a wide variation in baseline age (~50–85 y), and substantial loss to follow-up (11). Consuming tuna and dark-meat fish (∼50% nonconsumers) ≥1 times/wk was associated with slower verbal memory decline after 4 y of follow-up in the Women’s Health Study, with participants aged ≥65 y at enrollment (10). There was little evidence of added benefits of further increasing intakes. However, there was no association between cognitive decline and total seafood intake, including light-meat fish and shellfish, which contrasts with our finding of a relation with intakes of predominantly lean fish. The most commonly consumed fish types in Chinese older adults are fresh-water fish, which include silver carp, grass carp, and goldfish carp, as reported in the CHNS data. These species are typically lower in ω-3 PUFA content than marine fatty fish (35). Our findings indicate that the potential benefits of fish consumption for cognitive health could be applied to the Chinese population in which the fish types consumed differ from those in Western countries.
Our findings of a null association for adults aged 55–64 y were not directly reported in previous literature. Such null findings do not necessarily indicate that there is no cognitive benefit of fish consumption at younger ages, considering that the window for accruing cognitive benefits may occur over a long period, and that there may be diminished ability to detect associations at younger ages, when the rate of cognitive decline is less pronounced as observed in our study and others (36, 37).
Like studies on age-related cognitive decline, the evidence relating fish consumption and the risk of cognitive impairment or dementia was also limited and inconsistent. Some studies found an inverse association between fatty fish intake and prevalence of cognitive impairment among adults with a mean age of 56 y (38) or between fish intake and risk of dementia among the elderly (39). However, other studies found weak or no association (30, 40) or associations observed only among those without the apoE ε4 high-risk allele (41, 42).
ω-3 PUFAs such as DHA and EPA, found in high concentrations in fish, were hypothesized to be beneficial for cognitive health in part through reducing inflammation and oxidative stress in the central nervous system (2, 43). A meta-analysis suggests that findings from observational studies support a role of ω-3 PUFAs in the prevention of cognitive decline but perhaps not in the prevention or treatment of dementia (44). A recent fMRI study suggested that supplementation with EPA, found in relatively high concentrations in shellfish and lean fish, as well as in fatty fish, may be more effective than DHA (which tends to be concentrated in fatty fish) at improving neurocognitive function in young adults (45). Recent longitudinal structural MRI studies supported that circulating ω-3 PUFAs (46, 47), or supplementation with these nutrients (48), may also improve the aging brain in older adults. However, the benefits of fish consumption for cognitive health might be due to the interplay of a number of nutrients in fish beyond these FAs, such as vitamin D and B-complex vitamins, trace elements, such as magnesium and selenium, and essential amino acids, such as arginine and taurine (6, 7). In fact, the primarily contradictory results from randomized clinical trials on supplementation with ω-3 PUFAs and cognitive change or Alzheimer disease in healthy participants (48–50), patients with mild cognitive impairment (51), or patients with mild Alzheimer disease (52, 53) may suggest that nutrients from fish that may influence cognitive health are not limited to ω-3 PUFAs alone.
Major strengths of this study include the use of longitudinal data, the use of 2 scoring methods to evaluate overall cognitive function, and the use of multiple analytic models, which increased our confidence in the robustness of the findings. The population-based sample from the CHNS imparts the ability to generalize the results to the Chinese population.
Limitations of the current study should also be considered. Although adjusting for a wide array of covariates was found to have little effect, as with all observational research, some residual confounding is possible. Although 1 of our aims was to assess the fish intake–cognitive decline association in a population with different sociodemographic correlates of fish intake, we found that, similar to other populations, fish intake in our sample was correlated with higher socioeconomic status. Second, the cognitive screening items adopted in the CHNS were relatively narrow in the scope of cognitive assessments and may not be sensitive enough to detect changes in the younger age group. Besides, use of a limited number of 24-h recalls did not allow us to evaluate in greater detail the dose–response association of fish intake and cognitive decline. The use of 24-h recalls likely increased random measurement error in the estimate of fish consumption, which could attenuate associations (54–56). However, 24-h recalls supplemented with measured changes in household food inventories offers additional benefits in this older population because it is less reliant on memory or cognitive abilities than an FFQ (57).
In conclusion, the current study supports a favorable role of fish consumption in deterring cognitive decline. At least 1 serving/wk fish (i.e., 100 g) predicted slower cognitive decline among Chinese adults aged ≥65 y. Future studies should evaluate more precisely whether the beneficial factors are related to particular types of fish or specific nutrients.
Supplementary Material
Acknowledgments
All of the authors designed the research; L.S.A. and B.M.P. contributed to the data collection; B.Q. analyzed the data; L.J.E. provided statistical expertise; B.Q., B.L.P., and M.A.M. contributed to the data interpretation; B.Q. and M.A.M. wrote the paper; and B.Q. had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Wimo A, Prince MJ. World Alzheimer Report 2010: the global economic impact of dementia. London: Alzheimer’s Disease International; 2010. [Google Scholar]
- 2.Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease—but how and why? Prostaglandins Leukot Essent Fatty Acids 2008;78:11–9. [DOI] [PubMed] [Google Scholar]
- 3.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. VItamin d and risk of cognitive decline in elderly persons. Arch Intern Med 2010;170:1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 2012;27:592–600. [DOI] [PubMed] [Google Scholar]
- 5.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging 2006;10:377–85. [PubMed] [Google Scholar]
- 6.Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ 2012;345:e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease—eat fish or take fish oil supplement? Prog Cardiovasc Dis 2009;52:95–114. [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62:1849–53. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr 2007;85:1142–7. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Grodstein F, Rosner B, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Seafood types and age-related cognitive decline in the Women’s Health Study. J Gerontol A Biol Sci Med Sci 2013;68:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Rest O, Spiro A, Krall-Kaye E, Geleijnse JM, de Groot LC, Tucker KL. Intakes of (n-3) fatty acids and fatty fish are not associated with cognitive performance and 6-year cognitive change in men participating in the Veterans Affairs Normative Aging Study. J Nutr 2009;139:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Access Economics. Dementia in the Asia Pacific region: the epidemic is here. Published September 21, 2006. (cited 2013 Sept 17). Available from: http://www.alz.co.uk/research/files/apreport.pdf.
- 14.Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: the China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 2010;39:1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du S, Mroz TA, Zhai F, Popkin BM. Rapid income growth adversely affects diet quality in China–particularly for the poor! Soc Sci Med 2004;59:1505–15. [DOI] [PubMed] [Google Scholar]
- 16.He K, Xun P, Brasky TM, Gammon MD, Stevens J, White E. Types of fish consumed and fish preparation methods in relation to pancreatic cancer incidence: the VITAL Cohort Study. Am J Epidemiol 2013;177:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manerio E, Rodas VL, Costas E, Hernandez JM. Shellfish consumption: a major risk factor for colorectal cancer. Med Hypotheses 2008;70:409–12. [DOI] [PubMed] [Google Scholar]
- 18.Lee SA, Shu XO, Yang G, Li H, Gao YT, Zheng W. Animal origin foods and colorectal cancer risk: a report from the Shanghai Women’s Health Study. Nutr Cancer 2009;61:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong LY, Ho JH, Huang DP. Preserved foods as possible cancer hazards: WA rats fed salted fish have mutagenic urine. Int J Cancer 1979;23:542–6. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong RW, Imrey PB, Lye MS, Armstrong MJ, Yu MC, Sani S. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int J Cancer 1998;77:228–35. [DOI] [PubMed] [Google Scholar]
- 21.Plassman BL, Welsh KA, Helms M, Brandt J, Page WF, Breitner JC. Intelligence and education as predictors of cognitive state in late life: a 50-year follow-up. Neurology 1995;45:1446–50. [DOI] [PubMed] [Google Scholar]
- 22.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Cogn Behav Neurol 1993;6:103–10. [Google Scholar]
- 23.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011;66(Suppl 1):i162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender differences in cognition among older adults in China. J Hum Resour 2012;47:951–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss J, Lei X, Park A, Shen Y, Smith JP, Yang Z, Zhao Y. Health outcomes and socio-economic status among the elderly in China: evidence from the CHARLS Pilot. J Popul Ageing. 2010;3:111–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med 2010;71:1436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.StataCorp. Stata user’s guide, release 11. College Station (TX): Stata Press; 2009. [Google Scholar]
- 29.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 30.Devore EE, Grodstein F, van Rooij FJ, Hofman A, Rosner B, Stampfer MJ, Witteman JC, Breteler MM. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr 2009;90:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attard SM, Herring AH, Mayer-Davis EJ, Popkin BM, Meigs JB, Gordon-Larsen P. Multilevel examination of diabetes in modernising China: what elements of urbanisation are most associated with diabetes? Diabetologia 2012;55:3182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 33. Cochran WG, Rubin DB. Controlling bias in observational studies: a review. Sankhya Ser A 1973;35:417#x201346.
- 34.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33–8. [Google Scholar]
- 35.Yang Y. Chinese food composition table 2004. Beijing: Peking University Medical Press; 2005. [Google Scholar]
- 36.Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol 2003;39:535–50. [DOI] [PubMed] [Google Scholar]
- 37.Schaie KW. Intellectual development in adulthood. 4th ed. New York: Academic Press; 1990. [Google Scholar]
- 38.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004;62:275–80. [DOI] [PubMed] [Google Scholar]
- 39.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003;60:940–6. [DOI] [PubMed] [Google Scholar]
- 40.Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ 2002;325:932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Burke GL, Carlson MC. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology 2005;65:1409–14. [DOI] [PubMed] [Google Scholar]
- 42.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City Cohort Study. Neurology 2007;69:1921–30. [DOI] [PubMed] [Google Scholar]
- 43.Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White ME, Frautschy SA. Prevention of Alzheimer’s disease: omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging 2005;26:133–6. [DOI] [PubMed] [Google Scholar]
- 44.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 2009;5:140–52. [DOI] [PubMed] [Google Scholar]
- 45.Bauer I, Hughes M, Rowsell R, Cockerell R, Pipingas A, Crewther S, Crewther D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum Psychopharmacol 2014;29:133–44. [DOI] [PubMed] [Google Scholar]
- 46.Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, King IB, Mozaffarian D. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the cardiovascular health study. J Am Heart Assoc 2013;2:e000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 2012;79:642–50. [DOI] [PubMed] [Google Scholar]
- 48.Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex 2013;June 24 (Epub ahead of print; DOI:10.1093/cercor/bht163). [DOI] [PubMed] [Google Scholar]
- 49.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M, Investigators M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 2010;6:456–64. [DOI] [PubMed] [Google Scholar]
- 50.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 2008;71:430–8. [DOI] [PubMed] [Google Scholar]
- 51.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1538–44. [DOI] [PubMed] [Google Scholar]
- 52.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402–8. [DOI] [PubMed] [Google Scholar]
- 53.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304:1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keogh RH, White IR. Allowing for never and episodic consumers when correcting for error in food record measurements of dietary intake. Biostatistics 2011;12:624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keogh RH, Park JY, White IR, Lentjes MA, McTaggart A, Bhaniani A, Cairns BJ, Key TJ, Greenwood DC, Burley VJ, et al. Estimating the alcohol-breast cancer association: a comparison of diet diaries, FFQs and combined measurements. Eur J Epidemiol 2012;27:547–59. [DOI] [PubMed] [Google Scholar]
- 56.Orfanos P, Knuppel S, Naska A, Haubrock J, Trichopoulou A, Boeing H. Evaluating the effect of measurement error when using one or two 24 h dietary recalls to assess eating out: a study in the context of the HECTOR project. Br J Nutr 2013;110:1107–17. [DOI] [PubMed] [Google Scholar]
- 57.Willett W. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.