Abstract
Purpose of Review
To highlight recent advances in nutrition and protein research that have the potential to improve health outcomes and status in aging adults.
Recent Findings
The beneficial effects of dietary protein on muscle health in older adults continue to be refined. Recent research has bolstered support for moderately increasing protein consumption beyond the current RDA by adopting a meal-based approach in lieu of a less specific daily recommendation. Results from muscle protein anabolism, appetite regulation and satiety research support that contention that meeting a protein threshold (approximately 30 g/meal) represents a promising strategy for middle-aged and older adults concerned with maintaining muscle mass while controlling body fat.
Summary
Optimizing dietary protein intake to improve health requires a detailed consideration of topics including muscle protein anabolism, appetite control and satiety. While each area of research continues to advance independently, recent collaborative and translational efforts have highlighted broad, translational consistencies related to the daily distribution and quantity of dietary protein.
Keywords: aging, sarcopenia, nutrition, satiety, strength
Overview
Higher protein diets continue to gain scientific support as a strategy to preserve lean mass, promote weight loss, prevent weight-regain following weight loss, or to simply maintain a healthy weight throughout the lifespan. The effectiveness of these diets appears to be driven, in part, by improvements in protein anabolism, daily appetite control and satiety. This brief synopsis will first summarize the state of the research regarding the effects of increased protein consumption on changes in body weight/composition during controlled-feeding trials and in free-living older adult populations. Recent conceptual and mechanistic advances in protein synthesis, energy expenditure, and the regulation of food intake will also be explored. Finally, the impact of protein quantity and timing of consumption will be addressed.
Sarcopenia and protein recommendations
A reduction in muscle mass and functional capacity is a largely inevitable consequence of aging. The uncomplicated progression of sarcopenia results in a 3-8% reduction in muscle mass per decade, starting in the fourth or fifth decade of life. During this early period, small decrements in muscle mass or function may be readily masked by subtle lifestyle adaptations. However, advanced sarcopenia is synonymous with physical frailty and associated with an increased risk of falls and impairment in the ability to perform routine activities of daily living (1, 2).
The Recommended Dietary Allowance (RDA) for protein (i.e., 0.8 g protein/kg/day) describes the quantity of protein required daily to prevent deficiency for all adults, irrespective of age. In recent years, there has been increased support for the contention that the current RDA for protein is insufficient to promote optimal health. To this end, several recent reviews and consensus statements have suggested that a protein intake between 1.0 and 1.5 g/kg/day may confer health benefits beyond those afforded by simply meeting the minimum (3-8).
Analysis of large data sets such as the National Health and Nutrition Examination Survey (NHANES), suggests that many adult populations already consume a moderate-to-high protein diet (i.e., 1.3 g protein/kg/day), (9, 10). Unfortunately, older adults generally consume less protein and energy than their younger peers (6). Approximately one third of adults over 50 years of age fail to meet the RDA for protein, while approximately 10% of older women fail to meet even the Estimated Average Requirement (EAR) for protein (0.66 g protein/kg/day) (3, 11). For a 65 kg adult, the EAR represents a paltry 40 g of protein/day. Increasing and optimizing protein intake may be particularly important for older populations experiencing catabolic stressors such as illness, physical inactivity or injury (6, 12-14). In circumstances where the capacity or ability to exercise is limited, nutrition, and protein consumption in particular, represents one of the few remaining options to improve muscle protein anabolism and ultimately preserve muscle mass and function.
While there are compelling arguments supporting the role of higher protein diets for muscle health, it is equally clear that a growing proportion of the population chronically exceed their daily energy requirements (10, 15). In older adults, the outwardly paradoxical increase in the incidence of sarcopenic obesity highlights the need to look beyond the quantity of protein and energy consumed daily (16-18). Recent research efforts have clearly established the benefits of increased dietary protein within an energy controlled diet for weight management (16, 19). However, relatively few studies have examined issues such as protein distribution (20-22), or adopted an integrated approach to examine the concomitant effects of increased protein on a broad, cross-disciplinary array of outcomes/themes such as: protein metabolism, cell signaling, body composition, satiety, glucose regulation, and overall macronutrient consumption.
Body Weight/Composition
Three recent meta-analyses examined the effects of higher protein diets on changes in body weight and body composition. Wycherley et al. (23) analyzed 24 randomized controlled trials that compared higher protein or standard protein energy-restriction diets of 12.1 ± 9.3 weeks in duration in 1063 overweight and obese individuals between 18-80 years of age. The higher protein diets contained between 27-35% of daily energy intake as protein (1.07-1.60 g protein/kg/day), whereas the standard protein diets contained 16-21% protein (0.55-0.88 g protein/kg/day) (23). Across this broad age spectrum, the higher protein diets led to greater weight loss (mean difference: −0.79 kg; CI: −1.50, −0.08; p<0.03) and fat loss (mean difference: −0.87 kg; CI: −1.26, −0.48; p<0.001) compared to the standard protein diets (23). Of particular importance for middle-aged and older adults, the higher protein diets were also associated with greater lean mass retention (during energy restriction) (mean difference: +0.43 kg; CI: 0.09, 0.78; p<0.01) compared to the standard protein diets (23).
Similar findings were also reported in a meta-analyses of studies involving 418 middle-aged adults (46-63 y) with Type 2 Diabetes (24). In this analysis, the higher protein diets, containing 25-32% protein, led to greater weight loss (mean difference: −2.08 kg; CI: −3.25, −0.90 kg) compared to diets containing 15-20% protein (24). Santesso et al. (25) extended these findings to encompass 74 randomized controlled trials comparing higher protein (16-45%) vs. standard protein diets (5-23%). Irrespective of factors such as total energy consumption, health status and age, higher protein diets facilitated greater weight loss (mean difference: −0.36 kg; CI: −0.56, −0.17; p<0.001), reductions in BMI (mean difference: −0.37 kg/m2; CI: −0.56, −0.19; p<0.001) and reductions in waist circumference (mean difference: −0.43 cm; CI: −0.69, −0.16; p<0.001) compared to the standard protein diets. The magnitude of change, while modest, has clear clinical relevance in light of the on-going obesity epidemic as well as the increased prevalence of type 2 diabetes, metabolic syndrome, sarcopenia and sarcopenic obesity.
Muscle loss and inactivity: Is 50 the new 70?
Establishing healthy exercise and nutrition behaviors should not be viewed as a switch that can be turned on at a particular age or in response to a health scare. Age-related conditions such as osteoporosis and sarcopenia do not develop acutely. Rather, they are insidious conditions whose onset may be accelerated by less than optimal lifestyle practices in early-middle age (26). Nevertheless, middle-aged adults (i.e., 40-65 y) are comparatively under-represented in research, falling between the typical age-based group assignments of: “young” (20-40y) or “elderly” (65y +).
In a compilation of studies examining the effects of physical inactivity/bed rest on skeletal muscle, we nominally compared the rate and magnitude of muscle loss in young (27) and older adults (28) to a recently completed study in middle-aged adults (29) (Table 1.).
Table 1.
Comparison of leg lean mass loss per day during bed rest for young (27), middle-aged (29) and older adults (30).
| Bed Rest (days) |
Age (y) |
Muscle Loss (kg) |
Rate of Loss (g/day) |
|
|---|---|---|---|---|
| Young | 28 | 38±8 | −0.40±0.10 | −14 |
| Middle-age | 14 | 52±4 | −1.16±0.14 | −83 |
| Elderly | 10 | 67±5 | −0.95±0.15 | −95 |
While controlled experimental conditions are certainly needed to confirm these data, it appears that, while healthy middle age adults often have “normal” or “youthful” physiological responses during acute muscle metabolism studies (31, 32), a metabolic perturbation or catabolic insult (e.g., inactivity, injury, malnutrition) may facilitate anabolic resistance or an aging phenotype, increasing the rate of muscle loss.
These observations are consistent with the “Catabolic Crisis” model of muscle loss we recently put forward (13) and suggest that prevention and treatment strategies targeting older adults could be extended to middle-aged adults who may also be at increased risk of accelerated muscle loss during periods of physiological stress (33, 34).
Regulation of Protein Intake
Ingestive behavior is a complex system comprised of both physiological eating and reward-driven (i.e., hedonic) eating. Physiological eating occurs in response to acute and/or chronic fluctuations in energy balance during times of fasting, meal omission, and weight loss, whereas hedonic eating typically occurs in response to external (environmental) signals that stimulate memories and thoughts of food for enjoyment and/or reward. In the current, food-centric obesogenic environment, many support the premise that hedonic eating is the most significant factor in terms of overweight and weight gain; however, during weight loss (or following weight loss), the physiological signals that control hunger and satiety play a pivotal role. Thus, it is critical to identify the effects of dietary interventions on both types of eating. Recent evidence suggests that increased dietary protein modulates both systems.
Results from the majority of acute single-meal studies illustrate significant post-prandial reductions in perceived hunger and increased perceived fullness following the consumption of higher versus standard protein meals (35). These responses are accompanied by hormonal responses including reductions in the hunger-stimulating hormone ghrelin and increases in the satiety-stimulating hormones PYY and GLP-1 (35, 36). Although these findings are more physiological in nature, recent data from our lab also illustrates that the consumption of higher protein meals significantly impacts reward-driven eating behavior. Using functional magnetic resonance imaging (fMRI), we have identified neural activation in response to rewarding food stimuli prior to subsequent eating occasions. Compared to standard protein meals, the higher protein versions leads to reduced activation in select cortico-limbic brain regions, including the insula, hippocampus, parahippocampus, and/or middle pre-frontal cortex which control food motivation, reward, and cravings as well as executive function (37, 38). Collectively, these data support the beneficial effect of dietary protein to modulate appetite control, satiety, and food motivation/reward.
It is important to note that studies examining the regulation of food intake have been performed almost exclusively in young to middle-age men and women. Due to the well-documented anorexic effect of aging, the consumption of higher protein meals might exert an even larger reduction in appetite in the elderly. However, it is also possible that older individuals will be insensitive to increased protein consumption due to their already blunted, ‘non-responsive’ appetite signaling. A retrospective analysis comparing the post-meal appetite responses following standard protein versus higher protein meals across the lifespan is shown in Table 2. Although the older individuals experienced blunted appetite compared to their younger counterparts, the consumption of higher protein meals led to greater reductions in perceived appetite compared to a standard protein meal in all age groups (Table 2; all, p<0.05).
Table 2.
Age-related appetite responses following standard vs. high protein meals (39)
| Age (y) | 4-h Post-meal Appetite1 following a Standard Protein Meal (Area Under the Curve; mm*240 min) |
4-h Post-meal Appetite1 following a High Protein Meal (Area Under the Curve; mm*240 min) |
|
|---|---|---|---|
| Young | 38 ± 2 | 7878 ± 1310 | 6071 ± 1145* |
| Middle-aged | 50 ± 1 | 6065 ± 1205 | 5432 ± 1053* |
| Elderly | 64 ± 2 | 5551 ± 1254† | 4646 ± 1096*† |
Appetite was measured from 100 mm visual analog scales assessing perceived hunger as ‘how strong is your feeling of hunger’, with end anchors of ‘extremely’ to ‘not at all.’ Questionnaires completed every 30 min throughout the 4-h period.
High Protein vs. Standard Protein, p<0.05
Old vs. Young, p<0.05
However, irrespective of age, consumption of moderately higher protein meals still led to reduced post-prandial appetite responses compared to standard protein versions. While muscle protein metabolism research consistently demonstrates the benefits of a moderately higher dietary protein intake, they do not address complex behavioral issues such as the drive to eat. Studies examining the acute and chronic effects of increased dietary protein on physiological and hedonic eating are needed in the elderly to better understand the anorexia of aging to establish specific, age-appropriate protein recommendations.
Protein Quantity
One of the remaining overarching questions in the field of protein research centers on the quantity of protein required to optimize muscle protein anabolism, appetite control and body weight management. The difficulty in answering this question lies with the multiple ways in which protein consumption can been quantified. The dietary guideline recommendations are reported as grams of protein/kg of body weight per day with a minimal quantity of 0.8 g protein/kg/day established as the quantity to prevent deficiencies. The dietary guidelines also include an acceptable macronutrient distribution range (AMDR). The AMDR for protein represents 10%-35% of an individual’s total daily energy intake and is defined as “the range of intake for a particular energy source (i.e., protein) that is associated with reduced risk of chronic disease while providing intakes of essential nutrients”. Unfortunately, the prescriptive usefulness of the AMDR is diminished by the overly-restrictive and generous quantities of protein represented by both ends of the range. Nevertheless, the protein meta-analyses presented in this synopsis suggest that the quantity of protein necessary to promote improvements in body composition falls between 1.2-1.6 g protein·kg−1·d−1 (i.e., ~25-30% of daily intake as protein) (40). This is well within the acceptable macronutrient range for protein and allows for the ability to meet the recommendations for other requirements including fruits, vegetables, dairy, and fiber.
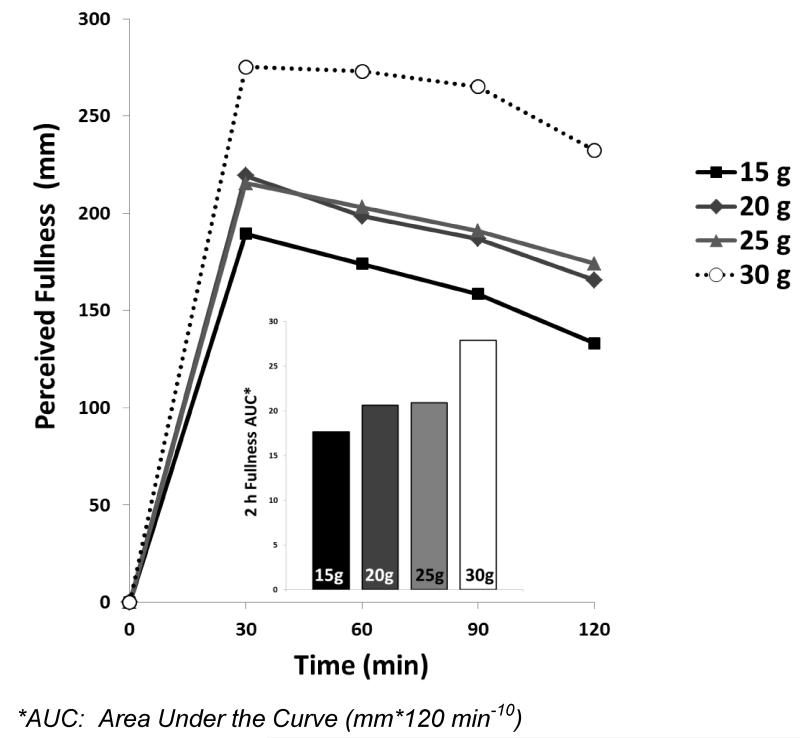
Many mechanistic studies and clinical trials examining protein synthesis, appetite control and satiety, focus on meal-specific protein consumption and not total daily intake. For some outcome measures, this may be a critical point. For example, the human body has limited capacity to temporarily store excess protein from a single large meal and use it to acutely stimulate muscle protein anabolism at a later date. In several recent studies and reviews, there is general agreement that approximately 30 g of protein/eating occasion is required to elicit an optimal or measureable change in a number of outcome variables (6, 41-44). For example, Figure 1 shows the 2 h post-prandial fullness response following the consumption of 350 kcal meals containing varying quantities of dietary protein (unpublished data, composite from previous studies with similar populations and experimental designs).
Figure 1.
Post-prandial fullness responses following 350 kcal meals varying in protein content
Although the consumption of all meals led to an immediate rise in fullness, the meal containing 30 g of protein elicited a larger (and more sustained) increase over the 2 h post-prandial period compared to the other quantities. Thus, these data support a within-meal protein threshold to elicit increases in satiety. If 3-4 meals containing 30 g of protein/meal are consumed throughout the day, the total amount of protein equates to the quantities shown to elicit body weight/body composition changes described above (22). Ingestion of larger protein meals may be justified for adults with increased energy demands or high protein turnover (e.g., athletes, some patient populations), but any potential benefit should be weighed against the risk of exceeding daily energy requirements.
Timing and Distribution of Consumption
Current diet trends continue to focus on the concept of eating frequency to promote optimal weight loss/body composition changes. Ironically, these trends cross the gamut of eating frequency with some advocating small ‘mini’ meals spread through the day, while others emphasis intermittent fasting which includes meal omission, particularly breakfast. However, based on the previous discussion, the quantity and distribution of protein embedded within this eating pattern may significantly impact the outcomes.
In older adults, two recent studies have focused on changes in muscle mass associated with protein distribution patterns (20, 21). Bollwein et al (20) performed a cross-sectional analysis of groups of frail, pre-frail and non-frail adults over the age of 75 y. They noted that while total daily protein consumption was similar in all groups, non-frail older adults consumed an evenly distributed protein diet (i.e., moderate amounts of protein at each meal). In contrast, frail and pre-frail cohorts followed a more skewed protein ingestion pattern, consuming the bulk of their total daily protein during the midday meal.
A recent clinical trial asked a similar question, but found quite different results. Bouillanne et al. (21), conducted a 6-week randomized trial in hospitalized, older adults (avg. 85 y). They reported that patients were provided with a “pulsed” protein diet (8am: 4.5 g, noon: 47.8 g, 4pm: 2.3 g, 7pm: 10.9 g) experienced a significant improvement in lean mass compared to the “spread” protein diet (8am: 12.2 g, noon: 21 g, 4pm: 13.5 g, 7pm: 21.2 g) that provided the same total amount of protein each day (i.e., 1.31 g protein/kg/day). Methodological differences aside, it is difficult to immediately reconcile the apparent discrepancy in the results of these studies. However, an obvious area for further investigation centers on the threshold amount of protein per meal required by various aging populations (healthy and clinical) to elicit a meaningful increase in muscle protein anabolism. In the case of the Bouillanne study, one could suggest that each of the spread protein meals (12-21 g protein) failed to meet the threshold required to optimally stimulate muscle protein anabolism (6, 41-44), whereas the noon meal in the pulsed protein likely had more than enough protein (~48 g) to elicit a single, robust anabolic response (41).
We previously examined whether 6 small meals (i.e., 350 kcal/meal) consumed every 2 h improves daily appetite and satiety responses compared to the standard 3 larger meals (i.e., 700 kcal/meal) (45, 46). Protein quantity was also varied within each meal pattern. Overall, we found that protein quantity had a more robust effect on satiety than eating frequency. Higher protein meals led to greater feelings of fullness and PYY concentrations throughout the day compared to the normal protein meals. These data refute claims that consuming ‘mini’ meals and/or frequent snacking throughout the day is beneficial in controlling appetite and satiety. Further, these data support the ‘protein threshold’ concept of ≥30 g protein to elicit satiety responses as the meals containing < 30 g protein, as was the case for the 6 small high protein meal and the 3 large normal protein meal patterns, led to the smallest satiety response.
A recent longer-term study by Arciero et al. (47) extended these findings to examine the effects of increased protein intake and meal frequency on changes in body weight and body composition. In this study, the higher protein diet groups, regardless of eating frequency, led to greater fat loss, particularly abdominal fat, compared to the normal protein diet. The higher protein diets also led to increased lean mass and reduced daily hunger vs. normal protein versions. The protein meal pattern in this study contained approximately 30 g of protein/eating occasion.
At the other end of the eating frequency spectrum is the concept of meal omission/skipping. Although intermittent fasting, which is a type of reduced eating frequency, is gaining traction in the lay press, limited data exist to support this dietary pattern. On the other hand, meal omission, particularly the breakfast meal, has been strongly associated with increased BMI, unhealthy weight gain, and obesity (48). We recently found that the consumption of breakfast led to increased appetite control, satiety, and reduced reward-driven eating behavior throughout the day compared to skipping the morning meal (38). However, a protein-rich breakfast, containing 35 g of protein, led to further improvements through greater increases in satiety throughout the day along with greater reductions in food motivation/reward compared to a normal protein breakfast containing only 13 g protein. Further, only the higher protein breakfast reduced unhealthy, evening snacking on high fat snacks by approximately 200 kcal compared to skipping breakfast or consuming a normal protein breakfast. These data suggest that substantial protein intake at the breakfast meal appears to have immediate and sustained effects throughout the day. Previous data from our lab further support these findings by illustrating that protein at the morning meal leads to greater immediate and sustained satiety throughout the day compared to protein at lunch or dinner (49).
Conclusions
Sarcopenia has debilitating consequences for the elderly community and considerable impact on health-care systems. The benefits of moderate protein diets for young and older adults have been well established and many ongoing research efforts seek to refine and incrementally add to our understanding. However, it is increasingly clear that multidisciplinary research efforts are needed to provide practical, translatable data on the regulation of body composition and maintenance of muscle mass and function during aging. A compilation of data from recent muscle protein anabolism, appetite regulation and satiety studies suggest that meeting a protein threshold (approximately 30 g/meal) represents a promising strategy or dietary-framework for middle-aged and older adults concerned with maintaining muscle mass while controlling body fat.
Extended Data
Table 1.
Comparison of leg lean mass loss per day during bed rest for young (27), middle-aged (29) and older adults (30).
| Bed Rest (days) |
Age (y) |
Muscle Loss (kg) |
Rate of Loss (g/day) |
|
|---|---|---|---|---|
| Young | 28 | 38±8 | −0.40±0.10 | −14 |
| Middle-age | 14 | 52±4 | −1.16±0.14 | −83 |
| Elderly | 10 | 67±5 | −0.95±0.15 | −95 |
Table 2.
Age-related appetite responses following standard vs. high protein meals (39)
| Age (y) | 4-h Post-meal Appetite following a Standard Protein Meal |
4-h Post-meal Appetite following a High Protein Meal |
|
|---|---|---|---|
| Young | 38 ± 2 | 7878 ± 1310 | 6071 ± 1145* |
| Middle-aged | 50 ± 1 | 6065 ± 1205 | 5432 ± 1053* |
| Elderly | 64 ± 2 | 5551 ± 1254† | 4646 ± 1096*† |
High Protein vs. Standard Protein, p<0.05
Old vs. Young, p<0.05
Key Points.
Sarcopenia has debilitating consequences for the elderly community and considerable impact on health-care systems.
Moderate protein diets for young and older adults have led to greater weight loss, fat loss, and preservation of lean mass compared to standard protein diets.
Potential protein-related mechanism(s)-of-action include the increase in protein anabolism, appetite regulation, and satiety.
Meeting a protein threshold (approximately 30 g/meal) represents a promising strategy or dietary-framework for middle-aged and older adults concerned with maintaining muscle mass while controlling body fat.
Multidisciplinary research efforts are needed to provide practical, translatable data on the regulation of body composition and maintenance of muscle mass and function during aging.
Acknowledgements and Conflicts of Interest
This research was supported by grants from the NIH/NINR RO1 NR012973 (DPJ), UTMB Claude D. Pepper Older Americans Independence Center # P30 AG024832 (DPJ), Pork Checkoff (HJL), Egg Nutrition Center (HJL), and Beef Checkoff (HJL).
D.P.J. has received compensation for speaking engagements with Abbott Nutrition, the National Cattlemen’s Beef Association, the American Egg Board and the National Dairy Council. H.L has received compensation for speaking engagements with the National Cattlemen’s Beef Association, the American Egg Board and the National Dairy Council.
References
- 1.Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652–8. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91–5. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. Jama. 2008;299(24):2891–3. doi: 10.1001/jama.299.24.2891. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27(5):675–84. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 6.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68(6):677–81. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips SM. Dietary protein requirements and adaptive advantages in athletes. Br J Nutr. 2012;108(Suppl 2):S158–67. doi: 10.1017/S0007114512002516. [DOI] [PubMed] [Google Scholar]
- 9.Fulgoni VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008;87(5):1554S–7S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 10.Layman DK. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr Metab (Lond) 2009;6:12. doi: 10.1186/1743-7075-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. JAMA. 2008;299(24):2891–3. doi: 10.1001/jama.299.24.2891. [DOI] [PubMed] [Google Scholar]
- 13.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13(1):34–9. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res Rev. 2013 doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Drewnowski A, Fulgoni VL, Young MK, Pitman S. Nutrient-rich foods: applying nutrient navigation systems to improve public health. J Food Sci. 2008;73(9):H222–8. doi: 10.1111/j.1750-3841.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang M, Armstrong CL, Leidy HJ, Campbell WW. Normal vs. high-protein weight loss diets in men: effects on body composition and indices of metabolic syndrome. Obesity (Silver Spring) 2013;21(3):E204–10. doi: 10.1002/oby.20078. [DOI] [PubMed] [Google Scholar]
- 17.Coker RH, Miller S, Schutzler S, Deutz N, Wolfe RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. 2012;11:105. doi: 10.1186/1475-2891-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding RA, Gunstad J, Gustafson DR, Heymsfield SB, Kral JG, Launer LJ, et al. The paradox of overnutrition in aging and cognition. Ann N Y Acad Sci. 2013;1287:31–43. doi: 10.1111/nyas.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leidy HJ, Tang M, Armstrong CL, Martin CB, Campbell WW. The effects of consuming frequent, higher protein meals on appetite and satiety during weight loss in overweight/obese men. Obesity (Silver Spring) 2011;19(4):818–24. doi: 10.1038/oby.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, et al. Distribution but not amount of protein intake is associated with frailty: a cross-sectional investigation in the region of Nurnberg. Nutr J. 2013;12(1):109. doi: 10.1186/1475-2891-12-109. This cross-sectional study highlights thatt even when older adults exceed the RDA for protien, an even protein distribution may be associated with muscle loss/fraility.
- 21.Bouillanne O, Curis E, Hamon-Vilcot B, Nicolis I, Chrétien P, Schauer N, et al. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin Nutr. 2013;32(2):186–92. doi: 10.1016/j.clnu.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition. 2012;96(6):1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 24.Dong JY, Zhang ZL, Wang PY, Qin LQ. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trials. Br J Nutr. 2013:1–9. doi: 10.1017/S0007114513002055. [DOI] [PubMed] [Google Scholar]
- 25.Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, Heels-Ansdell D, et al. Effects of higher-versus lower-protein diets on health outcomes: a systematic review and meta-analysis. European journal of clinical nutrition. 2012;66(7):780–8. doi: 10.1038/ejcn.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013;49(1):111–7. [PubMed] [Google Scholar]
- 27.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89(9):4351–8. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 28.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 29.English KL. Effects of Leucine on Skeletal Muscle During 14 d Bed Rest in Middle-aged Adults. The University of Texas Medical Branch; Galveston, Texas: 2013. [Google Scholar]
- 30.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–81. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 31.Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging. 2011;15(5):376–81. doi: 10.1007/s12603-010-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Current opinion in clinical nutrition and metabolic care. 2010;13(1):34–9. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.02.011. This study reports that a low volume supplement (HMB) partially preserved muscle mass during physical inactivity. This has potential application for clinical populations and individuals unable to consume sufficient protein during periods of metabolic stress.
- 34.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31(4):512–9. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leidy HJ. Evidence supporting a diet rich in protein to improve appetite control, satiety, and weight management across the lifespan; 65th Annual Reciprocal Meat Conference:1-5; American Meat Science Association. 2012. [Google Scholar]
- 36.van der Klaauw A, Keogh J, Henning E, Trowse V, Dhillo W, Ghatei M, et al. High protein intake stimulates GLP1 and PYY release. Obesity. 2013 doi: 10.1002/oby.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leidy HJ, Lepping RJ, Savage CR, Harris CT. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity (Silver Spring, Md. 2011;19(10):2019–25. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidy HJ, Ortinau LC, Douglas SM, Hoertel HA. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. The American journal of clinical nutrition. 2013;97(4):677–88. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity (Silver Spring, Md. 2007;15(5):1215–25. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- 40.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring, Md. 2007;15(2):421–9. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 41.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109(9):1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips BE, Hill DS, Atherton PJ. Regulation of muscle protein synthesis in humans. Curr Opin Clin Nutr Metab Care. 2011 doi: 10.1097/MCO.0b013e32834d19bc. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012;108(10):1780–8. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 44.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, et al. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab. 2013;38(2):120–5. doi: 10.1139/apnm-2012-0092. [DOI] [PubMed] [Google Scholar]
- 45.Leidy HJ, Armstrong CL, Tang M, Mattes RD, Campbell WW. The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity (Silver Spring, Md. 2010;18(9):1725–32. doi: 10.1038/oby.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leidy HJ, Tang M, Armstrong CL, Martin CB, Campbell WW. The Effects of Consuming Frequent, Higher Protein Meals on Appetite and Satiety During Weight Loss in Overweight/Obese Men. Obesity (Silver Spring, Md. 2010 doi: 10.1038/oby.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arciero PJ, Ormsbee MJ, Gentile CL, Nindl BC, Brestoff JR, Ruby M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity (Silver Spring, Md. 2013;21(7):1357–66. doi: 10.1002/oby.20296. [DOI] [PubMed] [Google Scholar]
- 48.Leidy HJ. The Benefits of Breakfast Consumption to Combat Obesity and Diabetes in Young People. American Journal of Lifestyle Medicine. 2013;7(2):98–104. [Google Scholar]
- 49.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2009;101(6):798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]