Abstract
Objective
To minimize maintenance immunosuppression in upper-extremity transplantation to favor the risk-benefit balance of this procedure.
Background
Despite favorable outcomes, broad clinical application of reconstructive transplantation is limited by the risks and side effects of multidrug immunosuppression. We present our experience with upper-extremity transplantation under a novel, donor bone marrow (BM) cell-based treatment protocol (“Pittsburgh protocol”).
Methods
Between March 2009 and September 2010, 5 patients received a bilateral hand (n = 2), a bilateral hand/forearm (n = 1), or a unilateral (n = 2) hand transplant. Patients were treated with alemtuzumab and methylprednisolone for induction, followed by tacrolimus monotherapy. On day 14, patients received an infusion of donor BM cells isolated from 9 vertebral bodies. Comprehensive follow-up included functional evaluation, imaging, and immunomonitoring.
Results
All patients are maintained on tacrolimus monotherapy with trough levels ranging between 4 and 12 ng/mL. Skin rejections were infrequent and reversible. Patients demonstrated sustained improvements in motor function and sensory return correlating with time after transplantation and level of amputation. Side effects included transient increase in serum creatinine, hyperglycemia managed with oral hypoglycemics, minor wound infection, and hyperuricemia but no infections. Immunomonitoring revealed transient moderate levels of donor-specific antibodies, adequate immunocompetence, and no peripheral blood chimerism. Imaging demonstrated patent vessels with only mild luminal narrowing/occlusion in 1 case. Protocol skin biopsies showed absent or minimal perivascular cellular infiltrates.
Conclusions
Our data suggest that this BM cell-based treatment protocol is safe, is well tolerated, and allows upper-extremity transplantation using low-dose tacrolimus monotherapy.
Keywords: bone marrow, cell therapy, composite tissue allotransplantation, hand transplantation, immunomodulation, immunomonitoring, immunosuppression, reconstructive transplantation, rejection, vascularized composite allotransplantation
Over 150 composite tissue allotransplantations have been performed to date with highly encouraging outcomes.1 In the combined American and European experience, graft survival is greater than 90%. However, 1 patient died from sepsis after combined hand and face transplantation and another developed avascular hip necrosis, emphasizing the importance of minimizing immunosuppression after such non-life-saving transplants. Donor bone marrow (BM) infusion has been attempted in selected cases of solid organ transplantation with the goal of reducing overall immunosuppression.2–7 After successful experimental trial of a similar strategy in a large animal composite tissue allotransplantation model,8 we implemented the treatment protocol in 5 upper-extremity transplant recipients. The 1-year results reported here suggest that the protocol is well tolerated and enables allograft survival with low-dose tacrolimus monotherapy.
METHODS
Inclusion and exclusion criteria for recipient and donor selection are listed at http://clinicaltrials.gov (NCT00722280). The study was approved by the University of Pittsburgh Institutional Review Board and by the Department of Defense Research Review Board.
PATIENTS
Patient 1: A 24-year-old Marine who lost his right, dominant hand in a training accident (January 2007) received a right-hand transplant at the distal forearm level on March 14, 2009. Patient 2: A 57-year-old Air Force veteran with bilateral mid-forearm and lower-limb amputations due to Streptococcus A sepsis (June 1999) became the first American bilateral hand/forearm transplant recipient on May 4, 2009. Patient 3: A 41 year-old National Guardsman injured in a farming accident (November 2008) was the first American recipient of a full forearm (right, above elbow) and hand transplant (left) on February 5, 2010. Patient 4: A 25-year-old female quadruple amputee due to Norovirus sepsis (2004) received a right distal-forearm-level hand transplant on September 11, 2010. Patient 5: A 33-year-old female quadruple amputee due to meningococcal sepsis (August 2003) underwent bilateral distal-forearm-level hand transplantation on September 18, 2010. Donor/recipient human leukocyte antigen (HLA) mismatch was 5/6, 5/6, 3/6, 2/6, and 5/6 for patients 1 to 5, respectively.
Treatment Protocol
Patients were pretreated (1 to 2 hours prior to transplantation) with alemtuzumab, 30 mg intravenously (Campath™, Millennium Pharma, Cambridge, MA) for lymphocyte depletion plus 250 mg methylprednisolone. Tacrolimus (Prograf, Astellas, Japan) monotherapy was commenced with target trough levels of 10 to 15 ng/mL (first month), 8 to 10 ng/mL (2–3 months), 5 to 10 ng/mL (4–12 months), and 3 to 7 ng/mL thereafter. In the event of skin rejection, tacrolimus dosage was modified to adjust whole-blood drug trough levels to the upper limit of target range (see also Fig. 1).
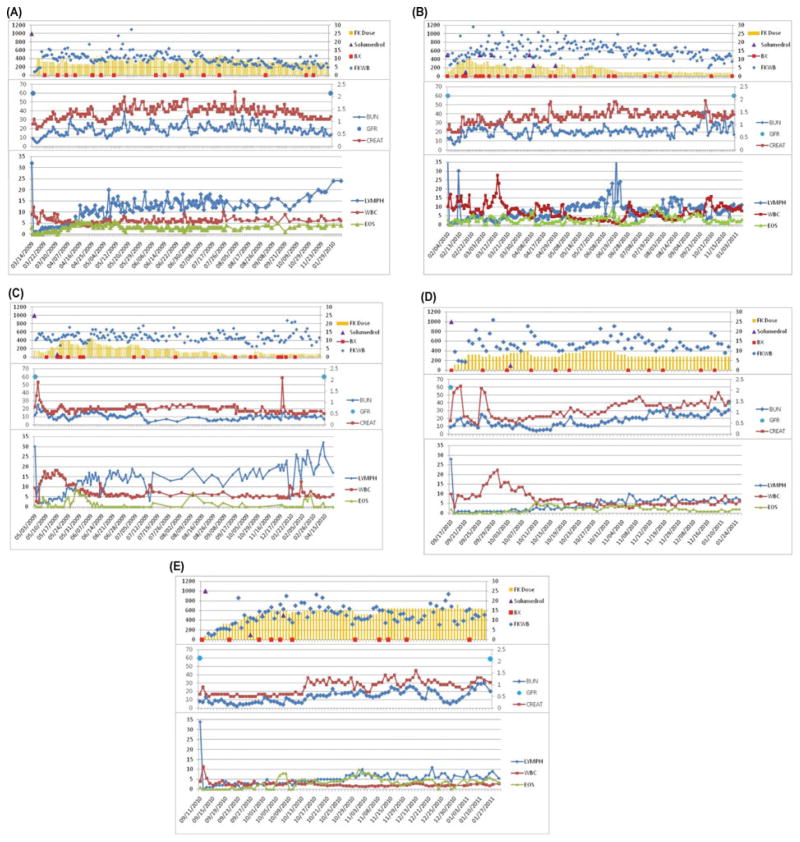
FIGURE 1.
Immunosuppression, kidney function, and peripheral blood WBC/lymphocyte/eosinophile counts are summarized for patients 1–5 (A–E). Tacrolimus dose was adjusted in individual patients to achieve target serum trough levels. The frequency of acute rejection episodes was 3 (patient 1), 1 (patient 2), 3 (patient 3), 1 (patient 4), and 1 (patient 5) (A–E). Patients received bolus steroids in the event of high-grade acute rejection that was nonresponsive to topical therapy. Rejection episodes resolved in all cases without requirement for a second maintenance immunosuppressive drug. In contrast to previous reports (1), no steroid-resistant episodes of acute rejection were observed with this regimen. All 5 patients are currently being maintained on tacrolimus monotherapy. Trough levels range between 4 and 6 ng/mL in patient 1 (A), 8 and 10 ng/mL in patient 2 (B) and patient 3 (C), and 10 and 12 ng/mL in patient 4 (D) and patient 5 (E). Side effects included transient increases in serum creatinine and hyperglycemia after transplant that initially required insulin but was then managed with glipizide 5 mg BID (patient 2, B), and a deep-vein thrombosis in the left lower extremity requiring coumadin treatment as well as a single episode of hyperuricemia that was treated with colchicine (patient 3, C). Patients 1 and 2 (A and B, respectively) required isoniazid prophylaxis with 300 mg QD after incidental and unanticipated exposure to a tuberculous patient while in the hospital.
Unmodified donor BM cells (5–10 × 108/kg body weight) were given intravenously on day 14. Skin rejections were treated with tacrolimus 0.1% topical BID (Protopic, Astellas, Japan) and clobetasol 0.05% topical BID (Fougera, Melville, NY), or methylprednisolone i.v. (500 mg for 1–3 days).
All patients received antibiotic prophylaxis for 5 to 10 days and trimethoprim/sulphamethoxazole (Bactrim, Roche; Mutual Pharmaceutical Company, Inc., Philadelphia, PA) 800 mg/160 mg thrice weekly for 12 months. No cytomegalovirus prophylaxis was given as all patients and donors were cytomegalovirus-negative. Acetylsalicylic acid was given at day 1 and continued for 1 year.
Surgery
Hand and forearm transplantations were performed as per previously described techniques in a bone – deep tendons – vessels –nerves – superficial tendons – skin sequence.1 For the above-elbow transplant, nerves were dissected and coapted just proximal to the first below-elbow motor branches, and the biceps, brachialis, and triceps were repaired.
Vertebral Body Retrieval and BM Isolation
The details of the procedures were recently published.9,10 In brief, vertebral bodies T8-L4 were retrieved and preserved in Custodiol™ (Koehler Chemie, Alsbach-Hähnlein, Germany) solution charged with gentamicin (50 μg/mL) and stored (37 ± 18 hours) until processing. Bone fragments were crushed and tumbled in DNase-enriched medium. BM cells obtained after mesh filtration were pooled and cryopreserved. The CD34 dose was ≥2 × 106/kg and CD34 viability prior to cryopreservation was ≥85% in all 5 samples. Multicolor flow cytometry revealed distinct populations of CD34+CD90+CD117(dim) hematopoietic stem cells (15.5 ± 7.5% of the CD34 + cells) and CD45−CD73+CD105+mesenchymal stromal cells (0.04 ± 0.04% of the total cells). Cultures were negative for bacteria and fungi except for 1 case of minimal contamination with the skin organism Propionibacterium acnes. Unmodified cells (5–10 × 108/kg body weight) were infused on day 14.
Immunomonitoring
Any human leukocyte antigens detected before transplant with corresponding antibodies (Luminex,™ One Lambda Inc., Canoga Park, CA) were considered unacceptable. Cytotoxic cross-matches were negative for all recipients. Global CD4 immune response following phytohemagglutinin stimulation was measured using the ImmuKnow™ assay (Cylex Inc., Columbia, MD). Short tandem repeat analysis (AmpF/STR ProfilerPlus; Applied Biosytems, Foster City, CA) was used to identify donor cells in peripheral blood. Samples were analyzed on an ABI 3130XL capillary electrophoresis system (Life Technologies, Carlsbad, CA).
For patients 1 to 3, T-cell phenotypic and functional analyses were performed at 1 year after transplant and compared with age-and sex-matched healthy controls. T-cell counts, phenotype of T-cell memory subset distribution, and carboxyfluorescein diacetate succinimidyl ester-MLR T-cell proliferation were assessed by flow cytometry.
Histopathology and Imaging
Specimens (84 protocol and clinically mandated skin biopsies) were stained with hematoxylin and eosin and C4d (C4d polyclonal antibody, Alpco Diagnostics, Windham, NH) and evaluated according to the Banff criteria.11 High-resolution ultrasound biomicroscopy of radial and ulnar arteries was performed at 3-month intervals. Computed tomography-angiography was done annually.
RESULTS
Immunological Course
The immunological courses are summarized in Figure 1. Patient 1 experienced 3 acute rejection episodes of the skin (day 43, grade II; 13 months, grade II–III; 21 months, grade I–II) that resolved with topical tacrolimus/clobetasol (rejection 1 and 2) or methylprednisolone (3 × 500 mg, rejection 3). Rejections 2 and 3 were timely correlated with transient nonadherence with immunosuppression. Patient 2 experienced 1 late episode of acute rejection in the skin (day 270, grade II–III) that resolved with a single steroid bolus (500 mg) and topical therapy. In patient 3, a total of 3 episodes of acute rejection (day 25, grade II–III; day 43, grade III; day 66, grade II) were observed. The third episode followed accidental scalding of the transplanted hand. All episodes were successfully reversed with steroid bolus therapy and topical tacrolimus/clobetasol treatment. Patient 4 experienced 1 early episode of acute rejection (day 18, grade II) that responded to steroid bolus. Patient 5 had an acute rejection episode (day 51, grade II–III) that responded to topical tacrolimus/clobetasol treatment.
Upon skin rejection, tacrolimus dose was adjusted to reach but not exceed the upper target range limit of the serum trough level in all patients. Latest protocol skin biopsies showed minimal or absent perivascular cellular infiltrates (Banff grade 0–I) and were C4d negative. Flow cytometry and short tandem repeat analysis for chimerism revealed no evidence of donor cells.
Infusion of BM Cells
Cryopreserved BM products contained 3.9 ± 0.7 × 1010 nucleated cells, 3.7 ± 2.3 × 108 CD34+ cells (5.4 ± 3.1 × 106/kg), and 2.5 ± 1.7 × 109 CD3+ T cells (3.7 ± 3.1 × 107/kg). CD34 viability was 98 ± 2%, and CD3 viability was 71 ± 28%. Frozen BM cell aliquots were thawed at bedside and infused without any untoward effects.
The infusion of BM cells was performed on day 14 with the intention to provide the donor cell pool at a time when the early and strong inflammatory response to ischemia/reperfusion and the surgical trauma had subsided. In a previous study, infusion of donor cells as an adjunct to treatment in solid organ transplantation at a similar time point was found to be safe.7
Histopathology
Histopathology of skin rejections is summarized in Figure 2. In all patients, biopsies obtained early after transplantation showed mild dermal edema, epidermal spongiosis, and rare apoptotic keratinocytes without inflammation, likely attributable to preservation/reperfusion injury.
FIGURE 2.
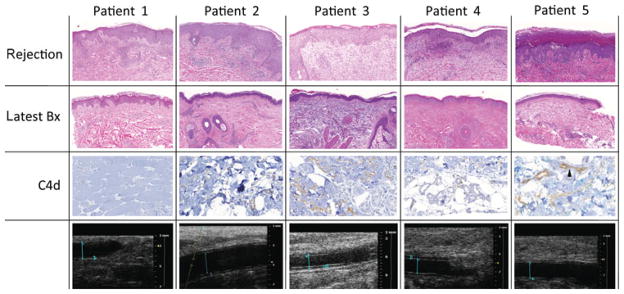
Rejections manifested as mild lymphohistiocytic and eosinophilic perivascular inflammation primarily involving the superficial dermal capillaries, and secondarily, the adnexal structures. acute rejection was associated with eosinophilic predominant infiltrates in 1 recipient (patient 1). The inflammation extended variably into the epidermis and/or adnexa, causing epithelial cell necrosis/apoptosis. Angiography and high-resolution ultrasound biomicroscopy did not reveal any vascular alterations. Adnexal and dermal fat capillaries were positive for C4d in patients 2, 3, and 5. Strong diffuse capillary C4d was noted in patient 5. Tissue and capillary endothelial C4d deposits were minimal (patient 2) and weak (patient 3), and negative (patients 1 and 4). C4d deposits were not accompanied by margination of monocytes and/or neutrophils in the microvasculature. None of the patients demonstrated evidence of luminal narrowing secondary to myointimal proliferation or increased intima medial thickness on high-resolution ultrasound biomicroscopy of radial and ulnar arteries at 1 year. Blood flow parameters remained unchanged. Patient 1: right ulnar artery luminal diameter 2.677 mm, intimal wall thickness 0.261 mm (January 18, 2011). Patient 2: right radial artery luminal diameter 1.96 mm, intimal wall thickness 0.22 mm (June 7, 2010). Patient 3: right radial artery luminal diameter 2.049 mm, intimal wall thickness 0.226 mm (February 3, 2011). Patient 4: right radial artery luminal diameter 1.642 mm, intimal wall thickness 0.326 mm (January 5, 2011). Patient 5: right radial artery luminal diameter 2.1 mm, intimal wall thickness 0.16 mm (January 5, 2011).
Deeper muscle, fat, and nerve tissue were obtained in patient 1 (at 22 months) and patient 2 (at 13 months). These tissue samples showed focal muscle atrophy but no evidence of arteriopathy.
Immunomonitoring
Donor-specific alloantibodies were detected in 4 of 5 recipients and were associated with skin rejection in most cases. Antibodies subsided with treatment (Fig. 3A). Adnexal and dermal fat capillaries were positive for C4d in patients 2, 3, and 5 and correlated well with circulating donor-specific alloantibodies. Strong diffuse capillary C4d deposits and strong donor-specific alloantibodies were noted in patient 5. Patient 2 showed no donor-specific alloantibodies and minimal C4d deposits, and patient 3 showed weak class I donor-specific alloantibodies and minimal-to-no tissue C4d deposits. Patients 1 and 4, in contrast, showed anti-class I and anti-HLA-DQ donor-specific alloantibodies without tissue C4d capillary endothelial deposits.
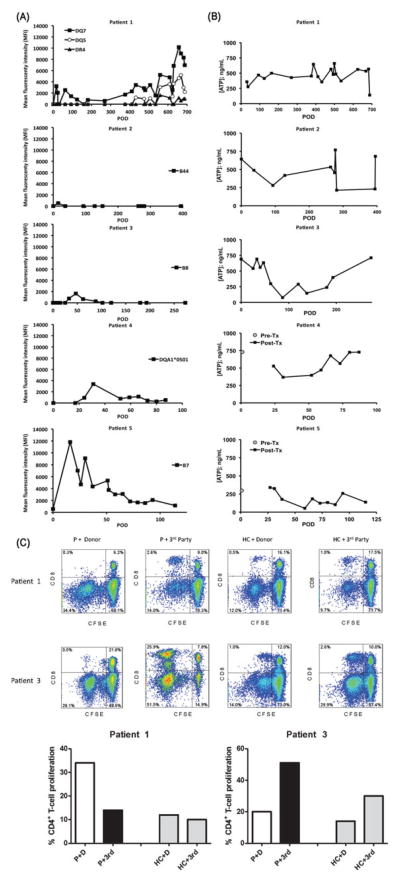
FIGURE 3.
A, Both human leukocyte antigen class I and class II donor-specific alloantibodies were first observed within the first month after transplant. Changes in donor-specific alloantibodies were limited to specific haplotypes in each patient, except for patient 1, who initially showed donor-specific alloantibodies against DQ7, which resolved with treatment but later recurred together with DR4 and DQ5 donor-specific alloantibodies. Such changes coincided with admitted noncompliance with immunosuppression. The donor-specific alloantibodies significantly subsided after compliance was re-established. B, Global CD4+ T-cell immune response were measured using the Immuknow™ assay and categorized into low (<225 ng/mL ATP), moderate (226–525 ng/mL ATP), and high (>525 ng/mL ATP). Patients 1, 2, and 4 demonstrated moderate responses over a majority of time points. In patients 2 and 3, the assay indicated a stronger cellular immune reactivity early after transplantation, with a dip during 12–18 months (patient 2) and 3 to 6 months (patient 3). However, recent values have trended to pretransplant levels. In patient 5, the response has varied between low and moderate despite stable target troughs on low-dose tacrolimus monotherapy. C, One-way carboxyfluorescein diacetate succinimidyl ester-MLR to assess T-cell allospecific proliferation. Proliferation of alloreactive CD3+ T cells was measured by carboxyfluorescein diacetate succinimidyl ester dilution (% of carboxyfluorescein diacetate succinimidyl ester -low cells) of CD4+ and CD8+ T cells after 5 days of in vitro stimulation of patient or healthy control peripheral blood mononuclear cells with donor (D) or third (3rd)-party peripheral blood mononuclear at 1:1 ratio. FACS analysis revealed that patient 1 displayed a significant CD4+ T-cell proliferation to donor antigens as opposed to third-party stimulation (donor-specific reactivity). Patient 3 demonstrated CD4+ and CD8+ T cells with normal memory subset distribution, a robust CD4+ and CD8+ T-cell proliferation to third-party stimulation, but lower levels of CD4+ and CD8+ T-cell proliferation to donor stimulation, denoting a quiescent status (donor-specific hyporesponsiveness).
The current/peak panel reactive antibodies based on ELISA panel reactive antibody results (class I/class II) are as follows:
Patient 1: Current post-Tx: 0/0; Peak post-Tx: 0/5
Patient 2: Current post-Tx: 0/0; Peak post-Tx: 4/7
Patient 3: Current post-Tx: 1/0; Peak post-Tx: 7/1
Patient 4: Current post-Tx: 0/0; Peak post-Tx: 83/3
Patient 5: Current post-Tx: 0/0; Peak post-Tx: 4/0
Global CD4+ T-cell immune responses are summarized in Figure 3B. Overall, responses correlated with adjustments in immunosuppression, or with other interventions or procedures after transplant. In patient 3, a stronger immune response and a total of 3 early acute rejection episodes were observed. After methylprednisolone administration, a decreased T-cell activity was observed and no more rejections occurred. In patient 4, the assay indicated a weak immune response; however, no infectious complications occurred.
Alemtuzumab preconditioning resulted in effective depletion of CD4+ (231 ± 41 cells/μL) and CD8+ (127 ± 38 cells/μL) T cells in all 3 patients (healthy controls: CD4, 880 ± 442 cells/μL; CD8, 375 ± 175 cells/μL). CD4+ T cells were more depleted than CD8+ T cells in patients 1 and 3, with decreased CD4/CD8 ratios. Patient 1 displayed increased proportion of effector memory (TEM) CD4+ T cells (27% vs 19% in healthy controls) and reduced terminally differentiated memory (TEMRA) CD8+ T cells (5% vs 17% in healthy controls), accompanied by an increased CD4+ T-cell proliferation to donor antigens as opposed to third-party stimulation (Fig. 3C). Although CD4+ and CD8+ T-cell memory subsets were distributed normally in patient 2, a significant loss in CD4+CD25hiFOXP3+CD127−T regulatory cells (1.5% vs 3.2% in healthy controls) was noted. Patient 3 had CD4+ and CD8+ T cells with normal memory subsets, and displayed robust CD4+ and CD8+ T-cell proliferation to third-party stimulation, but lower levels of CD4+ and CD8+ T-cell proliferation to donor stimulation (Fig. 3C).
Imaging
High-resolution ultrasound biomicroscopy of radial and ulnar arteries at 1 year revealed normal vascular diameter, lumen, and intima medial thickness in all patients, with 2 exceptions (see also Fig. 2): the luminal diameter in patient 1 was increased to 2.677 mm and intima-media thickness in patient 4 was increased to 0.326 mm. The reference for luminal diameter in healthy subjects is 1.7 ± 0.48 mm and for intima medial thickness is 0.21 ± 0.053 mm.12 Blood flow parameters remained unchanged in all patients during the observation period.
Functional Outcome
Functional outcomes varied depending on the level of amputation/transplantation, time after surgery, and participation in hand therapy. Patient 1: at 1 year, the patient had extrinsic flexion and extension of all digits, some intrinsic hand muscle reinnervation, a grip strength of 39 pounds, discriminatory sensation (Semmes-Weinstein score 4.16), warm/cold sensation, and satisfactory manual dexterity. Patient 2: at 1 year, the patient had warm/cold sensation but inconsistent protective sensation and no intrinsic reinnervation. Wrist and digit flexion/extension was active but weak. Patient 3: at 1 year, he had near normal flexion/extension and strength grade 4/5 in the transplanted elbow. Right-wrist flexion and extension was grade 3+/5, with active digital flexion/extension. The Tinel sign had progressed to 17 cm distal of the skin junction of the arm allograft. The left-hand allograft had intrinsic muscle reinnervation with extrinsic active flexion and extension strengths 3 to 4/5, grip strength 43 pounds, and warm/cold and discriminatory (Semmes-Weinstein score 3.61) sensation in all digits. Patient 4: at 4 months, she had a sensate thumb, index and long fingers, and more proximal ulnar digits. Her grip strength was 20 to 30 pounds, with wrist extension. Patient 5: at 4 months, warm/cold sensation in the palms and active wrist and digit flexion/extension were present.
DISCUSSION
In this study, the combination of pretransplant lymphoid depletion and a delayed posttransplant donor BM cell infusion resulted in graft survival using tacrolimus monotherapy in 5 hand/forearm transplant recipients. Functional outcomes were satisfactory and comparable to the world experience with hand transplantation.1 The early functional recovery after above-elbow transplantation is significantly better than anticipated.
The overall amount of maintenance immunosuppression was much less when compared with historical controls.1 Episodes of skin rejection were few and responsive to topical therapy alone or short courses of steroids. Adverse effects of immunosuppression were overall mild and transient. The limited number of recipients in this (patient’s) self-control trial, however, did not allow for statistical analysis. However, when compared with historical controls, the overall amount of immunosuppression after induction was limited to a single drug at the same or lower concentrations versus a triple-drug combination consisting of tacrolimus, mycophenolate mofetil, and steroids in the vast majority of previous cases.1,13
The BM cell infusion given 2 weeks after transplantation was well tolerated without side effects and did not result in graft-versus-host disease or sustained peripheral blood chimerism. This is in agreement with previous reports showing only transient chimerism after face transplantation or chimerism found in the BM compartment rather than the blood.14,15 Flow cytometry and carboxyfluorescein diacetate succinimidyl ester-MLR analyses identified a status previously shown to correspond with biopsy-proven acute rejection in kidney transplantation (patient 1, at a time when he had a skin rejection),16 a significant loss in circulating T regulatory cells previously identified to correspond to donor reactive kidney transplant patients in patient 2,16,17 and a robust T-cell proliferation to third party, but lower proliferation to donor resembling a quiescent status of relative donor-specific nonreactivity in patient 3.16,17
Alemtuzumab induction resulted in rapid pan-lymphocyte depletion and in contrast to solid organ transplantation, recovery of lymphocytes occurred early after transplantation but did not result in irreversible rejection.18 Calcineurin inhibitor dose reduction is further believed to prevent vasculopathy, interstitial fibrosis, and glomerulosclerosis, as described in serial kidney transplant biopsies.19
We found that the protocol employed in this patient self-control trial was safe and resulted in graft survival using little maintenance immunosuppression when compared with other hand transplant protocols and a small number of patients from another center where an attempt to reduce immunosuppression without the use of BM was attempted.20 The findings in this study indicate that (i) the treatment regimen had limited side effects and (ii) graft rejection was prevented despite reduction of maintenance immunosuppression to tacrolimus monotherapy. The causal relation between the BM cell infusion and reduction of immunosuppression or immunomodulation remains to be investigated.
A series of renal transplant studies using tacrolimus monotherapy without BM infusion have shown promising and favorable results in both adult and pediatric patients.21–26 In addition, the tacrolimus monotherapy protocols following alemtuzumab induction have been considered as simpler and more cost-effective regimen as compared with standard triple-drug therapy. In the longest published follow-up currently available, ranging between 3 and 5 years, studies demonstrated that the incidence of early acute rejection, patient and graft survivals as well as the incidence of infectious complications are similar or slightly lower as compared with triple-drug immunosuppression control groups.21,27 However, the long-term benefits of alemtuzumab pretreatment with tacrolimus monotherapy in particular with regard to incidence of chronic changes (chronic allograft nephropathy, delayed acute cellular rejection) or the incidence of malignancies (posttransplant lymphoproliferative disorder) clearly warrant further investigation. Furthermore, most recently, in patients with preformed donor-specific alloantibodies, a high risk of antibody-mediated rejection, impaired graft function, and graft loss were observed with tacrolimus monotherapy after alemtuzumab induction, indicating that such patients require augmented immunosuppression.28
However, in few and selected cases of solid organ transplantation, the additional use of donor BM infusion in different protocols has indicated the ability of such therapies to even further reduce maintenance immunosuppression or to induce tolerance and served as the rationale for the protocol used in this study.4,11,29–32 Also, evidence from small and large animal studies points to a beneficial effect of vascularized allogenic BM and BM induction regimens on graft survival and tolerance induction in composite tissue allotransplantation.33,34
Based on our preclinical trial, the patients in this study had received a lymphocyte-depleting induction therapy with alemtuzumab but no further conditioning prior to BM cell transfusion. Nonmyeloablative conditioning has recently been successfully introduced into solid organ transplantation and such a protocol may well be advantageous also in hand transplantation. As the safety of BM cell infusion in upper-extremity transplantation has now been illustrated, a conditioning protocol in hand or face transplantation could be considered.
Unmodified BM cell infusions without or with nonmyeloablative preconditioning6,7,31,32 have been previously used in solid organ transplant trials and a large animal limb transplant model. Based on the findings from these trials, we considered it safe to use unmodified BM cells in this study. Although we have not observed graft-versus-host disease in any of the patients in this study, strategies to prevent graft-versus-host disease should be revisited prior to expanding on this trial.
Skin-bearing composite tissue allotransplantations such as hand or face transplants allow for serial visual monitoring of rejection, directed biopsy, and targeted treatment with topical immunosuppression. This fundamental disparity from solid organs was essential for continuous and individual adjustment of tacrolimus dosage and indicates an intrinsic advantage of composite tissue allotransplantations for immunosuppression minimization and tolerance trials.
Donor-specific alloantibodies were found in conjunction with some but not all skin rejections, indicating that the cellular immune response is paralleled by antibody formation. High-resolution ultrasound allows for noninvasive, sensitive monitoring of the vasculature in upper-extremity transplantation. Intima-media thickness was within normal range in all except 1 patient (patient 4), where a slight increase was found. Although the visual impression of the vessel wall did not suggest major alterations and angiography indicated regular vasculature without narrowing or stenosis, close monitoring of all patients is required to rule out the onset or progression of vascular lesions.
Transient nonadherence with immunosuppression has been previously reported in hand transplantation.35 Prior to wait-listing, all patients had undergone thorough psychological and psychosocial investigation and passed all screening tests. Although no elements in the psychosocial constitution of our patients could have possibly predicting noncompliance, this remains a major challenge for reconstructive transplantation, especially in young patients. A positive correlation between younger age and noncompliance has been described in solid organ transplantation.36 This problem may be accentuated in hand transplantation because the ability to self-monitor the graft may falsely suggest the ability to self-treat.
In summary, hand/arm transplantation was performed successfully in 5 patients with tacrolimus monotherapy for maintenance using a novel treatment regimen including a donor BM cell infusion. Larger and/or randomized controlled trials with long-term follow-up need to be undertaken to confirm these findings.
Acknowledgments
This work was funded in part by the Armed Forces Institute for Regenerative Medicine (AFIRM) W81XWH-08-2-0032, Orthopedic Extremity Trauma Research Program (OETRP) W81XWH-08-1-0421, and the University of Pittsburgh Medical Center.
Daniel Earl Foust (Transplant Coordination), Kimberly Maguire, Marie Pace, and other rehabilitation specialists, Noriko Murase, MD (Thomas E. Starzl Transplantation Institute, Pittsburgh, PA), Susan Stewart and Kurt Shutterly (Center for Organ Recovery and Education, Pittsburgh, PA), Linda Moore (Hematopoietic Stem Cell Laboratory, Cambridge, United Kingdom), Kymberly Hambleton (Clinical Transplant Social Worker), and all members of surgical, anesthesia, and nursing teams. We also thank John McMichael for transplant database support.
Footnotes
Disclosure: The authors declare no conflicts of interest.
This work was presented in part at the American Transplant Congress 2011, Philadelphia, PA, April 30 to May 4, 2011.
ClinicalTrials.gov. Registration Number: NCT00722280.
References
- 1.Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2010;90:1590–1594. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE. Acquired immunologic tolerance: with particular reference to transplantation. Immunol Res. 2007;38:6–41. doi: 10.1007/s12026-007-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontes P, Rao AS, Demetris AJ, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciancio G, Miller J, Garcia-Morales RO, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001;71:827–835. doi: 10.1097/00007890-200104150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514–521. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 9.Gorantla VS, Schneeberger S, Moore LR, et al. Development and validation of a procedure to isolate viable bone marrow cells from the vertebrae of cadaveric organ donors for composite organ grafting. Cytotherapy. 2012;1:104–113. doi: 10.3109/14653249.2011.605350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg AD, Gorantla VS, Schneeberger S, et al. Clinical implementation of a procedure to prepare bone marrow cells from cadaveric vertebral bodies. Regen Med. 2011;6:701–706. doi: 10.2217/rme.11.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 12.Myredal A, Gan LM, Osika W, et al. Increased intima thickness of the radial artery in individuals with prehypertension and hypertension. Atherosclerosis. 2010;209:147–151. doi: 10.1016/j.atherosclerosis.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Dubernard JM, Lengelé B, Morelon E, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. 2007;357:2451–2460. doi: 10.1056/NEJMoa072828. [DOI] [PubMed] [Google Scholar]
- 14.Delis S, Ciancio G, Burke GW, III, et al. Donor bone marrow transplantation: chimerism and tolerance. Transpl Immunol. 2004;13:105–115. doi: 10.1016/j.trim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Macedo C, Orkis E, Elinoff B, et al. Long-term effect of alemtuzumab induction on T cell memory and T regulatory cell (Treg) subsets in kidney transplant recipients. Am J Transplant. 2010;10:489. [Google Scholar]
- 16.Macedo C, Orkis EA, Popescu I, et al. Contribution of naïve and memory T cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 17.Trzonkowski P, Zilvetti M, Chapman S, et al. Homeostatic repopulation by CD28-CD8 +T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8:338–347. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 18.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 19.Barber WH, Mankin JA, Laskow DA, et al. Long-term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman CL, Breidenbach W. World experience after more than a decade of clinical hand transplantation: update from the Louisville hand transplant program. Hand Clin. 2011;4:417–421. doi: 10.1016/j.hcl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Tan HP, Donaldson J, Basu A, et al. Two hundred living donor kidney transplantations under alemtuzumab induction and tacrolimus monotherapy: 3-year follow-up. Am J Transplant. 2009;2:355–366. doi: 10.1111/j.1600-6143.2008.02492.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas PG, Woodside KJ, Lappin JA, et al. Alemtuzumab (campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk renal transplantation. Transplantation. 2007;11:1509–1512. doi: 10.1097/01.tp.0000263344.53000.a1. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro R, Ellis D, Tan HP, et al. Alemtuzumab pre-conditioning with tacrolimus monotherapy in pediatric renal transplantation. Am J Transplant. 2007;12:2736–2738. doi: 10.1111/j.1600-6143.2007.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margreiter R, Klempnauer J, Neuhaus P, et al. Alemtuzumab (campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. Am J Transplant. 2008;7:1480–1485. doi: 10.1111/j.1600-6143.2008.02273.x. [DOI] [PubMed] [Google Scholar]
- 25.Chan K, Taube D, Roufosse C, et al. Kidney transplantation with minimized maintenance: alemtuzumab induction with tacrolimus monotherapy—an open label, randomized trial. Transplantation. 2011;7:774–780. doi: 10.1097/TP.0b013e31822ca7ca. [DOI] [PubMed] [Google Scholar]
- 26.Tan HP, Donaldson J, Ellis D, et al. Pediatric living donor kidney transplantation under alemtuzumab pretreatment and tacrolimus monotherapy: 4-year experience. Transplantation. 2008;12:1725–1731. doi: 10.1097/TP.0b013e3181903da7. [DOI] [PubMed] [Google Scholar]
- 27.Watson CJ, Bradley JA, Friend PJ, et al. Alemtuzumab (campath 1H) induction therapy in cadaveric kidney transplantation—efficacy and safety at five years. Am J Transplant. 2005;6:1347–1353. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 28.Willicombe M, Brookes P, Santos-Nunez E, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011;3:470–477. doi: 10.1111/j.1600-6143.2010.03421.x. [DOI] [PubMed] [Google Scholar]
- 29.Corry RJ, Chakrabarti PK, Shapiro R, et al. Simultaneous administration of adjuvant donor bone marrow in pancreas transplant recipients. Ann Surg. 1999;230:372–379. doi: 10.1097/00000658-199909000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham SM, Rao AS, Zeevi A, et al. A clinical trial combining donor bone marrow infusion and heart transplantation: intermediate-term results. J Thorac Cardiovasc Surg. 2000;119:673–681. doi: 10.1016/S0022-5223(00)70001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siemionow M, Klimczak A, Unal S, et al. Hematopoietic stem cell engraftment and seeding permits multi-lymphoid chimerism in vascularized bone marrow transplants. Am J Transplant. 2008;8:1163–1176. doi: 10.1111/j.1600-6143.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- 34.Arslan E, Klimczak A, Siemionow M. Chimerism induction in vascularized bone marrow transplants augmented with bone marrow cells. Microsurgery. 2007;27:190–199. doi: 10.1002/micr.20330. [DOI] [PubMed] [Google Scholar]
- 35.Schneeberger S, Gorantla VS, van Riet RP, et al. Atypical acute rejection after hand transplantation. Am J Transplant. 2008;8:688–696. doi: 10.1111/j.1600-6143.2007.02105.x. [DOI] [PubMed] [Google Scholar]
- 36.Gremigni P, Bacchi F, Turrini C, et al. Psychological factors associated with medication adherence following renal transplantation. Clin Transplant. 2007;6:710–715. doi: 10.1111/j.1399-0012.2007.00727.x. [DOI] [PubMed] [Google Scholar]