Abstract
Purpose
Combining cisplatin or cetuximab with radiation improves overall survival (OS) of patients with stage III or IV head and neck carcinoma (HNC). Cetuximab plus platinum regimens also increase OS in metastatic HNC. The Radiation Therapy Oncology Group launched a phase III trial to test the hypothesis that adding cetuximab to the radiation-cisplatin platform improves progression-free survival (PFS).
Patients and Methods
Eligible patients with stage III or IV HNC were randomly assigned to receive radiation and cisplatin without (arm A) or with (arm B) cetuximab. Acute and late reactions were scored using Common Terminology Criteria for Adverse Events (version 3). Outcomes were correlated with patient and tumor features and markers.
Results
Of 891 analyzed patients, 630 were alive at analysis (median follow-up, 3.8 years). Cetuximab plus cisplatin-radiation, versus cisplatin-radiation alone, resulted in more frequent interruptions in radiation therapy (26.9% v 15.1%, respectively); similar cisplatin delivery (mean, 185.7 mg/m2 v 191.1 mg/m2, respectively); and more grade 3 to 4 radiation mucositis (43.2% v 33.3%, respectively), rash, fatigue, anorexia, and hypokalemia, but not more late toxicity. No differences were found between arms A and B in 30-day mortality (1.8% v 2.0%, respectively; P = .81), 3-year PFS (61.2% v 58.9%, respectively; P = .76), 3-year OS (72.9% v 75.8%, respectively; P = .32), locoregional failure (19.9% v 25.9%, respectively; P = .97), or distant metastasis (13.0% v 9.7%, respectively; P = .08). Patients with p16-positive oropharyngeal carcinoma (OPC), compared with patients with p16-negative OPC, had better 3-year probability of PFS (72.8% v 49.2%, respectively; P < .001) and OS (85.6% v 60.1%, respectively; P < .001), but tumor epidermal growth factor receptor (EGFR) expression did not distinguish outcome.
Conclusion
Adding cetuximab to radiation-cisplatin did not improve outcome and hence should not be prescribed routinely. PFS and OS were higher in patients with p16-positive OPC, but outcomes did not differ by EGFR expression.
INTRODUCTION
Treatment of patients with locally advanced head and neck carcinomas (HNCs) remains a challenge. A thorough meta-analysis of randomized trials1 showed that adding cisplatin concurrently to radiotherapy improved progression-free survival (PFS), overall survival (OS), and organ preservation, but only approximately 50% of patients survived more than 5 years. Moreover, radiation-cisplatin regimens induce severe acute and late morbidity.2 These observations inspired the search for alternative therapy approaches.
Available data showed that most HNCs express high levels of epidermal growth factor receptor (EGFR),3–5 that high EGFR expression was associated with poor response to radiation4 or chemoradiotherapy,5 and that EGFR inhibitors sensitized tumors to cisplatin6 or radiation.7–9 A pivotal trial of the anti-EGFR antibody cetuximab and radiation therapy demonstrated that administering eight weekly doses of cetuximab concurrently with radiotherapy to patients with previously untreated locally advanced HNC significantly improved the median survival time and rates of locoregional control (LRC) and OS without increasing radiation-associated acute toxicity.10 Furthermore, in patients with metastatic disease, adding cetuximab to cisplatin increased the response rate.11 Another ongoing trial addressed the combination of cetuximab and platinum-based therapy, ultimately with positive results.12 Because cetuximab enhances HNC response to both radiation and cisplatin, it was hypothesized that adding cetuximab to the radiation-cisplatin platform would improve PFS of patients with locally advanced HNC. Although a phase II trial of a radiation-cisplatin-cetuximab triplet was closed early because of two deaths, one myocardial infarction, one case of bacteremia, and one case of atrial fibrillation,13 longer follow-up data revealed encouraging rates of 3-year OS and LRC. Therefore, Radiation Therapy Oncology Group (RTOG) investigators launched a phase III trial (RTOG 0522), with close monitoring, to examine the efficacy of this triplet. This article presents the overall outcome and results of planned correlative studies.
PATIENTS AND METHODS
Protocol and Treatment
Eligible patients had untreated, histologically confirmed, stage III or IV (T2N2-3M0 or T3-4, any N, M0) squamous cell carcinoma of the oropharynx, hypopharynx, or larynx; Zubrod performance status 0 to 1; age ≥ 18 years; any tobacco status; and adequate bone marrow, hepatic, and renal functions. Lifetime tobacco exposure was determined at enrollment using a standardized questionnaire.
Patients were stratified by tumor site (larynx v other), nodal stage (N0 v N1-N2b v N2c-N3), Zubrod performance status (0 v 1), use of intensity-modulated radiotherapy (IMRT; yes v no), and receipt of pretreatment fused positron emission tomography/computed tomography scan (yes v no), and were randomly assigned to radiotherapy with concurrent cisplatin without (arm A) or with cetuximab (arm B) in a 1:1 ratio using permuted block random assignment.14 Accelerated radiotherapy regimens included 72 Gy in 42 fractions given over 6 weeks, using twice-a-day irradiation for 12 treatment days as previously reported.15 When IMRT was used, a different accelerated schedule of twice-a-day dosing once a week for 5 weeks delivered 70 Gy in 35 fractions (2 Gy per fraction) over 6 weeks per the Danish Head and Neck Cancer Group (DAHANCA) 6 and 7 studies, which showed improved LRC and disease-specific survival compared with conventional fractionation.16
Cisplatin dose was 100 mg/m2 on days 1 and 22 of radiotherapy based on projected findings from RTOG 0129, which showed no significant difference between accelerated fractionation plus two cycles of cisplatin and standard fractionation plus three cycles of cisplatin.17 As in a previous trial, the cetuximab dose was 400 mg/m2 the week before radiotherapy and then 250 mg/m2 per week during radiotherapy.10 Toxicity was evaluated weekly during therapy using the Common Terminology Criteria for Adverse Events (version 3). Adverse events reported as definitely, probably, or possibly related were considered treatment-related events. Imaging was performed 8 to 9 weeks after treatment, at 6 months, and then annually, with physical examination every 3 months for 2 years, every 6 months through year 5, and then annually to assess tumor status and toxicity.
RTOG 0522 was registered with the National Cancer Institute (NCT00265941) and approved by the central and institutional review boards of the 151 participating centers. All patients provided written informed consent to participate.
Planned Laboratory Studies
As in the previous trial,17 immunohistochemical assays were used to assess p16 expression in specimens from oropharyngeal carcinomas (OPCs) by using a mouse monoclonal antibody (MTM Laboratories, Heidelberg, Germany) visualized with the Ventana XT autostainer using Ventana's one-view secondary detection kit (Ventana, Tucson, AZ). Stains were scored as positive when strong, diffuse nuclear and cytoplasmic staining was present in 70% of tumor cells.18
Specimens from OPCs and other primary tumors were available for EGFR immunohistochemical assay. Individual sections were deparaffinized in xylene, rehydrated with a serial alcohol gradient, and incubated in 3% hydrogen peroxide to block endogenous peroxidase. Antigen was retrieved by placing sections in 0.1 M of citrate buffer, steaming for 25 minutes, and incubating with anti-EGFR antibody (clone 31G7; Invitrogen, Grand Island, NY) diluted to 1:50 and a secondary conjugate antibody (EnVision polymer; DAKO, Carpinteria, CA) in buffer. Slides were developed with a 3,3′-diaminobenzidine chromogen kit and counterstained with hematoxylin. On the basis of the cut point defined from prior validation,19 EGFR expression was scored in the following two ways: as less than 80% or 80% of tumor cells staining positive for EGFR and by a semiquantitative method (0, +, ++, or +++).
Statistical Analysis
All time-to-event end points were measured from random assignment to date of event or censoring. Patients were grouped by intent-to-treat analysis. PFS failure, the primary end point, was defined as locoregional failure (LRF)/progression, distant metastasis (DM), or death. We further analyzed LRF (including neck dissection > 15 weeks after radiotherapy or salvage surgery for the primary site unless pathology showed no disease, and death as a result of cancer or unknown causes without a documented failure site) and DM as site of first failure. Other end points for this report included OS, adverse effects, compliance with protocol-defined treatment delivery, and p16 and EGFR expression. Quality-of-life end points and correlation of positron emission tomography findings with outcomes are being reported separately.
RTOG 0522 was initially designed with a sample size of 720 patients to detect a 25% reduction in the hazard associated with disease-free survival with 80% power and a one-sided test at the P = .025 level. The primary end point was changed to PFS in 2008 to allow comparisons with the end point in the international meta-analysis of event-free survival, which has been shown to be a surrogate for OS.20 In addition, because the control group had better-than-expected disease-free survival/PFS, the sample size was increased to 945 patients to allow detection of a 25% reduction in the risk of PFS failure with 84% statistical power and a one-sided final test at the P = .0238 significance level, after three interim analyses and a planned final analysis at 434 treatment failures. When the third planned interim analysis yielded a conditional power of less than 10%, the data monitoring committee recommended early reporting of results with 371 failure events. A planned subset analysis focused on treatment effect by p16 subgroups.
PFS and OS probabilities were estimated using the Kaplan-Meier method,21 and LRF and DM probabilities were estimated using the cumulative incidence method.22 PFS and OS were compared using log-rank tests,23 and LRF and DM were compared using failure-specific log-rank tests.24 Toxicity rates were compared using Fisher's exact tests, and hazard ratios (HRs) were estimated using Cox proportional hazards models.25 Outcomes were compared for patients with p16-positive versus p16-negative OPCs and for patients whose tumors had 80% versus less than 80% tumor cells staining positive for EGFR. Missing p16, EGFR, and pack-year values were imputed 20 times using conditional model specification for multivariable imputation via Gibbs sampling,26 the resulting data sets were combined using Rubin's formula, and sensitivity analyses were conducted to validate the robustness of the imputation procedure.
Study design, implementation, data collection, analysis, interpretation, and article preparation were performed by the authors as representatives of the RTOG Head and Neck Committee and the RTOG Statistical Center. The authors had complete access to all data. The first author (K.K.A., trial chair) and last author (R.S.A.) serve as guarantors of all analyses and article content.
RESULTS
Patient Characteristics and Treatment Parameters
Patients were accrued from November 2005 through May 2009. Of the 940 patients enrolled, 891 were analyzed (47 patients were excluded for not meeting inclusion criteria and two were excluded for lack of post–random assignment data; Fig 1). Table 1 lists demographic and baseline features of the 891 patients assigned to receive radiation-cisplatin alone (arm A; n = 447) or radiation-cisplatin plus cetuximab (arm B; n = 444) along with p16 and EGFR assay results. Briefly, 786 patients (88.2%) were men, 767 patients (86.1%) had stage IV disease, 625 patients (70.1%) had OPC, 258 patients (29.0%) had ≥ 5% weight loss during the preceding 6 months, and 121 patients (13.6%) had a feeding tube placed before treatment. Of the 321 OPCs assayed, 235 (73.2%) were positive for p16. Of the 380 tumors (261 OPCs and 119 other primary tumors) analyzed for EGFR expression, 145 (38.2%) had positive staining in ≥ 80% of tumor cells. Baseline characteristics and outcomes were compared between patients with and without known p16 status, EGFR status, and pack-years of smoking. Significant differences were present in race, T category, and OS between patients with and without known pack-years. However, sensitivity analysis for data missing not at random,27,28 in which the missing pack-years value was reimputed with mean pack-years varying from +20 pack-years to −20 pack-years for the nonresponders, showed consistent model estimates for treatment effects and other covariates.
Fig 1.
CONSORT diagram. RT, radiotherapy.
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Characteristic | All Eligible Patients |
Patients With Oropharyngeal Cancer and Tumor Specimens for p16 Assay |
Patients With Tumor Specimens for EGFR Assay |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm A: RT + Cisplatin (n = 447) |
Arm B: RT + Cisplatin + Cetuximab (n = 444) |
p16 Positive (n = 235) |
p16 Negative (n = 86) |
P | EGFR < 80%* (n = 235) |
EGFR ≥ 80%* (n = 145) |
P | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Treatment assigned | .46† | .98† | ||||||||||||
| RT + cisplatin | 447 | 100.0 | 0 | 0 | 112 | 47.7 | 45 | 52.3 | 117 | 49.8 | 72 | 49.7 | ||
| RT + cisplatin + cetuximab | 0 | 0 | 444 | 100.0 | 123 | 52.3 | 41 | 47.7 | 118 | 50.2 | 73 | 50.3 | ||
| Age, years | .14‡ | .69‡ | ||||||||||||
| Median | 57 | 58 | 56 | 58 | 57 | 58 | ||||||||
| Range | 31-79 | 34-76 | 36-76 | 40-75 | 36-79 | 31-79 | ||||||||
| Sex | .009† | .92† | ||||||||||||
| Male | 387 | 86.6 | 399 | 89.9 | 221 | 94.0 | 73 | 84.9 | 205 | 87.2 | 127 | 87.6 | ||
| Female | 60 | 13.4 | 45 | 10.1 | 14 | 6.0 | 13 | 15.1 | 30 | 12.8 | 18 | 12.4 | ||
| Race | .001§ | .33§ | ||||||||||||
| White | 411 | 91.9 | 399 | 89.9 | 223 | 94.9 | 74 | 86.0 | 211 | 89.8 | 129 | 89.0 | ||
| Nonwhite | 33 | 7.4 | 39 | 8.8 | 9 | 3.8 | 12 | 14.0 | 22 | 9.4 | 12 | 8.3 | ||
| Unknown | 3 | 0.7 | 6 | 1.4 | 3 | 1.3 | 0 | 0 | 2 | 0.9 | 4 | 2.8 | ||
| Zubrod performance status | .02† | .01† | ||||||||||||
| 0 | 292 | 65.3 | 295 | 66.4 | 174 | 74.0 | 52 | 60.5 | 162 | 68.9 | 81 | 55.9 | ||
| 1 | 155 | 34.7 | 149 | 33.6 | 61 | 26.0 | 34 | 39.5 | 73 | 31.1 | 64 | 44.1 | ||
| Weight loss in last 6 months | .01§ | .37§ | ||||||||||||
| < 5% of body weight | 290 | 64.9 | 290 | 65.3 | 173 | 73.6 | 51 | 59.3 | 166 | 70.6 | 93 | 64.1 | ||
| ≥ 5% of body weight | 130 | 29.1 | 128 | 28.8 | 50 | 21.3 | 30 | 34.9 | 59 | 25.1 | 41 | 28.3 | ||
| Unknown | 27 | 6.0 | 26 | 5.9 | 12 | 5.1 | 5 | 5.8 | 10 | 4.3 | 11 | 7.6 | ||
| Feeding tube use | .13§ | .80§ | ||||||||||||
| No | 380 | 85.0 | 388 | 87.4 | 209 | 88.9 | 71 | 82.6 | 202 | 86.0 | 126 | 86.9 | ||
| Yes | 66 | 14.8 | 55 | 12.4 | 26 | 11.1 | 15 | 17.4 | 33 | 14.0 | 19 | 13.1 | ||
| Unknown | 1 | 0.2 | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Anemia‖ | .009† | .46† | ||||||||||||
| No | 317 | 70.9 | 308 | 69.4 | 177 | 75.3 | 52 | 60.5 | 161 | 68.5 | 94 | 64.8 | ||
| Yes | 130 | 29.1 | 136 | 30.6 | 58 | 24.7 | 34 | 39.5 | 74 | 31.5 | 51 | 35.2 | ||
| Primary tumor site | < .001† | |||||||||||||
| Oropharynx | 313 | 70.0 | 312 | 70.3 | 235 | 100.0 | 86 | 100.0 | 180 | 76.6 | 81 | 55.9 | ||
| Hypopharynx | 33 | 7.4 | 29 | 6.5 | 0 | 0 | 0 | 0 | 12 | 5.1 | 14 | 9.7 | ||
| Larynx | 101 | 22.6 | 103 | 23.2 | 0 | 0 | 0 | 0 | 43 | 18.3 | 50 | 34.5 | ||
| T category | .004‡ | .13‡ | ||||||||||||
| T2 | 174 | 38.9 | 177 | 39.9 | 117 | 49.8 | 29 | 33.7 | 100 | 42.6 | 47 | 32.4 | ||
| T3 | 169 | 37.8 | 160 | 36.0 | 64 | 27.2 | 25 | 29.1 | 79 | 33.6 | 61 | 42.1 | ||
| T4 | 104 | 23.3 | 107 | 24.1 | 54 | 23.0 | 32 | 37.2 | 56 | 23.8 | 37 | 25.5 | ||
| N category | .76‡ | .71‡ | ||||||||||||
| N0 | 45 | 10.1 | 54 | 12.2 | 12 | 5.1 | 6 | 7.0 | 24 | 10.2 | 23 | 15.9 | ||
| N1 | 41 | 9.2 | 40 | 9.0 | 15 | 6.4 | 9 | 10.5 | 18 | 7.7 | 17 | 11.7 | ||
| N2a | 36 | 8.1 | 42 | 9.5 | 30 | 12.8 | 5 | 5.8 | 30 | 12.8 | 11 | 7.6 | ||
| N2b | 139 | 31.1 | 154 | 34.7 | 94 | 40.0 | 32 | 37.2 | 78 | 33.2 | 39 | 26.9 | ||
| N2c | 159 | 35.6 | 137 | 30.9 | 65 | 27.7 | 26 | 30.2 | 72 | 30.6 | 40 | 27.6 | ||
| N3 | 27 | 6.0 | 17 | 3.8 | 19 | 8.1 | 8 | 9.3 | 13 | 5.5 | 15 | 10.3 | ||
| AJCC stage¶ | .92‡ | .13‡ | ||||||||||||
| III | 59 | 13.2 | 65 | 14.6 | 13 | 5.5 | 5 | 5.8 | 29 | 12.3 | 26 | 17.9 | ||
| IV | 388 | 86.8 | 379 | 85.4 | 222 | 94.5 | 81 | 94.2 | 206 | 87.7 | 119 | 82.1 | ||
| Pretreatment PET/CT | .22† | .08† | ||||||||||||
| No | 159 | 35.6 | 156 | 35.1 | 73 | 31.1 | 33 | 38.4 | 85 | 36.2 | 40 | 27.6 | ||
| Yes | 288 | 64.4 | 288 | 64.9 | 162 | 68.9 | 53 | 61.6 | 150 | 63.8 | 105 | 72.4 | ||
| Tobacco-smoking history, pack-years# | < .001‡ | .74‡ | ||||||||||||
| Sample size | 390 | 380 | 215 | 72 | 214 | 124 | ||||||||
| Median | 22.5 | 20.7 | 5.25 | 29 | 18 | 20 | ||||||||
| Range | 0-150 | 0-162 | 0-150 | 0-104 | 0-150 | 0-162 | ||||||||
| p16 expression in oropharyngeal primary tumor | .06† | |||||||||||||
| Positive | 112 | 35.8 | 123 | 39.4 | 235 | 100.0 | 0 | 0 | 138 | 76.7 | 52 | 64.2 | ||
| Negative | 45 | 14.4 | 41 | 13.1 | 0 | 0 | 86 | 100.0 | 34 | 18.9 | 26 | 32.1 | ||
| Unknown | 156 | 49.8 | 148 | 47.4 | 0 | 0 | 0 | 0 | 8 | 4.4 | 3 | 3.7 | ||
| EGFR immunostaining | .009† | |||||||||||||
| < 80% of tumor cells positive | 117 | 26.2 | 118 | 26.6 | 138 | 58.7 | 34 | 39.5 | 235 | 100.0 | 0 | 0 | ||
| ≥ 80% of tumor cells positive | 72 | 16.1 | 73 | 16.4 | 52 | 22.1 | 26 | 30.2 | 0 | 0 | 145 | 100.0 | ||
| Unknown | 258 | 57.7 | 253 | 57.0 | 45 | 19.1 | 26 | 30.2 | 0 | 0 | 0 | 0 | ||
Abbreviations: AJCC, American Joint Committee on Cancer; CT, computed tomography; EGFR, epidermal growth factor receptor; PET, positron emission tomography; RT, radiotherapy.
Percentage of cells staining positive for EGFR.
Pearson χ2 test.
Wilcoxon rank sum test.
Pearson χ2 test excluding unknowns.
Anemia is defined as a hemoglobin level of ≤ 13.5 g/dL for men and ≤ 12.5 g/dL for women.
AJCC sixth edition staging system.
A pack-year is defined as the equivalent of smoking one pack of cigarettes a day for 1 year.
Appendix Table A1 (online only) lists the details of treatment delivered. Overall, IMRT was used in 86.4% and 86.7% of patients in arms A and B, respectively. In arms A and B, 97.5% and 94.8% of patients received at least 60 Gy, and 93.7% and 90.5% of patients received two cisplatin cycles, respectively. In arm B, 432 patients (97.3%) received the loading cetuximab, but only 327 patients (73.6%) received six or more weekly cetuximab doses as specified. The incidence of interruption of radiotherapy as a result of toxicity was significantly higher in arm B (26.9% v 15.1% in arm A; P = .001).
Toxicity End Points
More treatment-related grade 5 adverse events took place in the cetuximab arm (10 events in arm B v three events in arm A; P = .05). However, death rates within 30 days of treatment completion were similar between the two arms (2.0% with cetuximab v 1.8% without; P = .81). Table 2 lists the distribution of worst grade adverse effects. The cetuximab arm had significantly higher rates of grade 3 to 4 skin reactions (both inside and outside radiation volumes), radiation mucositis, fatigue, anorexia, and hypokalemia up to 90 days from the start of therapy. However, no significant differences were observed between the arms in rates of adverse effects after 90 days. In arms A and B, rates of feeding tube dependency were 21.2% (95% CI, 17.2% to 25.7%) and 18.8% (95% CI, 15.0% to 23.2%) at 1 year (P = .47), 13.5% (95% CI, 10.0% to 17.8%) and 11.9% (95% CI, 8.6% to 15.9%) at 2 years (P = .56), and 12.1% (95% CI, 8.4% to 16.8%) and 7.0% (95% CI, 4.2% to 10.8%) at 3 years (P = .05), respectively.
Table 2.
Treatment-Related Adverse Events by Assigned Treatment
| Adverse Event* | % of Patients |
P† |
||||
|---|---|---|---|---|---|---|
| Arm A: RT + Cisplatin |
Arm B: RT + Cisplatin + Cetuximab |
|||||
| All Grades | Grades 3-4 | All Grades | Grades 3-4 | All Grades | Grades 3-4 | |
| Acute period‡ | ||||||
| No. of patients | 447 | 444 | ||||
| Any event | 97 | 87 | 97 | 89 | .70 | .61 |
| Dysphagia | 86 | 57 | 82 | 53 | .08 | .25 |
| Radiation mucositis | 72 | 33 | 82 | 43 | < .001 | .002 |
| Skin reaction outside portal§ | 14 | 1 | 82 | 20 | < .001 | < .001 |
| Skin reaction inside portal‖ | 79 | 15 | 78 | 25 | .87 | < .001 |
| Fatigue | 60 | 9 | 65 | 14 | .17 | .03 |
| Nausea | 57 | 14 | 59 | 18 | .59 | .08 |
| Hemoglobin | 53 | 4 | 51 | 6 | .55 | .30 |
| Weight decreased | 50 | 2 | 52 | 2 | .74 | .80 |
| Leukopenia NOS | 49 | 19 | 50 | 19 | .79 | .80 |
| Mucositis/stomatitis (clinical exam): pharynx | 49 | 24 | 43 | 28 | .11 | .29 |
| Vomiting NOS | 38 | 9 | 42 | 10 | .17 | .56 |
| Hyponatremia | 34 | 10 | 42 | 13 | .01 | .29 |
| Dysgeusia | 39 | 0 | 36 | 0 | .33 | — |
| Dehydration | 36 | 15 | 37 | 18 | .63 | .24 |
| Dry mouth | 35 | 6 | 37 | 7 | .53 | .28 |
| Hypomagnesemia | 21 | 2 | 36 | 3 | < .001 | .26 |
| Neutrophil count | 33 | 16 | 33 | 17 | .89 | .65 |
| Pharyngolaryngeal pain | 32 | 8 | 26 | 7 | .05 | .70 |
| Anorexia | 32 | 11 | 32 | 16 | .89 | .04 |
| Salivary gland disorder NOS | 31 | 2 | 27 | 4 | .24 | .07 |
| Hypoalbuminemia | 25 | 1 | 30 | 2 | .11 | .09 |
| Oral pain | 24 | 7 | 28 | 10 | .17 | .19 |
| Hypocalcemia | 16 | 1 | 26 | 3 | < .001 | .09 |
| Hyperglycemia NOS | 23 | 3 | 25 | 3 | .48 | .84 |
| Hypokalemia | 18 | 5 | 25 | 10 | .007 | .005 |
| Constipation | 24 | 1 | 24 | 1 | .94 | .75 |
| Blood creatinine increased | 24 | 2 | 17 | 2 | .02 | 1.00 |
| Platelet count decreased | 21 | 2 | 22 | 2 | .74 | 1.00 |
| Lymphopenia | 18 | 13 | 18 | 14 | 1.00 | .63 |
| Pyrexia | 11 | 0 | 18 | < 1 | .003 | .50 |
| Laryngitis NOS | 17 | 2 | 16 | 2 | .59 | .64 |
| ALT increased | 14 | 1 | 16 | 2 | .35 | .30 |
| Tinnitus | 16 | 1 | 15 | < 1 | .85 | .12 |
| Diarrhea NOS | 10 | 1 | 16 | 2 | .02 | .58 |
| Mucositis/stomatitis (clinical exam): larynx | 13 | 5 | 13 | 5 | 1.00 | .76 |
| Alopecia | 13 | 0 | 11 | 0 | .40 | — |
| AST increased | 11 | < 1 | 12 | < 1 | .40 | 1.00 |
| Cough | 11 | < 1 | 12 | 1 | .67 | .37 |
| Headache | 4 | 0 | 12 | 1 | < .001 | .12 |
| Laryngeal edema | 11 | 2 | 10 | 1 | .83 | .77 |
| Late period‡ | ||||||
| No. of patients | 432 | 415 | ||||
| Any event | 97 | 54 | 97 | 60 | .85 | .11 |
| Dysphagia | 83 | 36 | 86 | 37 | .16 | .78 |
| Dry mouth | 75 | 4 | 75 | 5 | 1.00 | .40 |
| Skin fibrosis | 44 | 1 | 46 | 2 | .68 | .79 |
| Fatigue | 45 | 3 | 41 | 3 | .27 | .55 |
| Laryngeal edema | 42 | 3 | 40 | 4 | .58 | .85 |
| Dysgeusia | 41 | 0 | 42 | 0 | .94 | — |
| Radiation mucositis | 40 | 6 | 41 | 7 | .78 | .58 |
| Laryngitis NOS | 31 | 2 | 29 | 1 | .50 | .55 |
| Weight decreased | 29 | 7 | 29 | 8 | .94 | .61 |
| Dermatology/skin, other | 27 | 1 | 28 | 2 | .59 | .25 |
| Salivary gland disorder NOS | 28 | 1 | 25 | < 1 | .24 | .62 |
| Hemoglobin | 26 | 1 | 25 | 2 | .69 | .41 |
| Edema: head and neck | 25 | 1 | 26 | 1 | .64 | .73 |
| Pharyngolaryngeal pain | 26 | 2 | 22 | 2 | .30 | .63 |
| Mucositis/stomatitis (clinical exam): pharynx | 23 | 5 | 24 | 7 | .87 | .47 |
| Hearing impaired | 23 | 5 | 23 | 5 | 1.00 | .75 |
| Skin reaction inside portal‖ | 18 | 2 | 22 | 4 | .14 | .06 |
| Peripheral sensory neuropathy | 16 | < 1 | 20 | 1 | .15 | .68 |
| Trismus | 17 | 2 | 20 | 2 | .33 | 1.00 |
| Skin hyperpigmentation | 18 | 0 | 18 | 0 | 1.00 | — |
| Oral pain | 17 | 2 | 17 | 3 | .93 | .41 |
| Skin reaction outside portal§ | 3 | < 1 | 17 | < 1 | < .001 | 1.00 |
| Neuralgia NOS | 17 | 2 | 16 | 3 | .71 | .83 |
| Alopecia | 14 | 0 | 16 | 0 | .44 | — |
| Tinnitus | 15 | 1 | 13 | < 1 | .55 | .62 |
| Hypothyroidism | 14 | < 1 | 13 | 0 | .76 | 1.00 |
| Neck pain | 10 | 1 | 13 | 1 | .13 | 1.00 |
| Nausea | 12 | 3 | 13 | 3 | .60 | 1.00 |
| Anorexia | 13 | 2 | 12 | 3 | .83 | .67 |
| Cough | 10 | < 1 | 10 | 0 | .82 | 1.00 |
| Leukopenia NOS | 10 | 2 | 10 | 2 | .82 | 1.00 |
Abbreviations: NOS, not otherwise specified; RT, radiotherapy.
Definitely, probably, or possibly related to treatment and occurring in at least 10% of patients in either arm.
P values calculated using Fisher's exact test.
The acute and late periods are defined as ≤ 90 and > 90 days from start of radiation therapy, respectively.
Pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS.
Radiation dermatitis NOS; radiation recall syndrome.
Outcome End Points
At the time of analysis (June 2012), 630 patients were alive with a median follow-up time of 3.8 years. No significant differences were found between arms in PFS (primary end point), OS, LRF, or DM (Fig 2). The 3-year PFS probabilities were 61.2% (95% CI, 56.7% to 65.8%) for arm A and 58.9% (95% CI, 54.2% to 63.6%) for arm B (P = .76). The 3-year probabilities for OS were 72.9% (95% CI, 68.7% to 77.1%) for arm A and 75.8% (95% CI, 71.7% to 79.9%) for arm B (P = .32); the 3-year LRF probabilities were 19.9% (95% CI, 16.2% to 23.7%) for arm A and 25.9% (95% CI, 21.7% to 30.1%) for arm B (P = .97); and the 3-year DM probabilities were 13.0% (95% CI, 9.9% to 16.2%) for arm A and 9.7% (95% CI, 6.9% to 12.6%) for arm B (P = .08).
Fig 2.
Kaplan-Meier estimates of (A) progression-free and (B) overall survival and cumulative incidence estimates of (C) locoregional failure and (D) distant metastasis by assigned treatment. HR, hazard ratio; RT, radiotherapy.
Trends were noted toward differential cetuximab treatment effects in patients with OPCs with known p16 status. For PFS, the treatment effect HRs were 1.57 for p16-positive OPC and 0.86 for p16-negative OPC (P for interaction = .12); imputation and adjustment for known prognostic factors reduced these HRs (1.29 v 0.92, respectively; P for interaction = .31). For OS, the corresponding HRs were 1.42 for patients with p16-positive OPC and 0.69 for patients with p16-negative OPC (P for interaction = .13); after imputation and adjustment for covariates, the HRs were 1.10 and 0.63, respectively (P for interaction = .19).
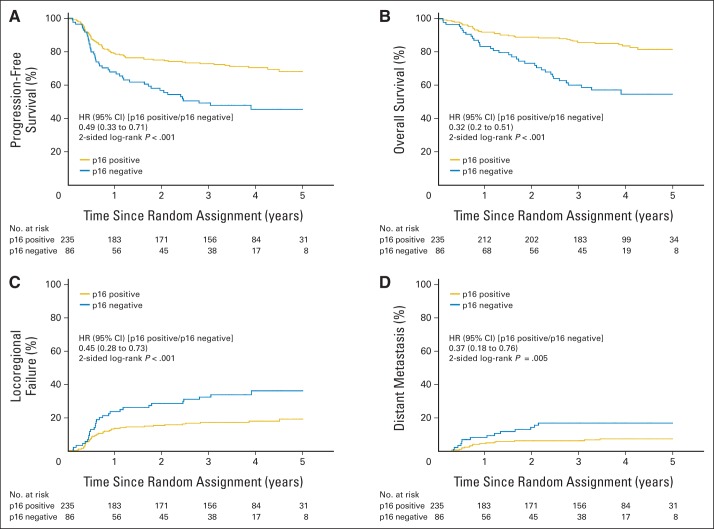
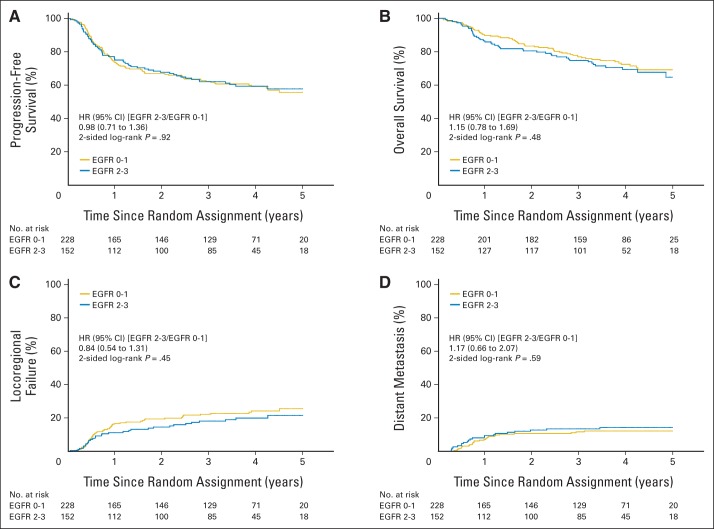
Because results were not significantly different between the two treatment arms, they were combined for the correlative analyses. Figure 3 shows that patients with p16-positive OPCs, compared with patients with p16-negative OPCs, had significantly better PFS (3-year probability, 72.8% v 49.2%, respectively; P < .001) and OS (3-year probability, 85.6% v 60.1%, respectively; P < .001) and a significantly lower probability of LRF (17.3% v 32.5%, respectively; P < .001) and DM (6.5% v 17.0%, respectively; P = .005). However, survival end points and pattern of relapse were not significantly different by tumor EGFR expression when scored with either our previous cut point of 80% tumor cells staining positive or using a four-level semiquantitative method (Appendix Figs A1 and A2, online only).
Fig 3.
Kaplan-Meier estimates of (A) progression-free and (B) overall survival and (C) cumulative incidence estimates of locoregional failure and (D) distant metastasis by p16 expression in patients with oropharyngeal carcinoma. HR, hazard ratio.
Figure 4 shows the forest plots of HRs of the effect of treatment by patient and tumor variables and by p16 and EGFR expression. With the exception of better OS for younger patients (age ≤ 50 years; HR, 0.45; 95% CI, 0.23 to 0.89; P for interaction = .02), the addition of cetuximab did not affect outcome. Primary laryngeal-hypopharyngeal carcinoma, p16-negative OPC, N2b-3 category, T4 tumor, more than 10 pack-years of cigarette smoking history, age greater than 50 years, and Zubrod performance status of 1 were associated with poorer PFS and OS in multivariable analysis (Table 3).
Fig 4.
Forest plots of treatment effect for (A) progression-free and (B) overall survival. A hazard ratio of less than 1 indicates a benefit with the addition of cetuximab. Vertical lines are shown at a hazard ratio of 1.0 and the observed hazard ratio for the entire study population. There is a significant interaction (P = .02) between assigned treatment and age (> v ≤ 50 years) for overall survival, indicating a treatment benefit for younger patients receiving cetuximab. EGFR, epidermal growth factor receptor; IMRT, intensity-modulated radiotherapy; 3DCRT, three-dimensional conformal radiotherapy.
Table 3.
Hazard Ratios for Progression-Free and Overall Survival According to Patient Group
| Covariates | Progression-Free Survival |
Overall Survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients With p16, EGFR, and Smoking Data |
All Patients With Imputed Data |
Patients With p16, EGFR, and Smoking Data |
All Patients With Imputed Data |
|||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| All primary tumor sites | ||||||||||||
| No. of patients | 328 | 891 | 328 | 891 | ||||||||
| Cetuximab (yes v no) | 1.29 | 0.91 to 1.83 | .16 | 1.13 | 0.92 to 1.38 | .26 | 1.01 | 0.66 to 1.55 | .96 | 0.98 | 0.77 to 1.25 | .87 |
| Age (> v ≤ 50 years) | 1.22 | 0.78 to 1.92 | .38 | 1.11 | 0.86 to 1.44 | .43 | 2.22 | 1.10 to 4.47 | .03 | 1.53 | 1.09 to 2.15 | .01 |
| Zubrod performance status (1 v 0) | 1.25 | 0.87 to 1.81 | .23 | 1.30 | 1.05 to 1.62 | .02 | 1.46 | 0.94 to 2.26 | .09 | 1.72 | 1.33 to 2.22 | < .001 |
| Pack-years of smoking (> v ≤ 10) | 1.53 | 1.00 to 2.34 | .05 | 1.35 | 1.03 to 1.75 | .03 | 2.17 | 1.21 to 3.88 | .009 | 1.55 | 1.11 to 2.17 | .01 |
| p16-negative v p16-positive OPC | 1.61 | 0.97 to 2.69 | .07 | 1.60 | 1.14 to 2.25 | .007 | 2.17 | 1.16 to 4.04 | .01 | 2.10 | 1.39 to 3.19 | < .001 |
| Non-OPC v p16-positive OPC | 1.63 | 1.05 to 2.54 | .03 | 1.79 | 1.34 to 2.38 | < .001 | 1.93 | 1.12 to 3.34 | .02 | 2.25 | 1.58 to 3.20 | < .001 |
| T category (T4 v T2-3) | 1.33 | 0.89 to 1.98 | .16 | 1.65 | 1.32 to 2.08 | < .001 | 1.73 | 1.09 to 2.73 | .02 | 1.86 | 1.43 to 2.43 | < .001 |
| N category (N2b-3 v N0-2a) | 1.62 | 1.08 to 2.43 | .02 | 1.75 | 1.36 to 2.26 | < .001 | 1.51 | 0.92 to 2.47 | .10 | 1.48 | 1.11 to 1.99 | .008 |
| EGFR (≥ v < 80% positive tumor cells) | 0.93 | 0.64 to 1.35 | .70 | 0.97 | 0.66 to 1.41 | .86 | 0.99 | 0.63 to 1.56 | .97 | 1.02 | 0.65 to 1.59 | .94 |
| OPC | ||||||||||||
| No. of patients | 222 | 625 | 222 | 625 | ||||||||
| Cetuximab (yes v no) | 1.46 | 0.92 to 2.32 | .11 | 1.14 | 0.88 to 1.48 | .33 | 0.92 | 0.50 to 1.69 | .79 | 0.87 | 0.63 to 1.21 | .41 |
| Age (> v ≤ 50 years) | 1.16 | 0.67 to 2.01 | .59 | 0.94 | 0.69 to 1.28 | .70 | 3.19 | 1.13 to 9.02 | .03 | 1.44 | 0.94 to 2.21 | .10 |
| Zubrod performance status (1 v 0) | 1.34 | 0.83 to 2.17 | .24 | 1.39 | 1.06 to 1.84 | .02 | 1.77 | 0.97 to 3.24 | .06 | 2.08 | 1.48 to 2.93 | < .001 |
| Pack-years of smoking (> v ≤ 10) | 1.76 | 1.07 to 2.88 | .03 | 1.45 | 1.07 to 1.96 | .02 | 2.47 | 1.22 to 5.00 | .01 | 1.73 | 1.17 to 2.57 | .007 |
| p16 negative v p16 positive | 1.44 | 0.86 to 2.41 | .17 | 1.51 | 1.06 to 2.15 | .02 | 1.91 | 1.02 to 3.57 | .04 | 1.94 | 1.26 to 2.98 | .003 |
| T category (T4 v T2-3) | 1.70 | 1.03 to 2.82 | .04 | 1.98 | 1.50 to 2.62 | < .001 | 2.40 | 1.31 to 4.43 | .005 | 2.55 | 1.81 to 3.58 | < .001 |
| N category (N2b-3 v N0-2a) | 1.54 | 0.87 to 2.74 | .14 | 1.72 | 1.21 to 2.44 | .003 | 1.35 | 0.64 to 2.86 | .43 | 1.32 | 0.87 to 2.00 | .20 |
| EGFR (≥ v < 80% positive tumor cells) | 1.17 | 0.71 to 1.93 | .53 | 1.07 | 0.67 to 1.72 | .77 | 1.32 | 0.70 to 2.50 | .39 | 1.14 | 0.67 to 1.93 | .63 |
Abbreviations: EGFR, epidermal growth factor receptor; HR, hazard ratio from multivariable Cox model; OPC, oropharyngeal carcinoma.
DISCUSSION
This large phase III trial yielded several clinically important findings. The impetus for the study stemmed from strong previous phase III data showing that combining cisplatin or cetuximab concurrently with radiation improved PFS and OS of patients with locally advanced HNC10,20 and that adding cetuximab to platinum-based chemotherapy improved OS of patients with recurrent or metastatic HNC.12 Therefore, it is disappointing to discover that adding cetuximab to the radiation-cisplatin platform had no significant impact on PFS, OS, LRF, or DM. One plausible explanation for these negative results is that the toxicity burden of radiation-cisplatin is at the maximum-tolerated level, such that adding cetuximab caused radiotherapy interruption(s) in 26.9% of patients despite incomplete cetuximab administration in 26.4%. These compromises in therapy could explain the trend toward a higher LRF rate in the experimental arm. Another potential explanation for lack of benefit is that platinum derivatives and cetuximab have similar mechanisms of radiation sensitization (i.e., inhibition of repair of radiation-induced DNA damage).29,30 Consequently, tumors having proficient repair machinery would be resistant to both agents, and sensitive tumors would derive no additional benefit. If true, combining cetuximab with agents having different mechanisms of action is logical. For example, the antitubulin drug docetaxel produced promising results in combination with cetuximab and radiation in a preclinical study.31 RTOG 1216 is currently comparing postoperative radiation plus docetaxel and cetuximab versus docetaxel versus cisplatin in high-risk patients.
In terms of toxicity, we did not observe severe cardiac or other events with the addition of cetuximab to radiation-cisplatin, as had been observed in a previous phase II trial.12 Cetuximab exacerbated acute mucositis, in contrast to findings in the radiation-cetuximab trial,10 and adding cetuximab was associated with more treatment-related deaths compared with radiation-cisplatin alone (10 v three deaths, respectively; P = .05). Cetuximab also increased the incidence of in-field skin reactions, without reaching the severity described in a previous case report.32 In addition, cetuximab increased hypokalemia, fatigue, and anorexia, all contributing to incomplete cetuximab dosing in 26.4% of patients and interruption of radiotherapy in 26.9% of patients (Appendix Table A1). Because of the higher incidences of these acute toxic effects without advantages in tumor control or survival, we advise against the routine use of cetuximab with cisplatin and radiation.
Although tissue collection and analysis were cumbersome before funding became available through formal mechanisms, we successfully collected biopsy specimens from 43% of patients. As summarized in a recent review,33 several studies have established p16-positive OPC as a distinct entity with an excellent prognosis with current standard therapies. Our results confirmed that patients with p16-positive OPCs had significantly better PFS and OS and lower LRF rates than their p16-negative counterparts. In contrast to our previous finding,17 however, we found in this study that patients with p16-positive OPCs also had a significantly lower DM rate. Lower tobacco exposure (5.25 pack-years, Table 1) is one possible explanation. Further analysis showed no significant interaction between treatment and p16 status, although trends were evident for worse PFS (HR, 1.57; P for interaction = .12) and OS (HR, 1.42; P for interaction = .13) for patients with p16-positive OPCs receiving cetuximab (Fig 3). The lack of such trends in the subset without tumor specimens for p16 assay is unexpected and may represent an imbalance in the arms for p16-positive status or a decrease in cisplatin/radiation delivery, which is perhaps more biologically relevant.
We previously validated that high tumor EGFR expression was associated with higher LRF and lower PFS and OS rates than low tumor EGFR expression in two groups of patients treated with radiation alone in a prospective trial.4,19 Assessments with the same method yielded no such association in the this study (Appendix Figs A1 and A2). It is plausible that cisplatin/cetuximab primarily sensitized tumors with high EGFR expression, thus annulling its prognostic significance. Unfortunately, this notion could not be addressed in this study because there was no radiation alone arm. However, a thorough analysis of the available residual tumor specimens, if made available, of patients enrolled onto the trial randomly assigning patients to receive radiation with or without cetuximab10 could properly test this hypothesis. Another immunofluorescence-based assay method, the automated in situ quantitative assay (AQUA),5 could be a better marker, but regrettably, no uncommitted residual tumor specimens are available for analysis.
Multivariable analysis again identified more than 10 pack-years of cigarette smoking as an independent predictor of poor prognosis; other predictors were p16-negative carcinoma, N2b-3 category, T4 tumor, and poor performance status. These consistent findings support the current strategy of designing trials for better biologically defined HNC entities. RTOG 1016, which is selectively enrolling patients with T1-2N2a-3 or T3-4 with any N category, p16-positive OPC, represents this new paradigm. It is anticipated that future trials will further refine study populations based on smoking status and other biologic tumor features. This means, however, that the number of patients eligible for a given trial will decrease progressively. Therefore, international collaborations are imperative to complete patient accrual in a timely fashion. Hence, it is desirable to commence discussions of funding for and logistics of establishing international cooperative group alliances.
Acknowledgment
We are all indebted to the vision and leadership of K. Kian Ang, MD, PhD, in the conception and execution of this study, and to William J. Spanos, MD, for his contribution to the design and execution of the study.
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Appendix
Table A1.
Protocol Treatment Delivered by Assigned Treatment
| Treatment Component | Arm A: RT + Cisplatin (n = 447) |
Arm B: RT + Cisplatin + Cetuximab (n = 444) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Type of radiation administered | .008* | ||||
| 3DCRT | 59 | 13.2 | 46 | 10.4 | |
| IMRT | 386 | 86.4 | 385 | 86.7 | |
| None | 2 | 0.4 | 13 | 2.9 | |
| Radiation dose, Gy | .03† | ||||
| Mean | 69.1 | 67.2 | |||
| Standard deviation | 8.0 | 13.2 | |||
| Median | 70.0 | 70.0 | |||
| Range | 0.0-72.6 | 0.0-72.0 | |||
| Q1-Q3 | 70.0-70.0 | 70.0-70.0 | |||
| < 60 | 11 | 2.5 | 23 | 5.2 | |
| ≥ 60 | 436 | 97.5 | 421 | 94.8 | |
| No. of fractions | .03‡ | ||||
| Mean | 35.3 | 34.2 | |||
| Standard deviation | 4.7 | 7.0 | |||
| Median | 35.0 | 35.0 | |||
| Range | 0.0-42.0 | 0.0-42.0 | |||
| Q1-Q3 | 35.0-35.0 | 35.0-35.0 | |||
| < 30 | 11 | 2.5 | 23 | 5.2 | |
| ≥ 30 | 436 | 97.5 | 421 | 94.8 | |
| Total duration of radiation, days | .42§ | ||||
| Mean | 42.5 | 42.0 | |||
| Standard deviation | 6.8 | 10.2 | |||
| Median | 42.0 | 42.0 | |||
| Range | 0.0-73.0 | 0.0-108.0 | |||
| Q1-Q3 | 40.0-44.0 | 40.0-45.0 | |||
| ≤ 56 | 436 | 97.5 | 429 | 96.6 | |
| > 56 | 11 | 2.5 | 15 | 3.4 | |
| Radiation interruptions | 445 | 431 | < .001* | ||
| No | 258 | 58.0 | 212 | 49.2 | |
| Yes, as a result of toxicity | 67 | 15.1 | 116 | 26.9 | |
| Yes, as a result of other reason | 120 | 27.0 | 103 | 23.9 | |
| Tumor volume contouring score | |||||
| Per protocol | 231 | 51.7 | 235 | 52.9 | |
| Acceptable variation | 169 | 37.8 | 152 | 34.2 | |
| Unacceptable variation | 27 | 6.0 | 23 | 5.2 | |
| Not evaluable | 20 | 4.5 | 34 | 7.7 | |
| Organs at risk contouring score (IMRT only) | 388 | 396 | |||
| Per protocol | 208 | 53.6 | 220 | 55.6 | |
| Acceptable variation | 153 | 39.4 | 138 | 34.8 | |
| Unacceptable variation | 11 | 2.8 | 13 | 3.3 | |
| Not evaluable | 16 | 4.1 | 25 | 6.3 | |
| Tumor-volume dose-volume analysis score (IMRT only) | 388 | 396 | |||
| Per protocol | 251 | 64.7 | 227 | 57.3 | |
| Acceptable variation | 87 | 22.4 | 117 | 29.5 | |
| Unacceptable variation | 37 | 9.5 | 26 | 6.6 | |
| Not evaluable | 13 | 3.4 | 26 | 6.6 | |
| Cisplatin given | .06‖ | ||||
| None | 4 | 0.9 | 16 | 3.6 | |
| One cycle | 24 | 5.4 | 26 | 5.9 | |
| Two cycles | 419 | 93.7 | 402 | 90.5 | |
| Cumulative cisplatin dose, mg/m2 | .43¶ | ||||
| Mean | 191.9 | 185.7 | |||
| Standard deviation | 32.0 | 43.8 | |||
| Median | 200.0 | 200.0 | |||
| Range | 0.0-282.7 | 0.0-239.4 | |||
| Q1-Q3 | 198.4-200.3 | 197.1-200.0 | |||
| < 160 | 44 | 9.8 | 51 | 11.5 | |
| ≥ 160 | 403 | 90.2 | 393 | 88.5 | |
| Cetuximab loading dose given | |||||
| No | 443 | 99.1 | 12 | 2.7 | |
| Yes | 4 | 0.9 | 432 | 97.3 | |
| Weekly cetuximab doses given | |||||
| None | 442 | 98.9 | 35 | 7.9 | |
| 1-5 doses | 1 | 0.2 | 82 | 18.5 | |
| 6-7 doses | 4 | 0.9 | 323 | 72.7 | |
| 8 doses | 0 | 0.0 | 4 | 0.9 | |
Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; Q1, first quartile; Q3, third quartile; RT, radiotherapy.
Pearson χ2 test.
Pearson χ2 test: < v ≥ 60 Gy.
Pearson χ2 test: < v ≥ 30 fractions.
Pearson χ2 test: ≤ v > 56 days.
Wilcoxon rank sum test.
Pearson χ2 test: < v ≥ 160 mg/m2.
Fig A1.
Kaplan-Meier estimates of (A) progression-free and (B) overall survival and (C) cumulative incidence estimates of locoregional failure and (D) distant metastasis by epidermal growth factor receptor (EGFR) expression using a cut point of 80% of tumor cells staining positive. HR, hazard ratio.
Fig A2.
Kaplan-Meier estimates of (A) progression-free and (B) overall survival and cumulative incidence estimates of (C) locoregional failure and (D) distant metastasis by epidermal growth factor receptor (EGFR) expression using a four-level semiquantitative method. HR, hazard ratio.
See accompanying editorial on page 2929
Support information appears at the end of this article.
The contents of the article are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00265941.
Support
Supported by Grants No. RTOG U10-CA21661, CCOP U10-CA37422, and R01-CA168485 and Cancer Center Support (Core) Grant No. CA016672 from the National Cancer Institute.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: David I. Rosenthal, Bristol-Myers Squibb (C), ImClone Systems (C); Eric J. Sherman, Bristol-Myers Squibb (C); James A. Bonner, Bristol-Myers Squibb (C), Merck Serono (C), Eli Lilly (C); Maura L. Gillison, GlaxoSmithKline (C), Bristol-Myers Squibb (U) Stock Ownership: None Honoraria: David I. Rosenthal, Bristol-Myers Squibb; James A. Bonner, Bristol-Myers Squibb, Merck Serono, Eli Lilly; Maura L. Gillison, Merck Serono; Sue S. Yom, ImClone Systems Research Funding: James A. Bonner, Bristol-Myers Squibb Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Maura L. Gillison, Merck Serono
AUTHOR CONTRIBUTIONS
Conception and design: Qiang Zhang, David I. Rosenthal, Eric J. Sherman, James M. Galvin, James A. Bonner, Jonathan Harris, Andre A. Konski, William J. Spanos, Rita S. Axelrod
Financial support: Maura L. Gillison
Provision of study materials or patients: Phuc Felix Nguyen-Tan, Sue S. Yom
Collection and assembly of data: K. Kian Ang, James M. Galvin, Jonathan Harris, Adel K. El-Naggar, Maura L. Gillison, Richard C. Jordan, Wade L. Thorstad, Andy Trotti, Jonathan J. Beitler, Adam S. Garden, Sue S. Yom
Data analysis and interpretation: K. Kian Ang, Qiang Zhang, David I. Rosenthal, Phuc Felix Nguyen-Tan, Eric J. Sherman, Randal S. Weber, James M. Galvin, James A. Bonner, Jonathan Harris, Maura L. Gillison, Richard C. Jordan, Andre A. Konski, Wade L. Thorstad, Andy Trotti, William J. Spanos, Sue S. Yom, Rita S. Axelrod
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomized trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin Grandis J, Melhem MF, Barnes EL, et al. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer. 1996;78:1284–1292. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1284::AID-CNCR17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 5.Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 6.Fan Z, Baselga J, Masui H, et al. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 7.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 8.Saleh MN, Raisch KP, Stackhouse MA, et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother Radiopharm. 1999;14:451–463. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 9.Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 12.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 13.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 14.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 15.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 16.Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begum S, Gillison ML, Ansari-Lari MA, et al. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- 19.Chung CH, Zhang Q, Hammond EM, et al. Integrating epidermal growth factor receptor assay with clinical parameters improves risk classification for relapse and survival in head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:331–338. doi: 10.1016/j.ijrobp.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–350. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Kalbfleish J, Prentice R. New York, NY: John Wiley & Sons; 1980. The Statistical Analysis of Failure Time Data. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 26.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, et al. Fully conditional specification in multivariate imputation. J Stat Comput Sim. 2006;76:1049–1064. [Google Scholar]
- 27.Rubin DB, editor. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 28.Resseguier N, Giorgi R, Paoletti X. Sensitivity analysis when data are missing not-at-random. Epidemiology. 2011;22:282–283. doi: 10.1097/EDE.0b013e318209dec7. [DOI] [PubMed] [Google Scholar]
- 29.Amorino GP, Freeman ML, Carbone DP, et al. Radiopotentiation by the oral platinum agent, JM216: Role of repair inhibition. Int J Radiat Oncol Biol Phys. 1999;44:399–405. doi: 10.1016/s0360-3016(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 30.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 31.Nakata E, Hunter N, Mason K, et al. C225 antiepidermal growth factor receptor antibody enhances the efficacy of docetaxel chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:1163–1173. doi: 10.1016/j.ijrobp.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 32.Budach W, Bölke E, Homey B. Severe cutaneous reaction during radiation therapy with concurrent cetuximab. N Engl J Med. 2007;357:514–515. doi: 10.1056/NEJMc071075. [DOI] [PubMed] [Google Scholar]
- 33.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]