Abstract
Purpose
Chemoimmunotherapy has been the standard of care for chronic lymphocytic leukemia (CLL). However, the introduction of B-cell receptor (BCR) kinase inhibitors such as ibrutinib has the potential to eliminate the role of chemotherapy in the treatment of CLL. How to best incorporate old and new therapies for CLL in this landscape is increasingly complex.
Methods
This article reviews current data available to clinicians and integrates these data to provide a strategy that can be used to approach the treatment of CLL in the era of BCR signaling inhibitors.
Results
Current strategies separate patients based on age or functional status as well as genetics [presence or absence of del(17)(p13.1)]. In the era of targeted therapy, this will likely continue based on current available data. Phase III studies support chemoimmunotherapy as the initial standard therapy for patients without del(17)(p13.1). Choice of chemotherapy (fludarabine plus cyclophosphamide, bendamustine, or chlorambucil) and anti-CD20 antibody (rituximab, ofatumumab, or obinutuzumab) varies based on regimen and patient status. For patients with del(17)(p13.1), no standard initial therapy exists, although several options supported by phase II clinical trials (methylprednisolone plus alemtuzumab or ibrutinib) seem better than chemoimmunotherapy. Treatment of relapsed CLL seems to be best supported by ibrutinib-based therapy. Completion of trials with ibrutinib and other new agents in the near future will offer opportunity for chemotherapy-free treatment across all groups of CLL.
Conclusion
Therapy for CLL has evolved significantly over the past decade with introduction of targeted therapy for CLL. This has the potential to completely transform how CLL is treated in the future.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) occurs most frequently in patients age > 70 years and is similar genetically to small lymphocytic lymphoma (SLL), where blood lymphocytosis is lacking. The natural history of CLL progression is variable and influenced in great part by genetic, epigenetic, and biochemical properties of the tumor cells and clinical features at time of diagnosis. On the basis of earlier studies demonstrating no benefit of early treatment with alkylator-based therapy, treatment of CLL is not recommended until symptoms develop.1 However, during the past 5 years, the application of genomic studies and introduction of many new therapies for CLL have greatly increased the complexity of treating symptomatic CLL.2,3 In addition, new targeted therapy offers the possibility of a paradigm shift in this disease. This review focuses briefly on the biology of highly promising targets that are being pursued and expands on treatment scenarios clinicians will encounter as we enter the new era of targeted therapy for CLL.
RELEVANT THERAPEUTIC TARGETS FOR CLL
Extensive basic scientific investigation over the past three decades has begun to unravel different immunologic, biochemical, and genetic features of malignancies, including CLL, that offer opportunity for therapeutic targeting. Outlined here are pathways relevant to CLL for which impactful therapies are emerging.
B-Cell Receptor Signaling and Microenvironment
Antigen-dependent and -independent B-cell receptor (BCR) signaling plays a central role in the pathogenesis of CLL (Fig 1).4,5 In addition, BCR signaling activates integrin signaling and enhances CLL cell adhesion to microenvironment stroma, thereby increasing resistance to apoptosis.6,7 BCR signaling in CLL is not driven by a specific mutation or rearrangement but instead by amplification of several survival pathways, including phosphatidylinositide 3-kinase (PI3K), NF-κB, and MAPK/ERK, which are constitutively active in the lymph node and bone marrow compartments of CLL, where disease expansion occurs.8 Although many of the components of BCR signaling are ubiquitous and therefore challenging to therapeutically target, mouse knockout or inactivation studies of both PI3Kδ and Bruton's tyrosine kinase (BTK) have demonstrated a predominately B-cell phenotype.9–11 These findings, combined with strong preclinical studies showing that inhibitors of p110δ PI3K12–14 and BTK7,15–17 prevent BCR-mediated proliferation, stromal protection, and signaling, provide justification for study of these agents in CLL. The two most mature therapeutic agents coming forward—idelalisib and ibrutinib—differ considerably from each other not only in target but also in mechanism. Idelalisib is a selective and reversible inhibitor of PI3Kδ,14 whereas ibrutinib irreversibly inactivates BTK by forming a covalent bond with a cytsteine residue (C481). Ibrutinib also inhibits several other kinases (interleukin-2–inducible T-cell kinase [ITK], TEC, BMX, EGFR, and HER4) with a similar cysteine-binding residue near the ATP binding pocket of the kinase.17
Fig 1.
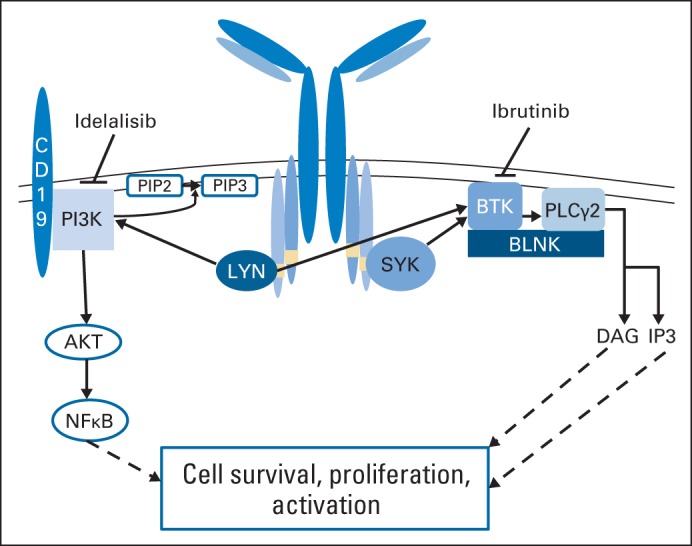
Simplified model of B-cell receptor signaling. Pathways demonstrated are actively targeted by therapeutic agents currently under late development or in postmarketing evaluation for chronic lymphocytic leukemia. BTK, Bruton's tyrosine kinase; PI3K, phosphatidylinositide 3-kinase.
Immune Dysfunction
One of the paramount ways cancer establishes itself is through suppressing both the acquired and innate immune systems. Immune suppression in CLL leads to disease progression, infection, and secondary malignancies and occurs through multiple mechanisms, including expansion of T regulatory cells, depression of antibody production, skewing of T cells toward a Th2 phenotype, and suppression of natural killer (NK) –cell and monocyte function.18–20 Immune dysregulation associated with CLL is present early in the disease and worsens in parallel with disease-related symptoms.20 Compounding this issue is that many of the therapies used in CLL, including fludarabine21,22 and bendamustine,23,24 are profoundly immune suppressive. Specific targeting of immune dysfunction in CLL is possible with lenalidomide, a multitargeted therapeutic now recognized to enhance E3 ubiquitin ligase degradation of Ikaros 1 and 3, the former of which represses interleukin-2 production, thereby enhancing T-cell and NK-cell activation and function.25 Lenalidomide also inhibits T regulatory cells26 and has been shown in several studies to promote T-cell synapse toward CLL cells.27,28 Passive use of immune therapy in CLL with monoclonal antibodies such as rituximab, when combined with chemotherapy, prolonged survival in this disease.29–31 Refinements in CD20 antibody properties have resulted in US Food and Drug Administration approval of two new antibodies (ie, ofatumumab and obinutuzumab). Similarly, although specifically developed to inhibit BCR signaling, the PI3Kδ inhibitor idelalisib likely has immune modulatory function through inhibition of T regulatory cells,32 and the BTK inhibitor ibrutinib was demonstrated by our group to also inhibit ITK.33 ITK is an essential kinase for immunosuppressive Th2 CD4 T cells, and therefore, ibrutinib skews CD4 T cells back to a Th1 phenotype, which responds more appropriately to infection or malignancy. Thus, it seems that many of the targeted therapies currently being explored in CLL directly or indirectly influence disease-associated immune suppression.33
Other Therapeutic Targets
Cell survival in CLL is currently targeted through BCL2 and MCL1. ABT263, which inhibits BCL2 function, has demonstrated dramatic clinical activity with durable remissions as part of phase I studies in relapsed CLL.34 A second-generation molecule more selective for BCL2 inhibition, ABT-199, lacks BCL-XL as a target and is currently in clinical trials with CLL.35 The dose-limiting toxicity of ABT-199 has been hyperacute tumor lysis syndrome, which has slowed development of this exciting compound but seems to be manageable with careful dose escalation. Therapeutics targeting MCL1 specifically are not currently available, but alternative therapeutics that inhibit gene transcription via CDK9 inhibition, such as pan-CDK inhibitors (eg, flavopiridol and dinaciclib), are clinically active in CLL and also have the dose-limiting toxicity of tumor lysis syndrome.36 Similarly, therapeutics targeting CD37 and promoting SHP1 activation, which inhibits BCR signaling, are also under study.37,38 However, because these agents are farther from regulatory approval, they will not be discussed in our review, which focuses solely on current treatment modalities employed for CLL.
SYMPTOMATIC, UNTREATED ELDERLY PATIENTS
Despite chlorambucil being an accepted therapy for elderly patients with CLL, transition to more superior combination therapy of either chlorambucil or bendamustine with rituximab has occurred (Table 1). New data on alternative CD20 antibodies and chlorambucil have also been reported, as summarized here.
Table 1.
Completed Treatment Trials Relevant to Untreated Elderly Patients With CLL
| Treatment | Phase | No. of Patients | ORR (%) | CR Rate (%) | Median PFS (months) | del(17)(p13.1) Worse |
|---|---|---|---|---|---|---|
| CLB plus rituximab | II | 100 | 82 | 9 | 24 | Yes |
| CLB plus rituximab | II | 97 | 82.4 | 18.9 | 34.7 | Yes |
| Bendamustine plus rituximab | II | 117 | 88 | 23 | 33.0 | Yes |
| Ibrutinib | IB/II | 31 | 71 | 13 | 96%* | Unknown |
| Idelalisib plus rituximab | IB/II | 64 | 97 | 19 | 93%* | Unknown |
| CLB v | III | 221 | 69 | 1 | 13.1 | Unknown |
| CLB plus ofatumumab | 226 | 82 | 12 | 22.4 | ||
| CLB v | III | 118 | 31.4 | 0 | 11.1 | Yes |
| CLB plus rituximab v | 330 | 65.3 | 7.3 | 16.3 | ||
| CLB plus obinutuzumab | 333 | 77.3 | 22.3 | 26.7 |
Abbreviations: CLB, chlorambucil; CLL, chronic lymphocytic leukemia; CR, complete response; ORR, overall response rate; PFS, progression-free survival.
At 24 months.
Bendamustine Chemoimmunotherapy
The Gynecologic Cancer Study Group (GCSG) CLL2M study represents the largest phase II study of bendamustine published and included 117 previously untreated patients with CLL who were treated with bendamustine plus rituximab (BR).39 Treatment was administered every 28 days for up to six cycles. The median age of patients was 64 years; 26% were age > 70 years; 46% had Binet stage C disease. The overall response rate (ORR) was 88%, and the complete response (CR) rate was 23%. Median event-free survival with this treatment was 33 months. Patients of all genomic groups except for del(17)(p13.1) responded favorably; for those with del(17)(p13.1), we do not recommend this treatment. Treatment was generally well tolerated, with cytopenias and infections being most problematic. Treatment-related mortality was 4%, mostly as a consequence of infections. The safety of this regimen in older patients was retrospectively confirmed in another study examining outcome in elderly patients treated with bendamustine.40 Although prospective randomized phase III data examining BR in this population do not exist, BR represents an acceptable initial treatment for elderly patients with CLL lacking del(17)(p13.1).
Chorambucil Chemoimmunotherapy
Several phase II trials have demonstrated the favorable efficacy and safety of the combination of chlorambucil and rituximab.41,42 These trials ultimately formed the basis for studying chlorambucil chemoimmunotherapy as part of two phase III trials in elderly untreated patients with CLL administered second-generation CD20 antibodies.
The first trial (COMPLEMENT 1 [Ofatumumab Plus Chlorambucil Versus Chlorambucil Monotherapy in Previously Untreated Patients With Chronic Lymphocytic Leukemia]), which has only been preliminarily reported, used ofatumumab, a type 1 humanized CD20 antibody that has a different binding site than rituximab and is much more effective than rituximab at complement mediation, direct killing with crosslinking, and antibody-dependent cellular cytotoxicity (ADCC).43 The trial examined the efficacy of adding ofatumumab to chlorambucil in patients for whom fludarabine therapy was inappropriate based on age or comorbidities.44 A total of 447 patients were enrolled, with a median age of 69 years; 82% were age ≥ 65 years and/or had ≥ two comorbidities. The combination arm had a significantly higher ORR (82% v 69%), CR rate (12% v 1%), and progression-free survival (PFS; median, 22.4 v 13.1 months) as compared with those receiving chlorambucil. The frequency of grade ≥ 3 adverse events occurred in 50% of patients receiving the combination treatment and 43% of patients receiving chlorambucil alone, with the most common events being neutropenia (26% v 14%), infection (15% v 14%), and infusion toxicity (10%, all in combined arm). No survival advantage was noted after short follow-up.
The second trial (GCSG CLL11) focused on the value of adding either rituximab or obinutuzumab to chlorambucil.45 Obtinutuzumab is a type 2 CD20 human antibody that is glycoengineered to enhance NK cell–mediated ADCC. This large phase III study did not use age as an eligibility criterion; rather, it used a high cumulative illness rating scale score, which denotes functional comorbidity, or modestly impaired renal function (glomerular filtration rate, 30 to 69 mL/min). The study showed that combination therapy yielded a significantly higher ORR (77.3% v 31.4%), CR rate (22.1 v 0%), PFS (median, 26.7 v 11.1 months), and overall survival (OS; death rate, 9% v 20%) as compared with chlorambucil. Only the subset of patients with del(17)(p13.1) did not benefit from this combined therapy. The combination of obinutuzumab plus chlorambucil was also statistically superior to that of rituximab plus chlorambucil with regard to ORR (78.6% v 65.1), CR rate (20.7% v 7%), and PFS (median, 26.7 v 15.2 months). No survival advantage was noted after short follow-up. The efficacy of the obinutuzmab combination is further exemplified by the significantly higher frequency of bone marrow minimal residual disease (MRD) negativity (obinituzumab, 19.5% v rituximab, 2.6%). Grade 3 and 4 infusion-related events were more frequent and severe with obinutuzumab (20% v 4%) than with rituximab, but in all cases, they were manageable. Similarly, grade 3 and 4 neutropenia was higher with obinutuzumab (33% v 28%) as compared with rituximab, but no increased risk in serious infections was noted. Overall, this study represents a major advance in the treatment of elderly patients with CLL lacking del(17)(p13.1) and establishes one standard of care for both elderly patients and those with comorbid conditions that make aggressive chemoimmunotherapy unfeasible.
Ibrutinib Therapy
Ibrutinib is an oral irreversible inhibitor of BTK that has demonstrated significant efficacy in relapsed CLL with durable remissions, including in patients with del(17)(p13.1), with modest toxicity. Limited data suggest even more impressive efficacy in previously untreated patients with CLL. A study of 31 patients age ≥ 65 years were treated with ibrutinib 420 mg daily until progression or unacceptable toxicity. The median age was 71 years, and more than half of patients had advanced Rai stage disease.46 Only 9% of patients had high-risk genomic features, either del(11)(q22.3) or del(17)(p13.1). The ORR according to 2008 International Workshop on Chronic Lymphocytic Leukaemia (IWCLL) criteria was 71%, and an additional 13% of patients achieved partial responses (PRs) with lymphocytosis. At a median follow-up of 24 months, PFS and OS rates were 96%. Toxicity was similar to that observed in patients treated for relapsed disease.47 These results are quite remarkable, but they only applied to predominately low–genomic risk patients. These promising data prompted several ongoing phase III trials of ibrutinib in the elderly (summarized in Table 2), which have the potential to change CLL treatment recommendations in the future. RESONATE II (A Multicenter, Open-Label, Phase III Study of the Bruton's Tyrosine Kinase Inhibitor PCI-32765 Versus Chlorambucil in Patients 65 Years or Older With Treatment-Naive Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma), which has completed enrollment, includes patients age ≥ 65 years and is comparing chlorambucil with ibrutinib in previously untreated CLL, with a primary end point of PFS. If durable remissions with ibrutinib are confirmed in this trial, this will likely become an acceptable therapy for patients with CLL. The Alliance A041202 trial, which is actively accruing patients, will compare chemoimmunotherapy (BR) with either ibrutinib or ibrutinib plus rituximab in patients age ≥ 65 years and represents the first comparison of chemoimmunotherapy with ibrutinib-containing regimens in elderly patients.
Table 2.
Ongoing Phase III Studies With Potential to Change Standard of Care in CLL
| Study | Treatment Comparison | Treatment Status | Age Group (years) |
|---|---|---|---|
| CLL12 | Ibrutinib v placebo | High risk, aymptomatic | ≥ 18 |
| RESONATE II | CLB v ibrutinib (crossover) | Symptomatic, untreated | ≥ 65 |
| A041202 | BR v ibrutinib plus rituximab v ibrutinib (crossover) | Symptomatic, untreated | ≥ 65 |
| E1912 | FCR v IR | Symptomatic, untreated | < 70 |
| NCT01980888 | BR plus placebo v BR plus idelalisib | Symptomatic, untreated | ≥ 18 |
| NCT01980875 | Idelalisib plus rituximab v placebo plus rituximab or idelalisib plus CLB v placebo plus CLB | Symptomatic, untreated | ≥ 65 |
| CLLM1 | Lenaldiomide v placebo for consolidation after initial therapy | Consolidation after first treatment | ≥ 18 |
| RESONATE | Ofatumumab v ibrutinib | Relapsed | ≥ 18 |
| NCT01611090 | BR plus placebo v BR plus ibrutinib | Relapsed | ≥ 18 |
| NCT01569295 | BR plus placebo v BR plus idelalisib | Relapsed | ≥ 18 |
| NCT02005471 | ABT199 plus rituximab v BR | Relapsed | ≥ 18 |
Abbreviations: BR, bendamustine plus rituximab; CLB, chlorambucil; CLL, chronic lymphocytic leukemia; FCR, fludarabine, cyclophosphosphamide, and rituximab; IR, ibrutinib plus rituximab; RESONATE, A Phase III Study of Ibrutinib (PCI-32765) Versus Ofatumumab in Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia; RESONATE II, A Multicenter, Open-Label, Phase III Study of the Bruton's Tyrosine Kinase Inhibitor PCI-32765 Versus Chlorambucil in Patients 65 Years or Older With Treatment-Naive Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma.
Other Therapies
Other agents potentially used include lenalidomide with or without rituximab, which in several phase II trials demonstrated responses and durable remissions in the majority of patients. Long-term follow-up in one study demonstrated minimal long-term toxicity with follow-up exceeding 4 years.48,49 A phase III study examining chlorambucil versus lenalidomide in elderly patients was terminated early because of early deaths in patients age > 80 years and likely futility in obtaining the study primary end point. Lenalidomide represents one of the only therapeutics used in CLL that is immune restorative, but unfortunately, current data do not support its initial use for CLL therapy outside the setting of a clinical trial. Similarly, idelalisib has been explored predominately in relapsed CLL. However, a phase IB study has been preliminarily reported in elderly treatment-naive patients treated with eight doses of rituximab once per week in combination with idelalisib administered continuously at 150 mg twice per day until progression.50 Outcome in this preliminary report included data on 50 patients, demonstrating an ORR of 97% (CR rate, 19%). PFS rate at 24 months was 93%, including all nine patients with del(17)(p13.1) or p53 mutation. Many patients discontinued therapy because of diarrhea or other nonhematologic toxicities, and only 45% continue to receive idelalisib maintenance. Further follow-up is required to determine the duration of remission and feasibility of this regimen for patients receiving continuous idelalisib therapy.
SYMPTOMATIC, UNTREATED YOUNGER PATIENTS
The genesis of treatment for younger patients with CLL has changed modestly over the past two decades. However, the addition of the CD20 antibody rituximab to either FR51 or FR plus cyclophosphamide (FCR)30 in two separate phase II studies with extended follow-up seemed to prolong survival over historical controls. Table 3 summarizes relevant trials.
Table 3.
Select Chemoimmunotherapy Trials Relevant to Untreated Young Patients With CLL
| Treatment | Phase | No. of Patients | ORR (%) | CR Rate (%) | Median PFS (months) | del(17p) Worse |
|---|---|---|---|---|---|---|
| Fludarabine → rituximab v | II | 51 | 77 | 28 | 42 | Yes |
| FR | 53 | 90 | 47 | 42 | ||
| FCR | II | 300 | 85 | 72 | 80 | Yes |
| BR | II | 117 | 88 | 23 | 33.0 | Yes |
| Pentostatin, cyclophosphamide, and rituximab | II | 64 | 91 | 41 | 32.6 | Yes |
| FC v | III | 31 | 88.4 | 21.8 | 32.8 | Yes |
| FCR | 95.1 | 44.1 | 51.8 | |||
| FCR v | III | 284 | 97.8 | 47.4 | 85% | Yes |
| BR | 280 | 97.8 | 38.1 | 78.1% |
Abbreviations: BR, bendamustine plus rituximab; CLL, chronic lymphocytic leukemia; CR, complete response; FC, fludarabine plus cyclophosphosphamide; FCR, fludarabine, cyclophosphosphamide, and rituximab; FR, fludarabine plus rituximab; ORR, overall response rate; PFS, progression-free survival.
Fludarabine Chemoimmunotherapy
The promising studies with FR51 and FCR30 prompted a large phase III study (GCSG CLL8) that enrolled 761 previously untreated patients with CLL, with random assignment between FC and FCR.31 This study reported an ORR (95.1% v 88.4%), CR rate (44.1% v 21.8%), PFS (median, 51.8 v 32.8 months), and OS rate (alive, 87% v 83%) that were statistically superior with FCR. Emerging from this study were numerous biologic correlative studies showing the most predictive test of long-term PFS and OS was attainment of MRD-negative disease by high-sensitivity flow cytometry. In addition, patients with unmutated IGHV disease had a shorter PFS and OS with both FCR and FR, although rituximab seemed to benefit both treatment groups. All interphase cytogenetic groups benefited from rituximab, with exception of those with del(17p13.1) and patients with no interphase cytogenetic abnormalities. Toxicity included regimen-related mortality of 2%, with cytopenias and infections being the most common causes of morbidity. Long-term follow-up presented at the IWCLL meeting in 2013, from both this study and the MD Anderson Cancer Center FCR experience, suggests that durable remissions seem to occur, with the appearance of a plateau lacking late relapses in patients with IGHV-mutated disease with favorable interphase cytogenetics [ie, del(13)(q14) and trisomy 12]. For this subset of patients with CLL, treatment with FCR seems most advantageous, because the potential to induce durable remissions exists. Although this favorable patient group also does well with FR-based chemoimmunotherapy, the extended remissions observed with FCR and current absence of randomized data showing similar outcomes with attenuated therapy support use of the FCR regimen.
Bendamustine Chemoimmunotherapy
Attempts to improve on the early and late toxicities observed with FCR have been a recent major focus of research in younger previously untreated patients with CLL. In addition to the data on BR we mentioned earlier in this article, the GCSG performed a phase III study (CLL10) comparing BR with FCR in fit patients with CLL. The preliminary outcomes have been presented, demonstrating inferior response and PFS with BR as compared with FCR52 across all genetic groups. On the basis of these results, the GCSG still considers FCR to be its standard therapy for young, fit patients with CLL.
PUTTING IT ALL TOGETHER FOR SYMPTOMATIC, UNTREATED PATIENTS: WHAT IS BEST?
The completion of several phase III studies has advanced therapy for CLL across several different ages, functional statuses, and genomic groups. Reliance on therapies shown to prolong survival in CLL represents the best standard therapeutic option. For older patients lacking del(17)(p13.1), strong consideration should be given to initial treatment with obinutuzumab plus chlorambucil or BR, unless a confounding comorbidity exists prohibiting this. The use of ibrutinib in this population is limited, and recommendations will best be made once RESONATE II is completed. For younger, more fit patients lacking del(17)(p13.1), consideration of FCR-based therapy seems most justified at the present time. With this therapy, assessment of bone marrow MRD-negative status offers the opportunity for extended remission and justifies this procedure after therapy. Patients with significant disease burden after completion of FCR treatment should be considered for clinical trials targeting elimination of MRD.
CLINICAL APPROACH FOR SYMPTOMATIC PATIENTS WITH del(17)(p13.1)
Although representing only 7% to 10% of patients with symptomatic previously untreated CLL, those with del(17)(p13.1) present perhaps the most challenging treatment decisions. There is no clear consensus among experts as to the best therapy for this patient group. Cytotoxic chemotherapy is much less effective, and the addition of CD20 antibody therapy does not significantly affect outcome. Treatment regimens using high-dose steroids with alemtuzumab, which have resulted in higher ORRs, CR rates, and PFS rates than expected with chemotherapy, have been put forward as an alternative, but outcomes remain suboptimal.53 To date, results with ibrutinib in this patient population in the relapse setting have been the most promising,54 although they are still limited. Given the improved toxicity profile of ibrutinib over high-dose steroids and alemtuzumab, this might represent the best available option for these patients. However, enrollment onto clinical trials should be of highest priority for this patient group. The role of allogeneic transplantation as part of consolidation therapy for those with del(17)(p13.1) should be considered as one option, particularly in younger patients, if a good donor exists. However, the durable remissions observed with ibrutinib have called into question this recommendation if other effective cytoreductive therapy is available should relapse of disease occur.
TREATMENT OF RELAPSED CLL
In many regards, the approach to relapsed CLL is similar to the evaluation before initial therapy. Treatment initiation should not occur until patients are symptomatic and meet one of the defined criteria for treatment. It is important to repeat interphase cytogenetics to evaluate clonal evolution, which may direct therapy decisions. Bone marrow biopsy should be repeated in patients with cytopenias, especially in patients who have received FCR, because therapy-related myeloid neoplasm (trMN) occurs more commonly in this group. Identification of coexisting trMN generally prompts immediate consideration of disease cytoreduction as appropriate and allogeneic stem-cell transplantation if a donor exists, because of poor outcomes in these patients and challenges in treating coexisting CLL and trMN. Although a host of therapies exists for relapsed CLL (outlined in National Comprehensive Cancer Network guidelines),55 recent trials with ibrutinib suggest it to be the best initial choice for this patient population.
Ibrutinib Therapy
Ibrutinib has shown exceptional activity in relapsed CLL. A phase I study of ibrutinib in relapsed B-cell malignancies was initiated, where durable clinical activity was noted in nine of 16 patients with CLL or SLL.56 Notable among these patients with CLL receiving ibrutinib was early lymphocytosis, which now is recognized to be a class effect of all BCR antagonists. This occurs early with therapy but is otherwise accompanied by reduction in organomegaly, lymph node size, and cytopenias, thereby differentiating it from typical progression seen with chemoimmunotherapy. No dose-limiting toxicity was identified with ibrutinib at any dose. This study was followed by a phase IB/II study of ibrutinib, where 85 patients with relapsed or refractory CLL or SLL were treated at two different doses (420 and 840 mg daily).54 This group was heavily pretreated (median, four prior therapies) and had a high proportion of patients with advanced-stage disease and del(17)(p13.1). Similar to the phase I study of ibrutinib, an early increase in lymphocytosis was typically noted by day 7 and persisted for 2 to 3 months before slowly declining over time, concomitant with notable reduction in lymph node size, spleen size, and improvement in cytopenia. The ORR by IWCLL 2008 criteria57 was 71% (CRs, two; PRs, 34) in the 420-mg cohort and 71% (PRs, 24) in the 840-mg cohort. In addition, 10 (20%) and five patients (15%) in the 420- and 840-mg cohorts, respectively, demonstrated a nodal response with persistent lymphocytosis. A subsequent report examining outcomes of patients with persistent lymphocytosis using a landmark analysis did not demonstrate adverse outcomes in this group as compared with those with a PR or better response.58 Response to ibrutinib did not vary based on cytogenetic adverse features previously identified in CLL. Most notably, of the 28 patients with del(17)(p13.1) enrolled onto this study, 18 (68%) responded. The 26-month estimated PFS rate for all patients enrolled onto this study was 75%. Unlike response, PFS did differ by genomic group; 26-month estimated PFS rate was 56% when either del(11)(q22.3) or del(17)(p13.1) was present versus 93% when neither of these abnormalities was present.
Extended therapy with ibrutinib was well tolerated in this trial, with common adverse events including grade 1 to 2 diarrhea, cough, fatigue, upper respiratory infections, nausea, fever, peripheral edema, myalgias, and petechiae or ecchymoses. Although grade ≥ 3 infections occurred frequently in this study, the occurrence decreased with time, where the average rate per 100 patient-months within the first 6 months was 7.1 but 2.6 thereafter. Most problematic was the low frequency of subarachnoid hemorrhages in several patients receiving concomitant warfarin treatment but no other anticoagulants or antiplatelet agents. This unusual, atypical CLL toxicity prompted prohibition of concurrent therapy with oral vitamin K antagonists in all subsequent studies of ibrutinib. In addition, ibrutinib is typically held for 3 to 7 days before and after surgical procedures in a further attempt to avoid bleeding problems. BTK does not seem essential for platelet activation, but a role for BTK inhibition in stable thrombus formulation has been postulated.59 Studies of platelet function in patients with BTK-inactivating mutations have demonstrated abnormalities of collagen- and collagen-related peptide–induced aggregation60 but not bleeding.61 Sorting out the role of ibrutinib in these low-frequency toxicities will require randomized phase III trials with standard therapies in this patient population.
These results demonstrating durable remissions prompted initiation of a large randomized phase III study (RESONATE) comparing ibrutinib monotherapy with ofatumumab in patients with relapsed CLL (Table 2). This trial was recently ended by the data safety and monitoring committee because of the significant benefit seen in both PFS and OS with ibrutinib. On the basis of these collective results, ibrutinib represents the best salvage therapy for relapsed CLL irrespective of genomic risk type unless long-term anticoagulation with warfarin is required. At the present time, data support continuous use of ibrutinib in the absence of progression or toxicity.
Ibrutinib Combination Therapy
The success of ibrutinib monotherapy has prompted several combination trials, in which ibrutinib is administered with therapeutic monoclonal antibodies (eg, rituximab and ofatumumab), chemotherapy (eg, FCR and BR), and select targeted immune therapies (eg, lenalidomide). Of the studies presented to date, combination approaches have generally yielded improved ORRs in part through earlier resolution of persistent lymphocytosis.62–64 However, none of these studies were randomized, and it is unclear if combination therapy will affect response duration versus single-agent therapy. Therefore, at the present time, the combination of ibrutinib with other therapies should not be considered outside of a well-designed clinical trial. Such trials should be directed at inducing MRD-negative CRs to facilitate cessation of therapy or alternatively at preventing resistance in the small subset predisposed to this, such as patients with del(17)(p13.1), del(11)(q22.3), or a complex karyotype.
Idelalisib Therapy
The initial phase I study with idelalisib enrolled 54 patients with relapsed or refractory CLL who were treated with continuous dosing until progression.65 Like with ibrutinib, early lymphocytosis was observed concomitantly with reduction in nodal size. After a median 9 months of drug exposure, the ORR was 39%, with nodal response observed in a larger proportion of patients (81%). The median PFS was 17 months. Dose-limiting toxicities were not observed, and potentially treatment-related adverse events (chiefly fatigue, rash, diarrhea, respiratory tract infections, and reversible elevations of hepatic transaminases) resulted in discontinuation of treatment in only 7% of patients. Subsequent combination studies with rituximab, bendamustine, BR, ofatumumab, and chlorambucil were performed, demonstrating all these approaches resulted in higher ORRs and acceptable toxicity.66 Several phase III studies were initiated, including combination approaches with BR, ofatumumab, and rituximab. The only fully published study administered rituximab with idelalisib or placebo as part of salvage therapy.67 This study was closed prematurely because the combination therapy demonstrated a significantly higher ORR (81% v 13%), PFS rate, and OS rate at 12 months (92% v 80%). Grade 3 diarrhea, rash, and liver function abnormalities were more common with the combination therapy. Although idelalisib is not yet approved for marketing, it is likely that its use will be more limited based on reported shorter duration of remission with the single agent compared with ibrutinib in patients with relapsed CLL with or without del(17)(p13.1). However, market approval is anticipated, and the role of idelalisib in CLL therapy will require further follow-up.
QUESTIONS POSED BY TARGETED THERAPY
As CLL therapy evolves from chemoimmunotherapy to integrate targeted therapy, several questions arise, for which there are few extant data to guide practitioners. Although data are insufficient to make definitive statements, our experience in treating more than 300 patients with ibrutinib or other related BCR signaling agents in CLL allows us to make preliminary comments.
When Should Allogeneic Transplantation Be Considered?
For most patients with CLL who relapse after chemoimmunotherapy, reduced-intensity allogeneic stem-cell transplantation is considered. Given that all patients with CLL without del(17)(p13.1) have a 2-year relapse risk ≤ 25% with ibrutinib, our practice has been to perform relevant preliminary evaluation and education regarding transplantation but not to pursue this unless other therapies are unavailable for cytoreduction in the rare event ibrutinib proves ineffective. However, if patients demonstrate progression with ibrutinib, our practice is always to immediately consider stem-cell transplantation after cytoreduction. Patients with both untreated and relapsed del(17)(p13.1) disease have a 57% 2-year PFS rate and present a more challenging transplantation decision. If transplantation is pursued in this setting, we generally treat with ibrutinib for 1 year to increase the depth of remission before transplantation to avoid rapid tumor flare, which can occur with early cessation of this agent. In addition, an exciting modality that will also be explored for these patients is chimeric antigen T-cell (CAR-T) therapy, which does not require an allogeneic donor. Preliminary activity with CAR-T therapy has been reported in CLL with demonstrated therapeutic efficacy.68 Although cytokine release and tumor lysis syndromes have been observed in the short term, and long-term hypogammaglobulinemia has followed, there is no risk of acute or chronic graft-versus-host disease with this treatment. Integrating ibrutinib with this approach represents an exciting potential opportunity for future clinical investigation.
How Should Patients With Ibrutinib Resistance Be Managed?
Ibrutinib relapse generally occurs in the context of Richter's transformation and, less commonly, CLL progression. In general, progression is manifested by persistent increasing enlargement of lymph nodes or rising lymphocyte count with ibrutinib therapy. Clinicians must use caution in assigning progression to intermittent mild excursions in lymphocyte counts or node size, because these can occur in some patients and do not represent progression. Similarly, transient cessation of ibrutinib because of surgical procedures, infections, or other causes can sometimes cause flare of node size, cytopenia, or lymphocytosis, which quickly resolves with reinstitution of therapy. In most cases of CLL relapse and some cases of Richter's transformation, mutations of either the BTK gene at the C481 residue, where the drug irreversibly binds, or the PLCGγ2 gene, the immediate downstream target of BTK, are found.69 Cessation of ibrutinib therapy in relapsing patients can sometimes result in rapid tumor progression, particularly for patients with previously heavily treated CLL or Richter's transformation. Therefore, our practice is not to discontinue ibrutinib therapy until immediately before the next treatment is initiated. In our experience thus far, patients with CLL with ibrutinib progression have been responsive to other CLL therapies. In contrast, the outcome of Richter's transformation after ibrutinib is poor; responses to other therapies, including investigational agents, have been observed but have often been of brief duration. Allogeneic stem-cell transplantation for Richter's transformation of CLL at any time point in the disease should be strongly considered.
DISCUSSION
After multiple decades of incremental therapeutic advances, new targeted therapies offer the opportunity to revolutionize the treatment of CLL. With new and more effective targeted and immune-directed therapies, it is possible that CLL therapy in the near future for most patients will evolve into treatment that does not involve chemotherapy.
Glossary Terms
- BCR:
B-cell antigen receptor that is a prerequisite for antigen recognition by B cells and for generation of a specific antibody immune response. BCR signaling is involved in the pathogenesis of several B-cell malignancies.
- BTK (Bruton's tyrosine kinase):
a B-cell signaling kinase best known for its involvement in B-cell receptor (BCR) signaling. BTK mutations are the cause for X-linked agammaglobulinemia, a rare primary immunodeficiency.
- ibrutinib:
an irreversible BTK inhibitor.
- minimal residual disease (MRD):
the low level of tumor cells (eg, after chemotherapy) that can only be detected with highly sensitive molecular methods (eg, polymerase chain reaction) or to molecularly defined relapse after long-term remission.
- PI3K (phosphatidylinositol-3 phosphate kinase):
adds a phosphate group to PI3, which is a downstream signaling molecule involved in survival/proliferative pathways mediated by growth factors such as the epidermal growth factor and the platelet-derived growth factors. PI3K is a heterodimeric molecule composed of a regulatory subunit and a catalytic subunit.
- Th2:
a categorization of helper (CD4+) T-cell responses, manifested typically by production of cytokines, including interleukin-4, interleukin-5, and interleukin-10, and with functional importance in supporting generation of B-cell and antibody responses.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by the Four Winds Foundation, D. Warren Brown Foundation, Mr and Mrs Michael Thomas, Harry Mangurian Foundation, Grants No. P50 CA140158, R01 CA177292, R01 CA183444, and R01 CA159296 from the National Institutes of Health, and the Leukemia and Lymphoma Society.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jeffrey J. Jones, Janssen Pharmaceuticals (C), Pharmacyclics (C), Gilead Sciences (C); Jennifer A. Woyach, Pharmacyclics (U) Stock Ownership: None Honoraria: None Research Funding: John C. Byrd, Pharmacyclics, Genentech, Emergent Biosciences; Jeffrey J. Jones, Pharmacyclics, Abott/Genentech, Gilead Sciences; Joseph M. Flynn, Merck, Genentech Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: John C. Byrd, Gilead Sciences
AUTHOR CONTRIBUTIONS
Conception and design: John C. Byrd, Jeffrey J. Jones, Jennifer A. Woyach
Financial support: John C. Byrd
Administrative support: John C. Byrd
Collection and assembly of data: John C. Byrd, Jennifer A. Woyach
Data analysis and interpretation: John C. Byrd, Jennifer A. Woyach, Amy J. Johnson, Joseph M. Flynn
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Chemotherapeutic options in chronic lymphocytic leukemia: A meta-analysis of the randomized trials—CLL Trialists' Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 2.Motiwala T, Zanesi N, Datta J, et al. AP-1 elements and TCL1 protein regulate expression of the gene encoding protein tyrosine phosphatase PTPROt in leukemia. Blood. 2011;118:6132–6140. doi: 10.1182/blood-2011-01-323147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: Justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson FK, Krysov S, Davies AJ, et al. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118:4313–4320. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 5.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quiroga MP, Balakrishnan K, Kurtova AV, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: Specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 8.Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaus GG, Holman M, Johnson-Léger C, et al. A re-evaluation of the effects of X-linked immunodeficiency (xid) mutation on B cell differentiation and function in the mouse. Eur J Immunol. 1997;27:2749–2756. doi: 10.1002/eji.1830271102. [DOI] [PubMed] [Google Scholar]
- 10.Hendriks RW, de Bruijn MF, Maas A, et al. Inactivation of Btk by insertion of lacZ reveals defects in B cell development only past the pre-B cell stage. Embo J. 1996;15:4862–4872. [PMC free article] [PubMed] [Google Scholar]
- 11.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 12.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison VA. Management of infectious complications in patients with chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2007;2007:332–338. doi: 10.1182/asheducation-2007.1.332. [DOI] [PubMed] [Google Scholar]
- 19.Morrison VA. The infectious complications of chronic lymphocytic leukemia. Semin Oncol. 1998;25:98–106. [PubMed] [Google Scholar]
- 20.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–249. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann L, Fenchel K, Jahn B, et al. Immunosuppressive effects and clinical response of fludarabine in refractory chronic lymphocytic leukemia. Ann Oncol. 1993;4:371–375. doi: 10.1093/oxfordjournals.annonc.a058515. [DOI] [PubMed] [Google Scholar]
- 22.Wijermans PW, Gerrits WB, Haak HL. Severe immunodeficiency in patients treated with fludarabine monophosphate. Eur J Haematol. 1993;50:292–296. doi: 10.1111/j.1600-0609.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 23.Kath R, Blumenstengel K, Fricke HJ, et al. Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J Cancer Res Clin Oncol. 2001;127:48–54. doi: 10.1007/s004320000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoda T, Yokoyama A, Yoneda M, et al. Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54:1327–1328. doi: 10.3109/10428194.2012.739285. [DOI] [PubMed] [Google Scholar]
- 25.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanafelt TD, Ramsay AG, Zent CS, et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL) Blood. 2013;121:4137–4141. doi: 10.1182/blood-2012-12-470005. [DOI] [PubMed] [Google Scholar]
- 29.Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: Long-term follow-up of CALGB study 9712. J Clin Oncol. 2013;29:1349–1355. doi: 10.1200/JCO.2010.31.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 32.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: The phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 33.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2013;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 36.Blachly JS, Byrd JC. Emerging drug profile: Cyclin-dependent kinase inhibitors. Leuk Lymphoma. 2013;54:2133–2143. doi: 10.3109/10428194.2013.783911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd JC, Pagel JM, Awan FT, et al. A phase 1 study evaluating the safety and tolerability of otlertuzumab (TRU-016), an anti-CD37 mono-specific ADAPTIRTM therapeutic protein in chronic lymphocytic leukemia. Blood. 2014;123:1302–1308. doi: 10.1182/blood-2013-07-512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapalombella R, Yeh YY, Wang L, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21:694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: A multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 40.Kolibaba KS, Sterchele JA, Joshi AD, et al. Demographics, treatment patterns, safety, and real-world effectiveness in patients aged 70 years and over with chronic lymphocytic leukemia receiving bendamustine with or without rituximab: A retrospective study. Ther Adv Hematol. 2013;4:157–171. doi: 10.1177/2040620713478629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillmen P, Gribben JG, Follows GA, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J Clin Oncol. 2014;32:1236–1241. doi: 10.1200/JCO.2013.49.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foà R, Del Giudice I, Cuneo A, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol. 2014;89:480–486. doi: 10.1002/ajh.23668. [DOI] [PubMed] [Google Scholar]
- 43.Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190:2702–2711. doi: 10.4049/jimmunol.1202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillmen PR, Janssens A, Govindbabu K, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): Results of the phase III study Complement 1. Blood. 2013:122. (abstr 528) [Google Scholar]
- 45.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: An open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrd JC, O'Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:1278–1279. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 48.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CI, Bergsagel PL, Paul H, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brien SM, Lamanna N, Kipps TJ, et al. A phase II study of the selective phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor idelalisib (GS-1101) in combination with rituximab (R) in treatment-naive patients (pts) ≥ 65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) J Clin Oncol. 2013;(suppl 15s):31. abstr 7005. [Google Scholar]
- 51.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 52.Eichhorst B, Fink AM, Busch R, et al. Chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus bendamustine and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): Results of a planned interim analysis of the CLL10 trial, an international, randomized study of the German CLL Study Group (GCLLSG) Blood. 2013:122. (abstr 21) [Google Scholar]
- 53.Pettitt AR, Jackson R, Carruthers S, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: Final results of the National Cancer Research Institute CLL206 trial. J Clin Oncol. 2012;30:1647–1655. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 54.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zelenetz AD, Wierda WG, Abramson JS, et al. Non-Hodgkin's lymphomas, version 1.2013. J Natl Compr Canc Netw. 2013;11:257–272. doi: 10.6004/jnccn.2013.0037. quiz 273. [DOI] [PubMed] [Google Scholar]
- 56.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Fitzgerald ME, Berndt MC, et al. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108:2596–2603. doi: 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quek LS, Bolen J, Watson SP. A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol. 1998;8:1137–1140. doi: 10.1016/s0960-9822(98)70471-3. [DOI] [PubMed] [Google Scholar]
- 61.Futatani T, Watanabe C, Baba Y, et al. Bruton's tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br J Haematol. 2001;114:141–149. doi: 10.1046/j.1365-2141.2001.02905.x. [DOI] [PubMed] [Google Scholar]
- 62.Burger JA, Keating MJ, Wierda WG, et al. Ibrutinib in combination with rituximab (iR) is well tolerated and induces a high rate of durable remissions in patients with high-risk chronic lymphocytic leukemia (CLL): New, updated results of a phase II trial in 40 patients. Blood. 2013:122. (abstr 675) [Google Scholar]
- 63.Jaglowski SM, Jones JA, Flynn JM, et al. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. J Clin Oncol. 2012;30(suppl):418s. abstr 6508. [Google Scholar]
- 64.Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: Final results of a phase 1b study. Blood. 2013:122. (abstr 525) [Google Scholar]
- 65.Brown JR, Furman RR, Flinn I, et al. Final results of a phase I study of idelalisib (GS-1101) a selective inhibitor of PI3Kδ, in patients with relapsed or refractory CLL. J Clin Oncol. 2013;(suppl 15s):31. abstr 7003. [Google Scholar]
- 66.Barrientos JC, Furman RR, Leonard J, et al. Update on a phase I study of the selective PI3Kδ inhibitor idelalisib (GS-1101) in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL. J Clin Oncol. 2013;(suppl 15s):31. abstr 7017. [Google Scholar]
- 67.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]


