Abstract
The onset and symptoms of the myotonic dystrophies are diverse, complicating their diagnoses and limiting a comprehensive approach to their clinical care. This report analyzes the diagnostic delay (time from onset of first symptom to diagnosis) in a large sample of myotonic dystrophy (DM) patients enrolled in the US National Registry [679 DM type 1 (DM1) and 135 DM type 2 (DM2) patients]. Age of onset averaged 34.0 ± 14.1 years in DM2 patients compared to 26.1 ± 13.2 years in DM1 (p<0.0001). The most common initial symptom in DM2 patients was leg weakness (32.6%) compared to grip myotonia in DM1 (38.3%). Pain was reported as the first symptom in 11.1% of DM2 and 3.0% of DM1 patients (p<0.0001). Reaching the correct diagnosis in DM2 took 14 years on average (double the time compared to DM1) and a significantly higher percentage of patients underwent extended workup including electromyography, muscle biopsies, and finally genetic testing. DM patients who were index cases experienced similar diagnostic delays to non-index cases of DM. Further evaluation of how to shorten these diagnostic delays and limit their impact on burdens of disease, family planning, and symptom management is needed.
Keywords: myotonic dystrophy, facioscapulohumeral muscular dystrophy, registry, muscular dystrophy, clinical care guidelines
Introduction
The myotonic dystrophies (DM) are the most common adult muscular dystrophy and can manifest at any age. They have some of the most diverse, disease related symptoms known in medicine [1]. Myotonic dystrophy type 1 (DM1) results from an unstable trinucleotide repeat expansion (CTG)n in the dystrophia myotonica-protein kinase (DMPK) gene located on chromosome 19q13.3 [2–4]. Myotonic dystrophy type 2 (DM2) is due to an unstable four nucleotide repeat expansion (CCTG)n in intron 1 of CNBP/ ZNF9 on chromosome 3q21.3 [5, 6]. Evidence suggests that manifestations of both DM1 and DM2 primarily result from abnormal regulation of alternative splicing and RNA toxicity [7–10].
Manifestations of DM1 and DM2 include dominant inheritance, myotonia, muscle weakness, cataracts, and multi-system complications involving the brain, smooth muscle, and cardiovascular and endocrine systems [1, 10]. Examples of multi-system effects of DM include hypersomnia, cardiac conduction defects, insulin resistance, and complications with anesthesia [1]. The onset of DM1 is commonly in the second and third decades [1, 10]. DM1, however, can manifest in childhood. In addition to muscle weakness, patients with childhood-onset DM1 often have mild to severe learning disability and other cognitive difficulties [11, 12]. The most severe cases are congenital DM1 with infant onset of manifestations, including respiratory distress, feeding problems, speech difficulty, and developmental delay in patients who survive the early months of life [12]. No congenital form exists in DM2. Other distinguishing manifestations of DM2 are proximal weakness, later onset of cognitive symptoms, and more severe muscle pain [13–16]. Cognitive symptoms in both disorders include compromised executive function and avoidant personality [17–22]. However, DM2 has less severe daytime sleepiness and lower risk of complications with anesthesia compared to DM1 [10].
The onset of symptoms in DM2 occurs later in adulthood than in DM1, but both diseases can share a spectrum of similar presenting symptoms. At recent international conferences, data from us [23] and colleagues in Germany [16] have described new insights into the spectrum of initial symptoms that occur in DM2 and new information on the delay in reaching an accurate diagnosis. More information is needed to substantiate these findings, compare diagnostic delays in DM1 versus DM2, and to increase awareness of the wide range of manifestations and onset of DM. Diagnostic delay in DM2 is often challenging because many patients lack core elements of DM, such as, clear dominant inheritance, myotonia on clinical exam, obvious electromyography (EMG) abnormalities, or cataracts [24, 25]. Many symptoms of DM2 resemble the diffuse muscle pain and stiffness associated with other disorders, such as fibromyalgia, and delay accurate diagnosis [26]. With many potential misdiagnosed and undiagnosed patients, the prevalence of DM2 is likely higher than currently assumed [26]. Indeed, new evidence suggests that DM2 may be as prevalent as DM1 in certain countries [27].
In light of these observations, and in the view of breakthroughs in the development of potential new treatments for myotonic dystrophies [28–30], we analyzed diagnostic delay and previous misdiagnoses in a large sample of DM patients (n=814) enrolled in the NIH sponsored National Registry of DM and Facioscapulohumeral Muscular Dystrophy Patients and Family Members. Understanding and preventing delays in diagnosis may reduce the burden of disease, facilitate family planning, improve symptom management in the short term, and facilitate more specific treatment in the long term.
Methods
This study received approval from the Research Subjects Review Board of the University of Rochester and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Registry membership is based on written consent, patient reported information (demographics, symptoms, etc.), and review of each member’s medical record including clinical examinations and laboratory results [31]. Approximately half of the members of the Registry have genetic confirmation of their disease. Patients without DNA testing are classified through stringent review of medical records and based upon current clinical guidelines and family history [31]. These clinically defined patients may more closely reflect the general population of DM, as many patients do not have genetic testing because of lack of health insurance, family history, and concerns about privacy [31].
This study analyzed de-identified data from Registry members classified with genetically proven or clinically defined DM1 and DM2. Congenital DM1 members were excluded from data analysis. Congenital DM1 is classified in the Registry as patients with facial and limb muscle weakness that is apparent within the first 4 weeks of life and who have positive DNA testing or a mother with DNA positive DM1 or a clinically probable diagnosis of DM1.
The medical records of genetically confirmed DM2 members of the Registry were also analyzed through a retrospective chart review to assess potential misdiagnoses. Registry members with DM1 and DM2 were compared with respect to first symptoms, diagnostic tests, and diagnostic delay (time from onset of first symptom to diagnosis). Members are allowed to report up to four first symptoms with only one corresponding age of onset (for example, members may report age of onset at 32 years with grip myotonia, weakness, and fatigue). First symptoms were classified into 28 categories to allow specific clinical details. For example, myotonia was subdivided into categories for grip, jaw, and leg myotonia.
To compare first symptoms with respect to their corresponding diagnostic delays, we chose six symptoms based on core manifestations of DM [1] and their high impact on quality of life [32]. The three core symptoms of DM chosen for analysis were myotonia (aggregated by grip, jaw, general, leg, and tongue), weakness (aggregated by arm, general, leg, facial, cough), and cataracts. The other symptoms analyzed were pain (aggregated by leg, arm, and general), fatigue, and sleep disturbances. Only the first symptom recorded by the patient was analyzed. Separate analyses were performed in DM1 and DM2.
Data are reported as means ± standard deviations or percentages. Group comparisons were performed using Wilcoxon rank sum tests and chi-square tests, as appropriate. Significance was set at α = 0.05 (two-tailed).
Results
The National Registry had 679 DM1 and 135 DM2 members who met our eligibility criteria at the time of data analysis. Average age of onset of symptoms was significantly greater in DM2 members (34.0 ± 14.1 years) compared to DM1 members (26.1 ± 13.2 years; p<0.0001). There were 7 members included in our analyses who reported having onset of symptoms within their first year of life, but they did not have specific muscle weakness and maternal history to meet our classification of congenital DM. Overall, 26% of members (n=215/814) had initial symptoms reported before age 18 years.
Diagnostic delay was greater in DM2 members (14.4 ± 12.8 years) compared to DM1 members (7.3 ± 8.2 years; p<0.0001; Table 1). DM members with initial symptoms reported before age 18 years had greater diagnostic delay (13.0 ± 11.3 years) compared to members with onset after age 18 years (delays of 6.9 ± 8.2 years; p<0.0001). A higher percentage of DM2 patients (49.6%) reported being the first member of their family diagnosed with DM compared to DM1 patients (39.3%; p=0.03). DM2 members who were index cases had similar diagnostic delays (14.8 ± 12.7 years) to non-index DM2 members (13.9 ± 13.2 years; p=0.70). Likewise, index case status in DM1 did not affect diagnostic delay (7.6 ± 8.3 years for index cases versus 7.2 ± 8.2 years for non-index cases; p=0.48).
Table 1.
Characteristics of DM members of the Registry
| DM1 (n=679) |
DM2 (n=135) |
p-value | |
|---|---|---|---|
| Mean ± SD (Range) |
|||
| Age at onset (years) | 26.1 ± 13.2 (0–70) | 34.0 ± 14.1 (6–66) | <0.0001 |
| Age at diagnosis (years) | 33.4 ± 12.5 (4–70) | 48.4 ± 12.2 (19–70) | <0.0001 |
| Diagnostic delay (years) | 7.3 ± 8.2 (0–48) | 14.4 ± 12.8 (0–48) | <0.0001 |
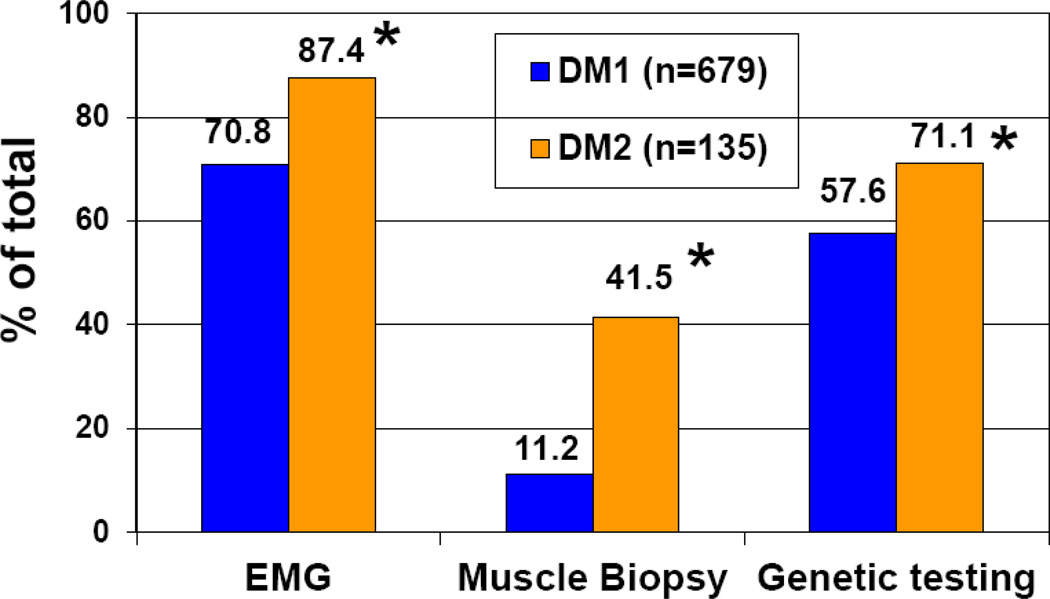
Compared to DM1 members, a significantly higher percentage of DM2 members of the Registry had genetic testing, EMG, and muscle biopsies (Figure 1). For example, 71.1% of DM2 members had received genetic confirmation of their disease compared to 57.6% of DM1 members (p=0.003). We reviewed the medical records of 96 genetically confirmed DM2 members. Based upon this review, the records for 25 (26%) of these DM2 members included a previous misdiagnosis, such as chronic fatigue syndrome, arthritis, limb girdle muscular dystrophy, and other disorders (Table 2).
Fig 1.
Percentages of enrollees who reported undergoing genetic testing, electromyography testing (EMG), and muscle biopsy. The asterisk (*) indicates a significant difference (2 test) between DM1 and DM2 patients for EMG (p<0.0001), muscle biopsy (p<0.0001) and genetic testing results (p=0.003).
Table 2.
Twenty-five of 96 (26%) genetically confirmed DM2 members of the Registry had a previous misdiagnosis based on review of medical records. Limb-girdle muscular dystrophy was the most common misdiagnosis (n=5).
| Previous misdiagnosis | Year of misdiagnosis |
Diagnosing physician |
Diagnostic delay (years) |
|---|---|---|---|
| Acid maltase deficiency | 1995 | Neurologist | 12 |
| Antibody negative Hashimoto's thyroiditis | 2007 | Neurologist | 2 |
| Chronic fatigue syndrome | 2006 | Neurologist | 2 |
| Chronic rhabdomyolysis | 2004 | N/Aa | 7 |
| Charcot-Marie-Tooth disease | 2001 | General practitioner | 48 |
| Degenerative joint disease | 2009 | General practitioner | 18 |
| Dystrophy unknown etiology | 1997 | Neurologist | 18 |
| Exertional muscle pain syndrome | 2003 | Neurologist | 13 |
| Fibromyalgia | 1980 | Neurologist | 28 |
| Graves’ disease and Addison's disease | 1975 & 1994 | Neurologist | 29 |
| Hypokalemic periodic paralysis | N/A | Neurologist | 7 |
| Inclusion body myositis | 2006 | Neurologist | 20 |
| Inclusion body myositis | 2007 | Neurologist | 9 |
| Limb-girdle muscular dystrophy | N/A | N/A | 36 |
| Limb-girdle muscular dystrophy | N/A | Neurologist | 24 |
| Limb-girdle muscular dystrophy | 1997 | Neurologist | 16 |
| Limb-girdle muscular dystrophy | 1996 | N/A | 25 |
| Limb-girdle muscular dystrophy | 2006 | Neurologist | 26 |
| Multiple sclerosis | 2004 | Neurologist | 1 |
| Myasthenia syndrome | 2002 | Neurologist | 6 |
| Nerve damage, unknown etiology | 1998 | N/A | 38 |
| Pseudo-myotonia | 2002 | Neurologist | 35 |
| Rheumatoid arthritis | 2003 | Neurologist | 2 |
| Shoulder girdle dystrophy | 2004 | Neurologist | 1 |
| Weakness due to pregnancy | 1980 | Neurologist | 20 |
N/A = Not available
The majority of patients (n = 510) reported only one first symptom There were 243 patients who reported two initial symptoms, 49 patients with three initial symptoms, 6 patients with four initial symptoms, and 6 patients with unavailable symptom data. The percentages of DM1 and DM2 subjects reporting each first symptom are provided in Table 3. With respect to initial symptoms, a higher percentage of DM2 members relative to DM1 members reported leg weakness (32.6% versus 9.0%; p<0.0001) and any type of pain (11.1% versus 3.0%; p<0.0001). In contrast, a higher percentage of DM1 members relative to DM2 members reported grip myotonia (38.3% versus 17.8; p<0.0001), arm weakness (19.4% versus 8.2%; p=0.002), and facial weakness (4.4% versus 0.7%, p=0.04) as initial symptoms. DM1 members who reported weakness as their first symptom had a shorter diagnostic delay (6.6 ± 8.2 years, n=214) than patients who reported their first symptom as myotonia (7.6 ± 7.6 years, n=271; p=0.02), fatigue (11.5 ± 10.9 years, n=18; p=0.04), and sleep disturbances (15.6 ± 13.4 years, n=5; p=0.05). Diagnostic delay did not differ significantly among the other initial symptom groups in DM1, nor were there any significant differences among groups of DM2 patients with various initial symptoms (p>0.50).
Table 3.
The most common first symptoms reported by patients with DM1 and DM2 are listed from highest to lowest prevalence, along with the corresponding diagnostic delay associated with each first symptom.
| DM1 | DM2 | ||||||
|---|---|---|---|---|---|---|---|
| First symptom | n | % | Diagnostic Delay (years) |
First symptom | n | % | Diagnostic Delay (years) |
| Mean ± SD | Mean ± SD | ||||||
| Myotonia grip | 260 | 38.3% | 7.3 ± 7.6 | Leg weakness | 44 | 32.6 % | 16.3 ± 12.9 |
| Arm weakness | 132 | 19.4% | 6.4 ± 7.3 | Myotonia grip | 24 | 17.8 % | 13.9 ± 10.8 |
| General weakness | 71 | 10.5% | 8.5 ± 9.7 | General weakness | 17 | 12.6 % | 14 ± 12 |
| Leg weakness | 61 | 9.0% | 6.0 ± 9.2 | Arm weakness | 11 | 8.1% | 14.6 ± 16 |
| Falling | 33 | 4.9% | 6.6 ± 9.2 | General myotonia | 9 | 6.7% | 17.8 ± 16.2 |
| Facial weakness | 30 | 4.4% | 9.1 ± 7.6 | Myotonia of the legs, ankle, or foot | 9 | 6.7% | 19.8 ± 15.8 |
| Cataracts | 30 | 4.4% | 6.4 ± 7.0 | Falling | 8 | 5.9% | 12 ± 11.8 |
| Myotonia jaw | 28 | 4.1% | 10 ± 7.6 | Fatigue | 7 | 5.2% | 5.3 ± 7.1 |
| Family member diagnosed | 26 | 3.8% | 7.7 ± 10.7 | Cataracts | 7 | 5.2% | 10.1 ± 9.1 |
| Fatigue | 24 | 3.5% | 11.4 ± 9.8 | General pain | 7 | 5.2% | 13.6 ± 11.4 |
| Cramps | 20 | 2.9% | 8.2 ± 8.9 | Leg pain | 7 | 5.2% | 14.4 ± 15.9 |
| General myotonia | 18 | 2.7% | 7.6 ± 6.2 | Cramps | 6 | 4.4% | 15.7 ± 13.9 |
| Instability or balance | 18 | 2.7% | 5.2 ± 5.7 | Instability/balance | 6 | 4.4% | 22.8 ± 15.1 |
| Myotonia of the legs, ankle, or foot | 15 | 2.2% | 7.5 ± 7.6 | Digestive | 4 | 3.0% | 11.2 ± 16.6 |
| Dysphagia | 13 | 1.9% | 6.8 ± 5.6 | Heart irregularities | 3 | 2.2% | 4.0 ± 4.4 |
| Myotonia tongue | 13 | 1.9% | 5.6 ± 4.1 | Arm or hand pain | 2 | 1.5% | 2.0 ± 2.8 |
| Digestive | 10 | 1.5% | 15.8 ± 10.3 | Hair loss/baldness | 1 | 0.7% | 48 |
| Sleep disturbances | 10 | 1.5% | 13.7 ± 4.3 | Dysphagia | 1 | 0.7% | 4.0 |
| Leg pain | 8 | 1.2% | 8.9 ± 9.7 | Sleep disturbances | 1 | 0.7% | 1.0 |
| Heart irregularities | 7 | 1.0% | 4.0 ± 4.8 | Facial Weakness | 1 | 0.7% | 26 |
| Arm or hand pain | 7 | 1.0% | 6.7 ± 9.8 | Family member diagnosed | 1 | 0.7% | 3.0 |
| Hair loss/baldness | 6 | 0.9% | 10.8 ± 7.0 | ||||
| General pain | 6 | 0.9% | 14.7 ± 18.5 | ||||
| Congenital | 4 | 0.6% | 11.8 ± 5.3 | ||||
| Cough weakness | 1 | 0.1% | 0.0 | ||||
Discussion
This report is our initial description of diagnostic delays in DM1 (7.3 ± 8.2 years) and DM2 (14.4 ± 12.8 years). In addition to these delays in diagnosis, we found that DM2 patients underwent a higher percentage of EMG evaluations and muscle biopsies before specific genetic testing than DM1 patients. Strengths of this study include a large sample of DM patients with confirmed diagnoses by medical record review; a broad age range; equally represented genders; and a broad spectrum of disease symptoms, onset, and severity [31]. Limitations of our report are that enrollment in the Registry is voluntary and, like in other studies, our sample may represent a more motivated patient population. Further studies to confirm our observations are needed. For example, there are opportunities to develop collaborations with the establishment of international registries in DM and other diseases [33, 34] and to develop more epidemiologically based studies, similar to the Center for Disease Control’s Muscular Dystrophy Surveillance, Tracking, and Research Network [35].
Additional studies are needed to evaluate if personal interviews with patients reveal even greater diagnostic delays. This approach allows patients to receive prompting during their interview and encourages them to report any subtle, earlier signs of the disease. Such interviews may reveal even greater diagnostic delays compared to our results. However, previous studies indicate that patient reports of first symptoms provide an appropriate basis for measuring of age of onset regardless of inclusion or exclusion of the index patient [36]. Researchers suggest that such patient reporting is accurate and distinctive because of the spectrum and severity of initial symptoms, especially between siblings and across generations [36]. Indeed, our data show that diagnostic delays were similar between index and non-index patients in both DM1 and DM2. In addition, our data on the age at onset of symptoms in DM2 members (mean = 34 years) were similar to earlier observations (mean = 37 years) [8].
A higher percentage of DM2 members of the Registry than DM1 members reported being the first person of their family diagnosed. These data suggest that previous generations of at-risk or affected DM2 family members were not correctly diagnosed, very mildly affected, or unaffected. Our medical record review of genetically confirmed DM2 members indicated a variety of previous misdiagnoses. Many of the symptoms of these misdiagnoses closely resemble the symptoms and signs observed in DM2, such as the pain and muscle weakness associated with arthritis, chronic fatigue syndrome, and limb girdle muscular dystrophy (Table 2). Most of these misdiagnoses were made by neurologists (76% total). The differing medical histories and potential multi-system manifestations of these patients who were misdiagnosed are not available. One other study has documented misdiagnosis in DM. A study by Finnish investigators tested 63 fibromyalgia patients for the DM2 mutation and reported that two patients tested positive [26]. While our methodological approach was different, we observed that one DM2 patient had a previous misdiagnosis of fibromyalgia. The full range of these misdiagnoses warrants further study to help clinicians and patients better assess the most common symptoms and disorders that may mimic and deter the diagnosis of DM.
Confirmatory clinical data on the types of misdiagnoses common in DM are needed to raise more awareness of early onset manifestations of DM. Improving the diagnosis of DM is crucial in order to meet the short term clinical needs of patients and to prepare for the long term prospects of promising experimental therapies. In the short term, standards of care are needed to facilitate the management of the multisystem manifestations of DM and their broad impact on quality of life. For example, Registry data suggest that pain is an important indicator of the onset of DM2 for patients and care providers to assess. Pain can be especially troublesome and more distinct in DM2 than DM1 as it can fluctuate over time and can be affected by exercise, palpation, and temperature [15, 37]. Another study indicated that 76% of DM2 patients (n=93) have experienced pain that has adversely affected their quality of life [38]. The pathomechanism for this type of pain that frequently occurs in DM2 remains unknown. Better understanding of pain amongst researchers and care providers may facilitate differential diagnoses through new clinical criteria or potential biomarkers for DM2. We need better ways to diagnose this pain, to measure its impact and progression, and to establish optimal pain treatment.
In addition, delays in diagnosis hinder appropriate care of several manifestations of DM that are often disabling, including excessive sleepiness, endocrine disorders, and cognitive symptoms [10]. Many of these symptoms require prompt detection and clinical care to ease burdens of disease, such as disability and unemployment. Delays in diagnoses also impact critical care of DM. More awareness is needed of treatment and preventative care for anesthesia risks [10, 39–41], cardiac conduction disorders and sudden death [42–46], and potential increased rates of certain cancers in DM [47–50].
The urgency for earlier detection of DM is also catalyzed by promising experimental therapies. For example, researchers have recently developed small molecule therapies that reverse myotonia and muscle fiber alterations in a mouse model of DM1 [30]. There is guarded optimism that these results can be translated into human clinical studies. Yet, much work remains to become “trial ready” and to better understand and measure the spectrum and progression of disease manifestations. It is imperative to diagnose patients earlier in the disease course if promising experimental therapies can reverse or delay onset of symptoms and potentially ward off the progression of many disabling manifestations.
In light of these short and long term prospects of care, there is an increased need to adopt new guidelines to lower the threshold of genetic testing and to broaden the consideration of a DM diagnosis in patients [16]. Better understanding of the spectrum of symptoms at onset will facilitate the development of new diagnostic guidelines. Our data suggest longer diagnostic delays if DM1 patients have myotonia, sleep disturbances, or fatigue as their first symptom compared to weakness, although the small sample sizes in some of these subgroups and the problem of multiple subgroup comparisons limit the conclusions that can be drawn. One reason for these longer delays might be that weakness is a complaint that physicians encounter commonly, and they are able to conduct the necessary clinical examinations to quickly identify the pattern and severity of weakness. Clinicians may be much less likely to perform grip myotonia evaluations or thenar muscle percussion testing for myotonia in patients who complain of stiffness or intermittent problems with their grip. Detection of myotonia is often difficult for physicians because grip myotonia is often subtle, variable, and less problematic to patients compared to weakness. Patients often adapt to their myotonia by using warm-up exercise to relieve myotonia, making “less than full effort” grip, or by maneuvering their hands and forearms to facilitate certain activities of daily living, for example, using more of their wrist extensors in combination with short and long finger flexors to overcome difficulty with buttons.
Longer diagnostic delays associated with fatigue and sleep disturbances as initial symptoms could be due to the fact that both symptoms are common and their impact is often high in a variety of neurological disorders, psychological problems, and systemic medical diseases. Follow-up studies with larger sample sizes are needed to determine if first symptoms are associated with other subtle clinical signs of DM. In evaluating patients who lack the core signs of DM (cataracts, myotonia or clear family history), it is important to discuss family history and look for any individuals with weakness or signs of multi-system involvement observed in DM [15].
Many patients with DM present with multi-system problems during their initial clinical exam, for example, reporting various severities of pain, stiffness, and fatigue [1]. These symptoms are commonly reported in the general population, but if reported with subtle weakness or myotonia, they may point to DM as a potential diagnosis. Inquiring about subtle manifestations is complicated in patients with DM because their cognitive symptoms may include apathy or an uncomplaining personality [51]. The patient may downplay or completely overlook their myotonia or mild weakness. The physician needs persistence in questioning the patient and family members about both their understated and most troublesome problems. Often, giving an example to the patient of seemingly unrelated complications (e.g., bowel dysfunction and excessive sleepiness) may help the patient recall these problems. Symptoms that may have the most impact on the lives of patients with DM include fatigue, limitations on mobility, problems with arms or hands, and daytime sleepiness [32]. These past data and our current report suggest that it may be valuable for neurologists to include fatigue, distal weakness, facial weakness, and hypersomnia in targeted questioning of patients to explore a potential DM1 diagnosis. More pertinent questions to probe for DM2 include those related to pain, trouble rising from a chair, or stiffness. These different queries may help guide the neurologist to consider DM and distinguish its subtypes.
The age of onset is another important consideration in the differential diagnosis of DM, with our data confirming previous reports of average onset in the 2nd decade for DM1 and in the 3rd decade for DM2. Our data also show greater diagnostic delays in members who report symptoms before age 18 years. These results indicate that symptoms may appear sooner than expected and awareness of these disorders remains too low amongst neurologists. Detecting cases earlier and preventing misdiagnosis can be facilitated by educating care providers, researchers, patients, and family members about the diverse timing of onset and diversity of symptoms of DM1 and DM2.
Acknowledgements
The Registry is supported through the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Neurological Disorders and Stroke (NIH Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant #U54-NS048843 and NIH contracts #N01-AR-5-2274 and #NO1-AR-0-2250).
Footnotes
Conflicts of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- 1.Harper PS. Myotonic Dystrophy. London: W.B. Saunders Company; 2001. [Google Scholar]
- 2.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T, Sohn R, Zemelman B, Snell RG, Rundle SA, Crow S, Davies J, Shelbourne P, Buxton J, Jones C, Juvonen V, Johnson K, Harper PS, Shaw DJ, Housman DE. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 3.Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P, Wieringa B, Korneluk R, Perryman MB, Epstein HF, Caskey CT. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, Neville C, Narang M, Barcelo J, O'Hoy K, Leblond S, Earle-MacDonald J, De Jong P, Wieringa B, Korneluk R. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 5.Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, Day JW, Ranum LP. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 6.Ranum LP, Rasmussen PF, Benzow KA, Koob MD, Day JW. Genetic mapping of a second myotonic dystrophy locus. Nat Genet. 1998;19:196–198. doi: 10.1038/570. [DOI] [PubMed] [Google Scholar]
- 7.Cho DH, Tapscott SJ. Myotonic dystrophy: emerging mechanisms for DM1 and DM2. Biochim Biophys Acta. 2007;1772:195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Day JW, Ricker K, Jacobsen JF, Rasmussen LJ, Dick KA, Kress W, Schneider C, Koch MC, Beilman GJ, Harrison AR, Dalton JC, Ranum LP. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–664. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 9.Osborne RJ, Thornton CA. RNA-dominant diseases. Hum Mol Genet. 2006;15(Spec No 2):R162–R169. doi: 10.1093/hmg/ddl181. [DOI] [PubMed] [Google Scholar]
- 10.Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- 11.Douniol M, Jacquette A, Cohen D, Bodeau N, Rachidi L, Angeard N, Cuisset JM, Vallee L, Eymard B, Plaza M, Heron D, Guile JM. Psychiatric and cognitive phenotype of childhood myotonic dystrophy type 1. Dev Med Child Neurol. 2012;54:905–911. doi: 10.1111/j.1469-8749.2012.04379.x. [DOI] [PubMed] [Google Scholar]
- 12.Schara U, Schoser BG. Myotonic dystrophies type 1 and 2: a summary on current aspects. Semin Pediatr Neurol. 2006;13:71–79. doi: 10.1016/j.spen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Ricker K, Koch MC, Lehmann-Horn F, Pongratz D, Otto M, Heine R, Moxley RT3. Proximal myotonic myopathy: a new dominant disorder with myotonia, muscle weakness, and cataracts. Neurology. 1994;44:1448–1452. doi: 10.1212/wnl.44.8.1448. [DOI] [PubMed] [Google Scholar]
- 14.Thornton CA, Griggs RC, Moxley RT. Myotonic dystrophy with no trinucleotide repeat expansion. Ann Neurol. 1994;35:269–272. doi: 10.1002/ana.410350305. [DOI] [PubMed] [Google Scholar]
- 15.Meola G, Moxley RT., III Myotonic dystrophy type 2 and related myotonic disorders. J Neurol. 2004;251:1173–1182. doi: 10.1007/s00415-004-0590-1. [DOI] [PubMed] [Google Scholar]
- 16.Udd B, Meola G, Krahe R, Wansink DG, Bassez G, Kress W, Schoser B, Moxley R. Myotonic dystrophy type 2 (DM2) and related disorders Report of the 180th ENMC Workshop including guidelines on diagnostics and management 3–5 December 2010, Naarden, The Netherlands. Neuromuscul Disord. 2011;21:443–450. doi: 10.1016/j.nmd.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Gaul C, Schmidt T, Windisch G, Wieser T, Muller T, Vielhaber S, Zierz S, Leplow B. Subtle cognitive dysfunction in adult onset myotonic dystrophy type 1 (DM1) and type 2 (DM2) Neurology. 2006;67:350–352. doi: 10.1212/01.wnl.0000225180.27833.c1. [DOI] [PubMed] [Google Scholar]
- 18.Minnerop M, Weber B, Schoene-Bake JC, Roeske S, Mirbach S, Anspach C, Schneider-Gold C, Betz RC, Helmstaedter C, Tittgemeyer M, Klockgether T, Kornblum C. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain. 2011;134:3530–3546. doi: 10.1093/brain/awr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franc DT, Muetzel RL, Robinson PR, Rodriguez CP, Dalton JC, Naughton CE, Mueller BA, Wozniak JR, Lim KO, Day JW. Cerebral and muscle MRI abnormalities in myotonic dystrophy. Neuromuscul Disord. 2012;22:483–491. doi: 10.1016/j.nmd.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornblum C, Reul J, Kress W, Grothe C, Amanatidis N, Klockgether T, Schroder R. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol. 2004;251:710–714. doi: 10.1007/s00415-004-0408-1. [DOI] [PubMed] [Google Scholar]
- 21.Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, Cattaneo E, Cotelli M, Gobbo C, Fazio F, Siciliano G, Mancuso M, Vitelli E, Zhang S, Krahe R, Moxley RT. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2) Neuromuscul Disord. 2003;13:813–821. doi: 10.1016/s0960-8966(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 22.Sansone V, Gandossini S, Cotelli M, Calabria M, Zanetti O, Meola G. Cognitive impairment in adult myotonic dystrophies: a longitudinal study. Neurol Sci. 2007;28:9–15. doi: 10.1007/s10072-007-0742-z. [DOI] [PubMed] [Google Scholar]
- 23.Hilbert JE, Luebbe EA, Martens WB, Moxley RT, III Registry Scientific Advisory Committee. Diagnostic odyssey of myotonic dystrophy type 2 (DM2) patients; Poster P4-08; 9th International Myotonic Dystrophy Consortium Meeting; Wurzburg, Germany. Medizinische Genetik. 2009. p. 444. [Google Scholar]
- 24.Milone M, Batish SD, Daube JR. Myotonic dystrophy type 2 with focal asymmetric muscle weakness and no electrical myotonia. Muscle Nerve. 2009;39:383–385. doi: 10.1002/mus.21150. [DOI] [PubMed] [Google Scholar]
- 25.Young NP, Daube JR, Sorenson EJ, Milone M. Absent, unrecognized, and minimal myotonic discharges in myotonic dystrophy type 2. Muscle Nerve. 2010;41:758–762. doi: 10.1002/mus.21615. [DOI] [PubMed] [Google Scholar]
- 26.Auvinen S, Suominen T, Hannonen P, Bachinski LL, Krahe R, Udd B. Myotonic dystrophy type 2 found in two of sixty-three persons diagnosed as having fibromyalgia. Arthritis Rheum. 2008;58:3627–3631. doi: 10.1002/art.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suominen T, Bachinski LL, Auvinen S, Hackman P, Baggerly KA, Angelini C, Peltonen L, Krahe R, Udd B. Population frequency of myotonic dystrophy: higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland. Eur J Hum Genet. 2011;19:776–782. doi: 10.1038/ejhg.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foff EP, Mahadevan MS. Therapeutics development in myotonic dystrophy type 1. Muscle Nerve. 2011;44:160–169. doi: 10.1002/mus.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JE, Bennett CF, Cooper TA. RNase H-mediated degradation of toxic RNA in myotonic dystrophy type 1. Proc Natl Acad Sci U S A. 2012;109:4221–4226. doi: 10.1073/pnas.1117019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler TM, Leger AJ, Pandey SK, MacLeod AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF, Thornton CA. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 2012;488:111–115. doi: 10.1038/nature11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilbert JE, Kissel JT, Luebbe EA, Martens WB, McDermott MP, Sanders DB, Tawil R, Thornton CA, Moxley RT., III If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD) Contemp Clin Trials. 2012;33:302–311. doi: 10.1016/j.cct.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heatwole C, Bode R, Johnson N, Quinn C, Martens W, McDermott MP, Rothrock N, Thornton C, Vickrey B, Victorson D, Moxley R., III Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1) Neurology. 2012;79:348–357. doi: 10.1212/WNL.0b013e318260cbe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinstein YR, Groft SC, Bartek R, Brown K, Christensen RA, Collier E, Farber A, Farmer J, Ferguson JH, Forrest CB, Lockhart NC, McCurdy KR, Moore H, Pollen GB, Richesson R, Miller VR, Hull S, Vaught J. Creating a global rare disease patient registry linked to a rare diseases biorepository database: Rare Disease-HUB (RD-HUB) Contemp Clin Trials. 2010;31:394–404. doi: 10.1016/j.cct.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson R, Schoser B, Monckton DG, Blonsky K, Lochmuller H. Patient Registries and Trial Readiness in Myotonic Dystrophy--TREAT-NMD/Marigold International Workshop Report. Neuromuscul Disord. 2009;19:860–866. doi: 10.1016/j.nmd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Ciafaloni E, Fox DJ, Pandya S, Westfield CP, Puzhankara S, Romitti PA, Mathews KD, Miller TM, Matthews DJ, Miller LA, Cunniff C, Druschel CM, Moxley RT. Delayed diagnosis in duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) J Pediatr. 2009;155:380–385. doi: 10.1016/j.jpeds.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider C, Ziegler A, Ricker K, Grimm T, Kress W, Reimers CD, Meinck H, Reiners K, Toyka KV. Proximal myotonic myopathy: evidence for anticipation in families with linkage to chromosome 3q. Neurology. 2000;55:383–388. doi: 10.1212/wnl.55.3.383. [DOI] [PubMed] [Google Scholar]
- 37.George A, Schneider-Gold C, Zier S, Reiners K, Sommer C. Musculoskeletal pain in patients with myotonic dystrophy type 2. Arch Neurol. 2004;61:1938–1942. doi: 10.1001/archneur.61.12.1938. [DOI] [PubMed] [Google Scholar]
- 38.Suokas KI, Haanpaa M, Kautiainen H, Udd B, Hietaharju AJ. Pain in patients with myotonic dystrophy type 2: a postal survey in Finland. Muscle Nerve. 2012;45:70–74. doi: 10.1002/mus.22249. [DOI] [PubMed] [Google Scholar]
- 39.Gagnon C, Chouinard MC, Laberge L, Veillette S, Begin P, Breton R, Jean S, Brisson D, Gaudet D, Mathieu J. Health supervision and anticipatory guidance in adult myotonic dystrophy type 1. Neuromuscul Disord. 2010;20:847–851. doi: 10.1016/j.nmd.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu J, Allard P, Gobeil G, Girard M, De Braekeleer M, Begin P. Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology. 1997;49:1646–1650. doi: 10.1212/wnl.49.6.1646. [DOI] [PubMed] [Google Scholar]
- 41.Veyckemans F, Scholtes JL. Myotonic Dystrophies type 1 and 2: anesthetic care. Paediatr Anaesth. 2013 doi: 10.1111/pan.12120. [DOI] [PubMed] [Google Scholar]
- 42.Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand R, Otten RF, Bhakta D, Nair GV, Marashdeh MM, Zipes DP, Pascuzzi RM. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- 43.Groh WJ, Bhakta D. Arrhythmia management in myotonic dystrophy type 1. JAMA. 2012;308:337–338. doi: 10.1001/jama.2012.6807. [DOI] [PubMed] [Google Scholar]
- 44.Sansone VA, Brigonzi E, Schoser B, Villani S, Gaeta M, De Ambroggi G, Bandera F, De Ambroggi L, Meola G. The frequency and severity of cardiac involvement in myotonic dystrophy type 2 (DM2): Long-term outcomes. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.11.076. [DOI] [PubMed] [Google Scholar]
- 45.Veyckemans F, Scholtes JL. Myotonic Dystrophies type 1 and 2: anesthetic care. Paediatr Anaesth. 2013 doi: 10.1111/pan.12120. [DOI] [PubMed] [Google Scholar]
- 46.Wahbi K, Meune C, Porcher R, Becane HM, Lazarus A, Laforet P, Stojkovic T, Behin A, Radvanyi-Hoffmann H, Eymard B, Duboc D. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA. 2012;307:1292–1301. doi: 10.1001/jama.2012.346. [DOI] [PubMed] [Google Scholar]
- 47.Das M, Moxley RT, III, Hilbert JE, Martens WB, Letren L, Greene MH, Gadalla SM. Correlates of tumor development in patients with myotonic dystrophy. J Neurol. 2012;259:2161–2166. doi: 10.1007/s00415-012-6476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadalla SM, Lund M, Pfeiffer RM, Gortz S, Mueller CM, Moxley RT, III, Kristinsson SY, Bjorkholm M, Shebl FM, Hilbert JE, Landgren O, Wohlfahrt J, Melbye M, Greene MH. Cancer risk among patients with myotonic muscular dystrophy. JAMA. 2011;306:2480–2486. doi: 10.1001/jama.2011.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller CM, Hilbert JE, Martens W, Thornton CA, Moxley RT, III, Greene MH. Hypothesis: neoplasms in myotonic dystrophy. Cancer Causes Control. 2009;20:2009–2020. doi: 10.1007/s10552-009-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Win AK, Perattur PG, Pulido JS, Pulido CM, Lindor NM. Increased cancer risks in myotonic dystrophy. Mayo Clin Proc. 2012;87:130–135. doi: 10.1016/j.mayocp.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meola G. Myotonic dystrophies as a brain disorder. Neurol Sci. 2010;31:863–864. doi: 10.1007/s10072-010-0414-2. [DOI] [PubMed] [Google Scholar]