Abstract
Objective
To evaluate the effectiveness and safety of GnRH antagonist and GnRH agonist in supposed normal ovarian responders undergoing IVF.
Methods
Data from 6 databases were retrieved for this study. The RCTs of GnRH agonist and GnRH antagonist use during IVF-EF therapy for patients with supposed normal ovarian response were included. A meta-analysis was performed with Revman 5.1software.
Results
Twenty-three RCTs met the inclusion criteria. The number of stimulation days (mean difference (MD): −0.66, 95% confidence interval (CI): −1.04∼−0.27), Gn amount (MD: −2.92, 95% CI: −5.0∼−0.85), E2 values on the day of HCG (MD: −330.39, 95% CI: −510.51∼−150.26), Number of oocytes retrieved (MD: −1.33, 95% CI: −2.02∼−0.64), clinical pregnancy rate (odds ratio (OR): 0.87, 95% CI: 0.75−1.0), and ovarian hyperstimulation syndrome (OHSS) incidence (OR: 0.59, 95% CI: 0.42∼0.82) were significantly lower in GnRH antagonist protocol than GnRH agonist protocol. However, the endometrial thickness on the day of HCG (MD: −0.04, 95% CI: −0.23∼0.14), the ongoing pregnancy rate (OR: 0.87, 95% CI: 0.74∼1.03), live birth rate (OR: 0.89, 95% CI: 0.64∼1.24), miscarriage rate (OR: 1.17, 95% CI: 0.85∼1.61), and cycle cancellation rate (OR: 1.11, 95% CI: 0.90∼1.37) did not significantly differ between the 2 groups.
Conclusions
During IVF treatment for patients with supposed normal responses, the incidence of OHSS were significantly lower, whereas the ongoing pregnancy and live birth rates were similar in the GnRH antagonist compared with the standard long GnRH agonist protocols.
Introduction
It has been over 15 years since gonadotropin-releasing hormone (GnRH) antagonists were first applied in clinical practice in 1999. The debate regarding the efficacy and safety of GnRH antagonists and agonists for in vitro fertilisation - embryo transfer (IVF-ET)continues even today.
The specific binding of the GnRH antagonist to the GnRH pituitary receptor can suppress the surges of luteinising hormone (LH), feature a shorter ovarian stimulation time than the long protocol with a GnRH agonist, require a small amount of Gn, and have no flare-up effect. A systematic review of 5 randomised controlled trials (RCTs), conducted by Al-Inany in 2001 [1], showed that compared with the GnRH agonist long protocol, the GnRH antagonist fixed protocol showed a significantly reduced stimulation time and Gn amount, along with lower oocyte retrieved numbers and clinical pregnancy rates, whereas the incidence of severe ovarian hyperstimulation syndrome (OHSS) was not significantly different between the 2 treatment regimens. A systematic review of 27 RCTs, conducted by Al-Inany in 2006 [2], showed that the clinical pregnancy rate was significantly lower with GnRH antagonist treatment than with the GnRH agonist long protocol, while the differences in the ongoing pregnancy and live birth rates did not significantly differ between the 2 groups; however, the incidence of severe OHSS was significantly lower in the GnRH antagonist group. The live birth rate in a systematic review of 22 RCTs, conducted by Kolibianakis [3], was consistent with the findings reported by Al-Inany [2]. Another systematic review of 45 RCTs, conducted by Al-Inany in 2011 [4], reaffirmed the earlier results by the same author [2] with regard to the ongoing pregnancy and live birth rates and the incidence of severe OHSS. However, a review by Orvieto [5] stated that the ongoing pregnancy and live birth rates were significantly higher in the group treated according to the GnRH agonist long protocol compared to those treated with the GnRH antagonist and that the agonist protocol remained significantly better than the GnRH antagonist protocol. A meta-analysis by Pundir [6] showed that the incidence of moderate and severe OHSS was significantly lower in the GnRH antagonist group than in the GnRH agonist long protocol, while the incidence of severe OHSS was not significantly different.
Controlled ovarian hyperstimulation (COH) is an important component of IVF-ET technology. Different COH protocols would result in different ovarian responses in the same patient. The ovarian response to COH is an important factor that affects the pregnancy outcome, and different ovarian responses would produce different effects on pregnancy. Among the above-described systematic reviews, only the 2011 study by Al-Inany [4] conducted an analysis of all included patients, as well as of low-response and polycystic ovary syndrome (PCOS) subgroups. For all patients, the clinical pregnancy rate was significantly lower with the GnRH antagonist treatment than with the GnRH agonist long protocol, whereas the clinical pregnancy rates in the low-response and PCOS subgroups did not significantly differ, suggesting that the same COH protocol would cause different pregnancy outcomes in patients with different ovarian responses. Other studies [1]–[3], [5], [6] did not perform subgroup analyses based on the different ovarian responses of the patients. Those studies only compared the GnRH agonist and GnRH antagonist treatment regimens while ignoring the patients' characteristics and different pregnancy outcomes due to the different ovarian responses, and therefore, it is difficult to reach a consensus.
This dispute might be effectively resolved by evaluating the differences in the effects of the GnRH antagonist and GnRH agonist protocols based on the predicted ovarian responses of the patients. This study included RCTs of patients with supposed normal ovarian responses to systematically evaluate the effectiveness and safety of the GnRH antagonist and GnRH agonist long protocols for IVF.
Methods
Inclusion and exclusion criteria
The title and abstract of each study were read to filter out literature that obviously did not meet the inclusion criteria. Next, the full text of each study for possible inclusion was read to evaluate the included literature according to the inclusion and exclusion criteria.
All comparisons of the effectiveness and safety of GnRH agonists and GnRH antagonists for IVF in the context of RCTs were included, regardless of whether the blinding method was applied. The literature search was restricted to Chinese- and English-language articles.
Comparative studies of GnRH antagonists and GnRH agonists with other ovulation induction drugs, studies of GnRH antagonists without controls, and studies unrelated to the application effects of GnRH antagonists were excluded.
Studies of patients with a history of more than 3 IVF cycles, low or high ovarian response, PCOS, and severe endometriosis were excluded. Studies of GnRH antagonist in the context of minimal stimulation protocols and oocyte donation cycles were excluded.
The efficacy outcome measures included the number of stimulation days, given as the number of days of simulation; the Gn amount; the E2 value on the day of HCG; the number of oocytes retrieved; the endometrial thickness on the day of HCG; the clinical pregnancy rate, calculated as the number of pregnancies/the number of patients, for which clinical pregnancy was determined according to the detectable foetal heart beat in the intrauterine gestational sac by ultrasound; the ongoing pregnancy rate, which referred to pregnancies with over 12 weeks of gestation; and the live birth rate. The outcome measures of the safety included the incidence of OHSS, the miscarriage rate, and the cycle cancellation rate.
Search strategy
Electronic databases, including PubMed (1997–2013), Cochrane Library (–2013), ProQuest Medical Library (PML; 1997–2013), Foreign Medical Journal Service (FMJS; 2000–2013), the Chinese Biomedical Database (CBM, 1979–2013), China National Knowledge Infrastructure (CNKI; 1994–2013), were all searched using the following keywords: “GnRH antagonist, GnRH-ant, GnRHA, GnRH agonist, GnRHa, IVF, Normal responders, Normoresponder”. The retrieval time was from the first publication of the journal until the end of December 2013. References included in the literature were also searched.
Data extraction and quality assessment
The literature data extraction and quality assessment were independently completed and crosschecked by at least 2 trained qualified reviewers (XJS and SCM). If a disagreement occurred, a solution was achieved in a discussion with the third reviewer (ZXT).
The quality assessment of RCT complied with the assessing standards of risk of bias in the Cochrane Handbook for Systematic Reviews [7] (Version 5.1.0), including six aspects such as sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other biases.
Statistical analysis
The statistical analysis was performed with Revman 5.1 software (Cochrane IMS; available at http://ims.cochrane.org/revman) using Version 5.1.0 of the Cochrane Handbook for Systematic Reviews [7] as a reference. Dichotomous variables were represented as odds ratios (OR), and continuous variables were expressed as mean differences (MD). The 95% confidence interval (CI) was used for all evaluation indicators, with the test level α = 0.05. Heterogeneity was evaluated by means of I2 test, when I 2>50%, the included studies were considered to have large heterogeneity. The studies of non-statistical heterogeneity used the fixed effects model; the others of statistical heterogeneity used the subgroup analysis to find out the reason of the heterogeneity. If there was no clinical heterogeneity or methodological heterogeneity, the random effects model would be used. If there was significant clinical heterogeneity, the descriptive analysis was used. If needed, the sensitivity analysis was used to test stability of results. Publication bias was assessed with Begg's funnel plot carried out using Stata/SE, version 12.
Results
Screening Results
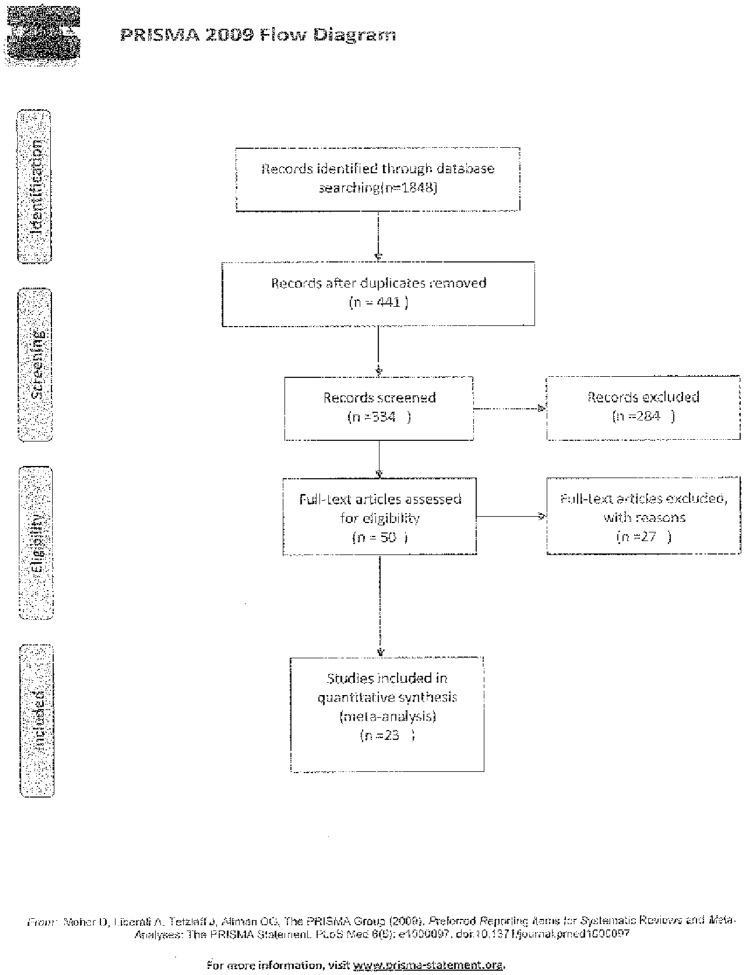
A total of 1,848 studies were initially included in our study. After reading the titles and abstracts, 1,798 studies that did not meet the inclusion criteria or were duplicates were excluded. After reading the full text, 27 papers were excluded, and 23 published studies were ultimately included. The literature screening process and the results are shown in Figure 1. The basic characteristics of the included studies are shown in Table 1.
Figure 1. Prisma flow diagram.
Table 1. Characteristics of included studies.
| Study | Inclusion/Exclusion criteria | No. of patients | Age (y) | bFSH(IU/L) | BMI(kg/m2) | IVF Protocol | |||||
| GnRH-ant | GnRH-a | GnRH-ant | GnRH-a | GnRH-ant | GnRH-a | GnRH-ant | GnRH-a | GnRH-ant | GnRH-a | ||
| Albano et al.,2000 [8] | Normal menstrual cycle, FSH levels <10 IU/L, and previous IVF cycle <3. | 198 | 95 | 31.9±3.7 | 31.6±3.8 | N/A | N/A | N/A | N/A | Multiple dose (cetrorelix) | Long, multiple dose (buserelin) |
| European orgalutran, 2000 [9] | Age<39, with normal menstrual cycles, BMI18–29 kg/m2 | 463 | 238 | 31.9±3.6 | 31.9±3.8 | N/A | N/A | 23.0±2.9 | 23.0±2.7 | Multiple dose (ganirelix) | Long, multiple dose (buserelin) |
| Olivennes et al., 2000 [10] | Normal menstrual cycle, FSH levels <10 IU/L, normal uterus, previous IVF cycle <3. | 126 | 43 | 31.4±3.7 | 31.8±3.8 | 6.3±2.0 | 6.3±1.9 | N/A | N/A | Single dose (cetrorelix) | Long, single dose (triptorelin) |
| Eroupean-Middle East, 2001 [11] | Age<39, with normal menstrual cycles, BMI18–29 kg/m2 | 226 | 108 | N/A | N/A | N/A | N/A | N/A | N/A | Multiple dose (ganirelix) | Long, single dose (triptorelin) |
| North American,2001 [12] | Age <39, with normal menstrual cycles | 198 | 99 | 33.0±3.4 | 32.8±4.0 | N/A | N/A | 23.0±3.0 | 23.0±3.0 | Multiple dose (ganirelix) | Long, multiple dose (leuprorelin) |
| Hohmann et al.,2003 [13] | Previous IVF cycle <3, no previous IVF cycle with a poor response or OHSS. | 48 | 45 | 33(26–38)* | 33(25–39)* | 6.3(2–16)* | 5.5(1.0–10.8* | 24.2 (19.7–28.4)* | 23.0(19.6–28.1)* | Multiple dose (cetrorelix) | Long, single dose (triptorelin) |
| Check et al., 2004 [14] | No description | 24 | 30 | 38.0±5.0 | 32.7±3.8 | N/A | N/A | N/A | N/A | Multiple dose (ganirelix) | Long, multiple dose (leuprorelin) |
| Lee et al.,2004 [15] | Regular menstruation cycles; no history of poor ovarian response or reserve. | 20 | 20 | 31.7±3.8 | 32.8±4.4 | 6.68±1.75 | 6.33±1.41 | 21.76±3.63 | 20.98±2.45 | Multiple dose (cetrorelix) | Long, multiple dose (buserelin) |
| Loutradis et al., 2004 [16] | No low response in a previous treatment cycle, regular menstrual cycles. | 58 | 58 | 35.8±4.9 | 34.9±4.7 | 6.3±1.5 | 6.0±1.3 | N/A | N/A | Multiple dose (cetrorelix) | Long, multiple dose (triptorelin) |
| Sauer et al.,2004 [17] | Regular menstrual cycles, both ovaries present. | 93 | 98 | 32.6±4.0 | N/A | N/A | 24.2±4.5 | Single dose (cetrorelix) | Long, multiple dose (leuprorelin) | ||
| Barmat et al., 2005 [18] | AFC>5 with a menstrual cycle, and failed IVF or IVF/ICSI cycle<1. Patients were excluded from the study if they had a history of previous poor response. | 39 | 41 | 32.4±0.4 | 32.2±0.4 | 6.6±0.3 | 6.3±0.3 | 24.7±0.6 | 23.8±0.5 | Multiple dose (ganirelix) | Long, multiple dose (leuprorelin) |
| Xavier et al., 2005 [19] | previous IVF cycle <3. | 53 | 59 | 31.8±3.0 | 30.6±2.8 | 6.4±1.2 | 6.3±1.0 | N/A | N/A | Multiple dose (cetrorelix) | Long, multiple dose (buserelin) |
| Friedler et al.,2006 [20] | The patients were excluded from the study if they had previous IVF or ICSI, | 56 | 40 | 28.36±3.1 | 28.71±2.8 | 5.54±1.1 | 5.77±1.2 | 27.54±4.3 | 28.1±3.4 | Multiple dose (ganirelix) | Long, multiple dose (buserelin) |
| Serafini et al.,2006 [21] | The presence of two functional ovaries; previous IVF cycle <3; no history of low ovarian response in previous IVF/ICSI treatment. | 93 | 98 | 34.4±0.4 | 33.4±0.3 | 8.0±2.3 | 8.8±2.7 | ≤25 | ≤25 | Multiple dose (cetrorelix) | Long, multiple dose (leuprorelin) |
| Rombauts et al., 2006 [22] | Exclusion criteria included endocrineabnormalities(e.g.PCOS),unsuccessful COS cycles>3, low or no ovarian response. | 110 | 111 | 32.1±3.7 | 32.2±4.0 | N/A | N/A | 23.4±3.0 | 24.2±3.6 | Multiple dose (ganirelix) | Long, multiple dose (Nafarelin) |
| Baart et al., 2007 [23] | Regular menstrual cycles, BMI between 19 and 29 kg/m2 | 56 | 40 | 33.2(22–37)* | 34.1(28–37)* | 7.6(5.5–18.4* | 8.1(4.4–13.8* | N/A | N/A | Multiple dose (orgalutran) | Long, multiple dose (triptorelin) |
| Hsieh et al., 2008 [24] | Age 18–39 years; and body weight of 40–70 kg. | 86 | 58 | 33.9±4.4 | 30.9±2.5 | 4.0±1.8 | 3.8±1.4 | 20.6±1.4 | 20.7±2.1 | Multiple dose (cetrorelix) | Long, multiple dose (leuprorelin) |
| Moraloglu et al.,2008 [25] | Patients were excluded from the study;a history of previous poor response, previous IVF cycles>3, and PCOS. | 45 | 48 | 30.91±5.52 | 30.25±4.94 | 6.63±1.33 | 6.32±1.77 | 29.36±4.45 | 26.58±3.32 | Multiple dose (cetrorelix) | Long, multiple dose (leuprorelin) |
| Depalo et al.,2009 [26] | Absence of uterine or ovarian abnormalities or severe endometriosis or PCOS, previous IVF cycles≤3. | 67 | 69 | 34.4±4 | 34±3.9 | 6.4±2.4 | 5.7±2 | 23.7±4.1 | 22.7±3.4 | Multiple dose (cetrorelix) | Long, multiple dose (triptorelin) |
| Ye et al., 2009 [27] | Previous IVF cycles <3, and no previous poor response to ovarian stimulation; normal ovulatory cycles. | 109 | 111 | 30.3±2.8 | 30.2±2.8 | 6.2±1.6 | 6.5±1.3 | 20.7±1.9 | 21.0±1.8 | Multiple dose (cetrorelix) | Long, multiple dose (triptorelin) |
| Firouzabadi et al.,2010 [28] | The first cycle of the ART, age <35 years, and bFSH <10 IU/L. | 110 | 100 | 28.36±3.1 | 28.71±2.8 | 5.54±1.1 | 5.77±1.2 | 27.54±4.3 | 28.1±3.4 | Multiple dose (ganirelix) | Long, multiple dose (buserelin) |
| Qiao et al.,2012 [29] | Aged ≥18 and ≤35 years, with BMI18–29 kg/m2, a normal menstrual cycle. | 113 | 120 | 29.3±2.8 | 29.1±3.0 | N/A | N/A | 21.3±2.2 | 21.3±2.4 | Multiple dose (ganirelix) | Long, multiple dose (triptorelin) |
| Papanikolaou et al. 2012 [30] | Age<39 years; FSH<12 mIU/ml, previous IVF cycles <3. | 96 | 94 | 32.2±0.3 | 32.8±0.3 | N/A | N/A | N/A | N/A | Multiple dose(ganirelix/cetrorelix) | Long, multiple dose (buserelin) |
bFSH = basal follicle stimulating hormone; BMI = body mass index; IVF = in vitro fertilization; AFC = antral follicle count; ICSI = intracytoplasmic sperm injection; COS = controlled ovarian stimulation; OHSS = ovarian hyperstimulation syndrome; PCOS = polycystic ovary syndrome; ART = assisted reproductive technology; N/A = Not available.
*median.
Quality Assessment
A total of 23 (3,961 cases) RCTs that compared GnRH antagonist and GnRH agonist long protocol treatments were included, The quality assessment of RCT complied with the assessing standards of risk of bias in the Cochrane Handbook for Systematic Reviews V5.1.0, including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and the baseline consistency., as shown in Figure S1 in File S1.
Outcome Measures of the Effectiveness
Number of stimulation days
This outcome measure was included in 16 studies [8]–[12], [15]–[20], [22], [25]–[27], [30] (3,118 cases), and heterogeneity was observed among various trials (P<0.0001, I 2 = 91%). Therefore, a random-effect model was used for the meta-analysis. The results showed that the number of stimulation days was significantly less in the GnRH antagonist group than in the GnRH agonist group; this difference was statistically significant (MD: −0.66, 95%CI: −1.04∼−0.27; P = 0.008; Figure S2 in File S1).
Gn amount
This outcome measure was included in 15 studies [8], [10], [15]–[17], [19]–[28] (2,086 cases), and heterogeneity was observed among various trials (P<0.00001, I 2 = 96%). Therefore, a random-effect model was used for the meta-analysis. The results showed that the Gn amount was significantly less in the GnRH antagonist group than in the GnRH agonist group; this difference was statistically significant (MD: −2.92,95%CI:−5.0∼−0.85; P = 0.006; Figure S3 in File S1).
Endometrial thickness on the day of HCG
This outcome measure was evaluated in 5 studies [15], [19], [20], [27], [28] (655 cases), and no statistical heterogeneity was observed among various trials (P = 0.79, I 2 = 0%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that there was no statistically significant difference in endometrial thickness on the day of HCG between the GnRH antagonist group and the GnRH agonist group (MD: −0.04,95%CI:−0.23∼0.14; P = 0.64; Figure S4 in File S1).
E2 value on the day of HCG
This outcome measure was evaluated in 15 studies [8]–[12], [15]–[19], [21], [24]–[26], [28] (2,807 cases; the unified international standard unit pg/ml was adopted, with a conversion factor of 3.67), and heterogeneity was observed among various trials (P<0.00001, I 2 = 96%). Therefore, a random-effect model was used for the meta-analysis. The results showed that the E2 value on the day of HCG was lower in the GnRH antagonist group than in the GnRH agonist group, and this difference was statistically significant (MD: −330.39,95%CI: −510.51∼−150.26; P = 0.0003; Figure S5 in File S1).
Number of oocytes retrieved
This outcome measure was included in 20 studies [8]–[12], [14]–[16], [19]–[30] (4,328 cases), and heterogeneity was observed among various trials (P<0.00001, I 2 = 88%). Therefore, a random-effect model was used for the meta-analysis. The results showed that the number of oocytes retrieved was lower in the GnRH antagonist group than in the GnRH agonist group, and this difference was statistically significant (MD: −1.33,95%CI: −2.02∼−0.64; P = 0.0001; Figure S6 in File S1).
Clinical Pregnancy rate
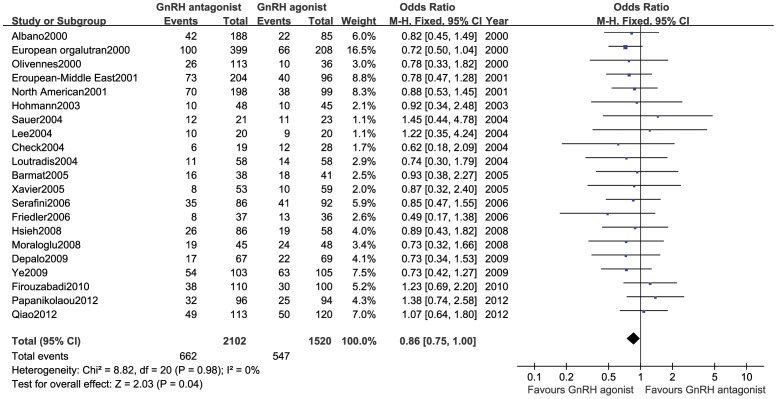
This outcome measure was included in 21 studies [8]–[21], [24]–[30] (3,622 cases), and no statistical heterogeneity was observed among various trials (P = 0.98, I 2 = 0%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that the clinical pregnancy rate was lower in the GnRH antagonist group than in the GnRH agonist group, and this difference was statistically significant (OR:0.86,95%CI:0.75∼1.00; P = 0.04; Figure 2).
Figure 2. Forest plot of the comparison of the GnRH antagonist group versus the GnRH agonist group for clinical pregnancy rate.
Ongoing pregnancy rate
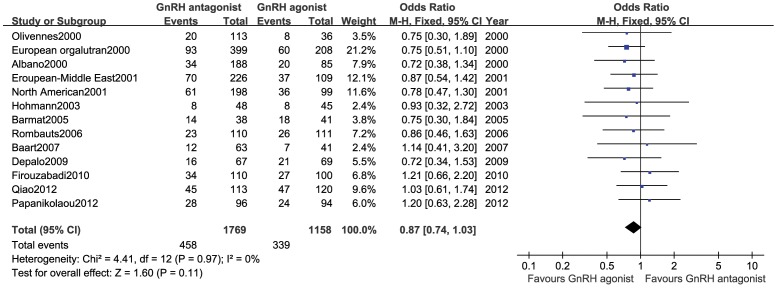
This outcome measure was included in 14 studies [8]–[13], [18], [22], [23], [26], [28]–[30] (2,927 cases), and no statistical heterogeneity was observed among various trials (P = 0.97, I 2 = 0%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that there was no statistically significant difference in the ongoing pregnancy rate between the GnRH antagonist group and the GnRH agonist group (OR:0.87,95%CI:0.74∼1.03; P = 0.11; Figure 3).
Figure 3. Forest plot of the comparison of the GnRH antagonist group versus the GnRH agonist group for ongoing pregnancy rate.
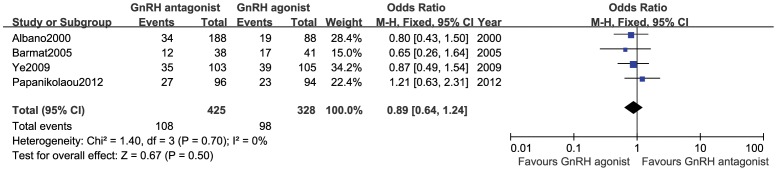
Live birth rate
This outcome measure was included in 4 studies [8], [18], [27], [30] (753 cases), and no statistical heterogeneity was observed among various trials (P = 0.84, I 2 = 0%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that there was no statistically significant difference in the live birth rate between the GnRH antagonist group and the GnRH agonist group (OR:0.89,95%CI:0.64∼1.24; P = 0.50; Figure 4).
Figure 4. Forest plot of the comparison of the GnRH antagonist group versus the GnRH agonist group for live birth rate.
Outcome Measures of the Safety
Incidence of OHSS
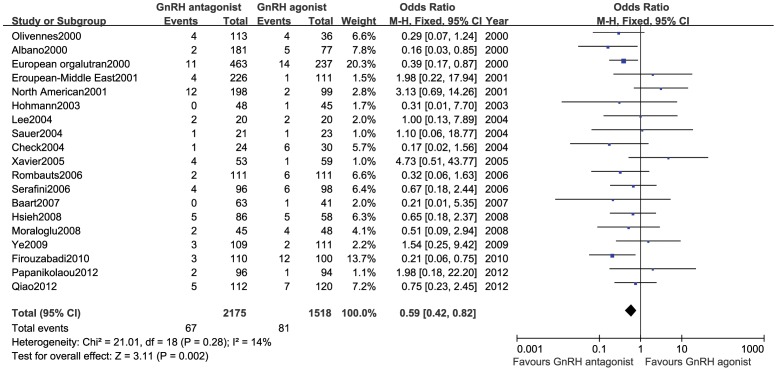
This outcome measure was included in 20 studies [8]–[15], [17], [19], [21]–[25], [27]–[30] (3,693 cases), and no statistical heterogeneity was observed among various trials (P = 0.28, I 2 = 14%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed that the incidence of OHSS was lower in the GnRH antagonist group than in the GnRH agonist group, and this difference was statistically significant (OR:0.59,95%CI:0.42∼0.82; P = 0.002; Figure 5).
Figure 5. Forest plot of the comparison of the GnRH antagonist group versus the GnRH agonist group for incidence of OHSS.
Miscarriage rate
This outcome measure was included in 17 studies [8]–[14], [18], [20], [22], [24], [26]–[30] (2,953 cases), and no statistical heterogeneity was observed among various trials (P = 0.73, I 2 = 0%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed no statistically significant difference in the miscarriage rate between the GnRH antagonist group and the GnRH agonist group (OR:1.17,95%CI:0.85∼1.61; P = 0.34; Figure S7 in File S1).
The cycle cancellation rate
This outcome measure was included in 21 studies [8]–[14], [17]–[23], [25]–[30] (3,823 cases), and no statistical heterogeneity was observed among various trials (P = 0.11, I 2 = 29%). Therefore, a fixed-effect model was used for the meta-analysis. The results showed no statistically significant difference in the cycle cancellation rate between the GnRH antagonist group and the GnRH agonist group (OR:1.11,95%CI:0.90∼1.37; P = 0.33; Figure S8 in File S1).
Sensitivity Analysis and Publication Bias
Excluding the maximum weight studies [9], [21], [27], [28], [30] in the following outcomes, sensitivity analysis showed that there was no statistically significant difference in clinical pregnancy rate between the GnRH antagonist group and the GnRH agonist group. (OR:0.89,95%CI:0.76∼1.04; P = 0.14), but the results of HCG endometrial thickness (MD: −0.12, 95%CI: −0.43∼0.19; P = 0.44), number of oocytes retrieved (MD: −1.56, 95%CI: −2.05∼−1.07; P<0.0001), ongoing pregnancy rate (OR: 0.90, 95%CI: 0.75∼1.09; P = 0.29), live birth rate (OR:0.91,95%CI:0.60∼1.36; P = 0.63), OHSS rate (OR:0.64,95%CI:0.44∼0.92; P = 0.02), miscarriage rate (OR:1.24,95%CI:0.87∼1.76; P = 0.24) and cycle cancellation rate(OR:1.00,95%CI:0.80∼1.26; P = 0.99)were steady. Begg's funnel plot was symmetrical and there was no notable publication bias (Begg's Test P >0.05, Figure S9 in File S1).
Discussion
The results of this systematic review showed that in IVF-EF patients with supposed normal responses, the number of stimulation days, Gn amount, E2 value on the day of HCG, number of oocytes retrieved, and incidence of OHSS were significantly lower with the GnRH antagonist protocol than with the GnRH agonist long protocol. The endometrial thickness on the day of HCG, ongoing pregnancy rate, live birth rate, miscarriage rate, and cycle cancellation rate were similar in the 2 groups. The difference in clinical pregnancy rate between the two groups was uncertain.
Definition of patients with supposed normal responses
The ovarian reserve function is the foundation of ovarian responses to COH. Predictive indicators of this function include the basal follicle stimulating hormone (bFSH), anti-Mullerian hormone (AMH), and inhibin B levels, as well as the antral follicle count (AFC). However, no method is currently available to accurately measure ovarian reserve. Ovarian responses can be roughly divided as high response, normal response, and low response. There remain no clear diagnostic criteria for these 3 types of response. Therefore, definitions of the scopes of these 3 responses are vague. In addition, it is difficult to accurately predict ovarian responses using the currently available approach. Patients defined as having normal responses often presented with the 3 above-described response types to COH. Therefore, the definition of a supposed normal response for the patients included in this study is, to some extent, merely an assumption.
Interpretation of the findings
PCOS, a high AMH level, and a younger age (<35 years) are considered primary risk factors for the occurrence of OHSS; a high blood E2 level during the COH process and an excessive number of follicles on the oocyte retrieved day are considered secondary risk factors for the occurrence of OHSS. Compared with the GnRH agonist protocol, the GnRH antagonist protocol had a shorter stimulation time, lower required Gn amount, lower number of oocytes retrieved, and lower E2 level on the day of HCG, and therefore, the incidence of OHSS was lower in this group.
The patients included in this study were assumed to have normal responses, and the clinical pregnancy rate was significantly lower with the GnRH antagonist protocol than with the GnRH agonist protocol. But the sensitivity analysis showed there was no statistically significant difference in clinical pregnancy rate between the two groups. So it could not conclude that the clinical pregnancy rate of GnRH antagonist protocol was lower than that of GnRH agonist protocol.
Among the patients with low responses, the clinical pregnancy rates were similar in the GnRH antagonist and GnRH agonist groups [4], [31]. Among the PCOS patients, the clinical pregnancy rate of the GnRH antagonist group was similar to that of the GnRH agonist group [4], [32]. A comprehensive analysis of the patients with all response types showed that the clinical pregnancy rate was significantly lower with the GnRH antagonist protocol than with GnRH agonist treatment [2], [4], suggesting that the clinical pregnancy rates could differ between the 2 groups of patients with different response types.
The differences in the ongoing pregnancy and live birth rates between the 2 groups were not statistically significant, suggesting that the final outcome of pregnancy was similar, regardless of whether the GnRH antagonist or GnRH agonist protocol was used.
The differences in the miscarriage and cycle cancellation rates between the 2 groups were not statistically significant, suggesting that these 2 outcome measures were not causes of the difference in the clinical pregnancy rate between the 2 protocols.
The effects of the GnRH antagonist and GnRH agonist protocols on the endometrium have not been clarified [33], [34]. In this study, the difference in endometrial thickness on the day of HCG between the 2 groups was not statistically significant; however, this is insufficient to demonstrate a difference in the impacts of the 2 protocols on the endometrium.
Comparison with existing reviews
In the 2011 study conducted by Al-Inany [4], 45 RCTs (n = 7511) were included to compare the GnRH antagonist and standard long GnRH agonist protocols. However, the patients included in the study of Al-Inany represented all response types, with no exclusion of studies including patients with PCOS and low responses. The patients included in our study were assumed to have normal ovarian responses. The studies of patients with low response and high response (PCOS), GnRH antagonist in the context of minimal stimulation protocols and oocyte donation cycles were excluded. The other two RCTs [29], [30] after 2011 were included in our study. In addition, the study by Al-Inany did not analyse outcome measures, such as the number of stimulation days, Gn amount, E2 value on the day of HCG, number of oocytes retrieved, and endometrial thickness on the day of HCG.
Strength and limitations of this study
The studies included in this systematic review and meta-analysis were screened according to strict criteria. The subjects included patients with supposed normal ovarian responses. Baseline consistency among the treated patients was analysed with regard to age, body mass index (BMI), bFSH, and other factors. The test and control groups in the 23 RCTs were comparable, with a consistent baseline. Single-dose or multiple-dose protocols were used in the GnRH antagonist group, and the standard long protocol was used in the GnRH agonist group. The protocols used in the 2 groups were equivalent and consistent. However, explicit randomised and concealment methods and the blinding method were not used in some studies, and the clinical drug protocols were not identical. All of these discrepancies might lead to bias.
Implications for clinical practice
Regarding efficacy, the overall clinical results obtained with the GnRH antagonist and GnRH agonist standard long protocols were similar, and the GnRH antagonist protocol had the added characteristics of a shorter stimulation time and smaller required amount of Gn. Regarding safety, the incidence of OHSS was significantly lower with the GnRH antagonist protocol than with the GnRH agonist standard long protocol. In general, before determining a clinical treatment plan, the patient's ovarian responsiveness to COH should be considered when developing a personalised treatment regimen based on biological indicators.
Implications for future research
The difference between the GnRH antagonist protocol and GnRH agonist standard long protocol for patients with the same response type requires further clarification; however, RCTs based on patients with different response types to compare the GnRH antagonist protocol and GnRH agonist standard long protocol are currently lacking. Therefore, a multi-centre RCT with a rigorous design is expected in the future.
Conclusion
In conclusion, when used during IVF treatment for patients with supposed normal responses, the GnRH antagonist protocol could significantly reduce the incidence of OHSS while yielding similar ongoing pregnancy and live birth rates compared with those of the GnRH agonist standard long protocol. But it was not sure that there was any difference in clinical pregnancy rate between the two groups. To further clarify the differences between the GnRH antagonist protocol and the GnRH agonist standard long protocol for patients with different types of ovarian responses, a multi-centre RCT with a rigorous design is needed in the future.
Supporting Information
Figure S1 in File S1. Risk of bias for each included study. + = low risk; ? = Unclear; – = high risk. Figure S2 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the number of stimulation days. Figure S3 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the Gn amount(amp). Figure S4 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the endometrial thickness on the day of HCG(mm). Figure S5 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the E2 value on the day of HCG(pg/ml). Figure S6 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the number of oocytes retrieved. Figure S7 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the miscarriage rate. Figure S8 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the cycle cancellation rate. Figure S9 in File S1. Begg's funnel plot of the comparison of the GnRH antagonist group versus GnRH agonist group for the clinical pregnancy rate.
(RAR)
(DOC)
Acknowledgments
The authors thank American Journal Experts for her English translation and editing.
Funding Statement
The authors have no support or funding to report.
References
- 1. Al-Inany HG, Aboulghar M (2001) Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev 4: CD001750. [DOI] [PubMed] [Google Scholar]
- 2. Al-Inany HG, Abou-Setta AM, Aboulghar M (2006) Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev 19: CD001750. [DOI] [PubMed] [Google Scholar]
- 3. Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, et al. (2006) Among patients treated for IVF with gonadotrophins and GnRH analogues,is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update 12: 651–671. [DOI] [PubMed] [Google Scholar]
- 4. Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, et al. (2011) Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 11: CD001750. [DOI] [PubMed] [Google Scholar]
- 5. Orvieto R, Patrizio P (2013) GnRH agonist versus GnRH antagonist in ovarian stimulation: an ongoing debate. Reprod Biomed Online 26: 4–8. [DOI] [PubMed] [Google Scholar]
- 6. Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y (2012) Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online 24: 6–22. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration 2011.
- 8. Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, et al. (2000) Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. European Cetrorelix Study Group. Hum Reprod 15: 526–531. [DOI] [PubMed] [Google Scholar]
- 9. The European Orgalutran Study Group (2000) Borm G, Mannaerts B (2000) Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarianstimulation with recombinant follicle stimulating hormoneis effective, safe and convenient: results of a controlled,randomized, multicentre trial. Hum Reprod 15: 1490–1498. [DOI] [PubMed] [Google Scholar]
- 10. Olivennes F, Belaisch-Allart, Emperare J, Dechaud H, Alvarez S, et al. (2000) Prospective randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LHRH) antagonist (cetrorelix) or a depot formula of an LHRH agonist (triptorelin). Fertil Steril 73: 314–320. [DOI] [PubMed] [Google Scholar]
- 11. European and Middle East Orgalutran Study Group (2001) Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod 16: 644–651. [DOI] [PubMed] [Google Scholar]
- 12. Fluker M, Grifo J, Leader A, Levy M, Meldrum D, et al. (2001) North American Ganirelix Study Group. Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertil Steril 75: 38–45. [DOI] [PubMed] [Google Scholar]
- 13. Hohmann FP, Macklon NS, Fauser BC (2003) A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. The Journal of Clinical Endocrinology and Metabolism 88: 166–173. [DOI] [PubMed] [Google Scholar]
- 14. Check ML, Check JH, Choel JK, Davies E, Kiefer D (2004) Effect of antagonists vs agonists on in vitro fertilization outcome. Clinical and Experimental Obstetrics & Gynecology 31: 257–259. [PubMed] [Google Scholar]
- 15. Lee TH, Wu MY, Chen HF, Chen MJ, Ho HN, et al. (2005) Ovarian response and follicular development for single-dose and multiple-dose protocols for gonadotropin-releasing hormone antagonist administration. Fertil Steril 83: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 16. Loutradis D, Stefanidis K, Drakakis P, Milingos S, Antsaklis A, et al. (2004) A modified gonadotropin-releasing hormone(GnRH) antagonist protocol failed to increase clinical pregnancy rates in comparison with the long GnRH protocol. Fertil Steril 82: 1446–1448. [DOI] [PubMed] [Google Scholar]
- 17. Sauer MV, Thornton MH 2nd (2004) Schoolcraft W, Frishman GN (2004) Comparative efficacy and safety of cetrorelix with or without mid-cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reprod Biomed Online 9: 487–493. [DOI] [PubMed] [Google Scholar]
- 18. Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, et al. (2007) Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22: 980–988. [DOI] [PubMed] [Google Scholar]
- 19. Xavier P, Gamboa C, Calejo L, Silva J, Stevenson D, et al. (2005) A randomised study of GnRH antagonist(cetrorelix) versus agonist (busereline) for controlled ovarian stimulation: effect on safety and efficacy. Eurpean Journal of Obstetrics, Gynecology, and Reproductive Biology 120: 185–189. [DOI] [PubMed] [Google Scholar]
- 20. Friedler S, Gilboa S, Schachter M, Raziel A, Strassburger D, et al. (2006) Luteal phase characteristics following GnRH antagonist or agonist treatment - a comparative study. Reprod Biomed Online 12: 27–32. [DOI] [PubMed] [Google Scholar]
- 21. Serafini P, Yadid I, Motta EL, Alegretti JR, Fioravanti J, et al. (2006) Ovarian stimulation with daily late follicular phase administration of low-dose human chorionic gonadotropin for in vitro fertilization: a prospective, randomized trial. Fertil Steril 86: 830–838. [DOI] [PubMed] [Google Scholar]
- 22. Rombauts L, Healy D, Norman RJ (2006) A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod 21: 95–103. [DOI] [PubMed] [Google Scholar]
- 23. Barmat LI, Chantilis SJ, Hurst BS, Dickey RP (2005) A randomized prospective trial comparing gonadotropin releasing hormone (GnRH) antagonist/recombinant follicle-stimulating hormone (rFSH) versus GnRH-agonist/rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertil Steril 83: 321–330. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh YY, Chang CC, Tsai HD (2008) Comparisons of different dosages of gonadotropin-releasing hormone (GnRH) antagonist, short-acting form and single, half-dose, long acting form of GnRH agonist during controlled ovarian hyperstimulation and in vitro fertilization. Taiwan Journal of Obstetrics and Gynecology 47: 66–74. [DOI] [PubMed] [Google Scholar]
- 25. Moraloglu O, Kilic S, Karayalçin R, Yuksel B, Tasdemir N, et al. (2008) Comparison of GnRH agonists and antagonists in normo-responder IVF/ICSI in Turkish female patients. Advances in Therapy 25: 266–273. [DOI] [PubMed] [Google Scholar]
- 26. Depalo R, Lorusso F, Palmisano M, Bassi E, Totaro I, et al. (2009) Follicular growth and oocyte maturation in GnRH agonist and antagonist protocols for in vitro fertilisation and embryo transfer. Gynecol Endocrinol 25: 328–334. [DOI] [PubMed] [Google Scholar]
- 27. Ye H, Huang G, Zeng P, Pei L (2009) IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. Journal of Assisted Reproduction and Genetics 2: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Firouzabadi RD, Ahmadi S, Oskouian H, Davar R (2010) Comparing GnRH agonist long protocol and GnRH antagonist protocol in outcome the first cycle of ART. Arch Gynecol and Obstet 281: 81–85. [DOI] [PubMed] [Google Scholar]
- 29. Qiao J, Lu G, Zhang HW, Chen H, Ma C, et al. (2012) A randomized controlled trial of the GnRH antagonist ganirelix in Chinese normal responders: high efficacy and pregnancy rates. Gynecol Endocrinol 28: 800–804. [DOI] [PubMed] [Google Scholar]
- 30. Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, et al. (2012) GnRH-agonist versus GnRH-antagonist IVF cycles:is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod 27: 1822–1828. [DOI] [PubMed] [Google Scholar]
- 31. Xiao J, Chang S, Chen S (2013) The effectiveness of gonadotropin-releasing hormone antagonist in poor ovarian responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril 100: 1594–1560. [DOI] [PubMed] [Google Scholar]
- 32. Xiao J, Chen S, Zhang C, Chang S (2013) Effectiveness of GnRH antagonist in the treatment of patients with polycystic ovary syndrome undergoing IVF: a systematic review and meta analysis. Gynecol Endocrinol 29: 187–191. [DOI] [PubMed] [Google Scholar]
- 33. Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, et al. (2010) Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod 82: 679–686. [DOI] [PubMed] [Google Scholar]
- 34. Kuć P, Kuczyńska A, Topczewska M, Tadejko P, Kuczyński W (2011) The dynamics of endometrial growth and the triple layer appearance in three different controlled ovarian hyperstimulation protocols and their influence on IVF outcomes. Gynecol Endocrinol 27: 867–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 in File S1. Risk of bias for each included study. + = low risk; ? = Unclear; – = high risk. Figure S2 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the number of stimulation days. Figure S3 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the Gn amount(amp). Figure S4 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the endometrial thickness on the day of HCG(mm). Figure S5 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the E2 value on the day of HCG(pg/ml). Figure S6 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the number of oocytes retrieved. Figure S7 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the miscarriage rate. Figure S8 in File S1. Forest plot of the comparison of the GnRH antagonist versus GnRH agonist protocol for the cycle cancellation rate. Figure S9 in File S1. Begg's funnel plot of the comparison of the GnRH antagonist group versus GnRH agonist group for the clinical pregnancy rate.
(RAR)
(DOC)