Abstract
Objective
To systematically evaluate the effect of extracorporeal membrane oxygenation (ECMO) on survival in adults with acute respiratory failure (ARF), to help inform institutional decisions about implementing an ECMO program or transferring patients to experienced ECMO centers during the H1N1 influenza pandemic.
Data Sources
National Guideline Clearinghouse, Medline, EMBASE, AHRQ Evidence-based Practice reports, National Institute for Health and Clinical Excellence, Cochrane Library, International Network of Agencies for Health Technology Assessment, citation review.
Study Selection
Studies of ECMO in adult ARF, reporting mortality rates for at least 10 patients in ECMO and non-ECMO groups.
Data Extraction
Mortality rates were abstracted for all patients and for influenza patients. Risk ratios were meta-analyzed using random-effects methods and assessed for heterogeneity.
Data Synthesis
There are no evidence-based clinical guidelines on the use of ECMO in influenza patients. Three randomized controlled trials and three cohort studies evaluated ECMO in ARF patients; none reported specifically on influenza patients. Meta-analysis of the RCTs revealed significant heterogeneity in risk of mortality. The summary risk ratio found by the meta-analysis was 0.93 [95% confidence interval (CI): 0.71 – 1.22]. The most recent trial found a reduction in mortality and severe disability at 6 months among patients in whom ECMO was considered. Observational studies suggest that ECMO for ARF due to viral pneumonia is associated with improved mortality compared with other etiologies of ARF.
Conclusions
The best evidence to guide decisions regarding the use of ECMO for influenza patients stems from trials of ECMO for ARF of all etiologies, among which significant heterogeneity exists, and from case series describing outcomes of ECMO in patients with influenza. Thus, there is insufficient evidence to provide a recommendation for ECMO use among patients with respiratory failure due to influenza. However, clinicians should consider ECMO within the context of other salvage therapies for ARF.
Introduction
In the United States, 700,000 adult patients require mechanical ventilation annually, and mortality rates for the most severe cases of respiratory failure approach 40-50% (1). Based on the experience in Canada (2), Mexico (3), and Australia and New Zealand (4), many young adults who contract the 2009 novel influenza A (H1N1) virus will be admitted to the intensive care unit (ICU) with acute, severe respiratory failure. In addition, when compared historically to other etiologies of acute lung injury, patients with H1N1 who require mechanical ventilation have the potential to rapidly deteriorate and develop refractory hypoxemia necessitating use of salvage therapies, i.e. neuromuscular paralysis, prone positioning, inhaled vasodilators, high-frequency ventilation, and extracorporeal membrane oxygenation (ECMO) (2, 4).
ECMO is a technique that uses a modified heart-lung machine at the bedside to support patients with acute, severe, reversible cardiorespiratory failure. A more general term for this technology is extracorporeal life support (ECLS). ECMO outcomes differ by respiratory failure etiology and an apparent survival benefit exists for ECMO use in potentially reversible cases of respiratory failure, such as status asthmaticus and viral pneumonia such as influenza (5-8). Recently, as evidenced by the ongoing international registry compiled by the Extracorporeal Life Support Organization (ELSO), ECMO has been used more frequently as a rescue modality for patients with refractory hypoxemia due to H1N1-associated respiratory failure (9). Aside from recent advances in ECMO technology and equipment, this popularity likely relates to the epidemiology of critically ill H1N1 cases with a preponderance of young patients who are without significant comorbid conditions. Based on the New Zealand and Australian ECMO published experience for H1N1-associated respiratory failure (4), ECMO has emerged as a viable rescue therapy option that may save lives during the current pandemic. Regrettably, many institutions do not have the equipment, nor the expertise and experience, to offer ECMO as a rescue option. This reality has led to much discussion within and between institutions, as well as within professional societies, regarding the difficult decisions of whether to initiate an ECMO program and whether, and when, to transfer critically ill patients to ECMO-capable institutions. However, there are no published evidence-based clinical practice guidelines or evidence reviews on ECMO for adult patients with acute respiratory failure (ARF) in general or adult patients with influenza more specifically. To address this gap, we performed a systematic review of the literature regarding the use of ECMO in ARF to inform the debate in regards to the use of ECMO for influenza.
Materials and Methods
Literature search
We first searched for evidence-based clinical practice guidelines relating to ECMO. Databases searched included the National Guideline Clearinghouse, Medline, EMBASE, the AHRQ Evidence-based Practice Center evidence reviews, and the National Institute for Health and Clinical Excellence (NICE, UK). Searches used the indexing terms specific to each database for ECMO and practice guidelines. Details of the search strategies are provided in the Appendix.
We then searched for systematic reviews on ECMO. Databases searched included Medline, EMBASE, the Cochrane Library, DARE (Database of Abstracts of Reviews of Effects), and HTA (Health Technology Assessments). In addition, we searched the published reports of 25 different health technology assessment agencies (members of the International Network of Agencies for Health Technology Assessment) for those relating to ECMO. A complete list of agencies is provided in the Appendix, along with search terms and strategies.
We searched for primary studies in the Medline and EMBASE databases. Before searching, we studied each database's indexing terms. Both the MeSH and EMTREE indexing structures used “extracorporeal membrane oxygenation” as their controlled vocabulary term for ECLS, and mapped searches for ECLS to the ECMO indexing term. Test searches verified that the search terms were sensitive. Searches used an “AND” command to combine search results for ECMO with search results for influenza “OR” the keyword ‘H1N1.’ We chose not to restrict our searches for studies of a particular design because we anticipated that few high-quality studies of any single design would be available given the narrowness of our technology and patient criteria.
We also sought to identify studies on ECMO in ARF patients from the evidence tables in published systematic reviews and technology assessment reports. All titles and abstracts were screened by an experienced Center for Evidence-based Practice analyst (MDM), and full text was retrieved for 1) any study that reported on the use of ECMO in influenza patients or 2) controlled trials and cohort studies that reported comparisons between patients with ARF managed with and without ECMO. Only studies that reported mortality rates for patients managed with and without ECMO and included a minimum of 10 patients in each group were included in the systematic review and meta-analysis. Neonatal and pediatric studies (patients under 18 years of age) were excluded. There were no language or data restrictions on the primary literature searches. All literature searches were conducted in October 2009.
Data Analysis
Data from each included study was abstracted by a single research analyst (MDM); abstraction was not performed in duplicate. Mortality was the only patient outcome consistently reported across all the studies. The analyst also graded the methodologic and reporting quality of each included RCT using a nine-point scale combining elements from Jadad's (10) and Chalmers' scales (11). Details of the scoring system are provided in the Appendix. Data abstraction forms were used for study quality assessment, and outcomes data were abstracted directly into evidence tables.
Meta-analysis of randomized trial results followed the methods of the Cochrane Collaboration (12). Mortality rates from each study for ECMO and control groups were expressed as risk ratios, then a summary risk ratio and confidence interval was calculated using random-effects methods in RevMan 5 software. Heterogeneity of effect sizes was assessed using both the chi-squared test and the Higgins I2 test. Critical values were p = 0.10 for the chi-squared test and 50% for the I2 test (13). If either test showed significant heterogeneity, then the summary effect size was considered invalid. There was insufficient data to analyze the possible causes of heterogeneity in study results.
Results
Literature searches
A total of 91 guidelines were screened. None pertained to ECMO in management of patients with influenza or other infectious diseases. Out of 250 reviews screened, 13 relating to ECMO for adult patients with respiratory failure were retrieved, and none were found to meet the inclusion criteria. The reviews did not address ECMO use in ARF due to influenza or other infectious diseases; the most frequent ECMO indication discussed in the reviews was neonatal respiratory distress. Based on title screening, a total of 4 technology assessment reports were retrieved. None of them made specific conclusions about ECMO for patients with influenza or other infectious disease.
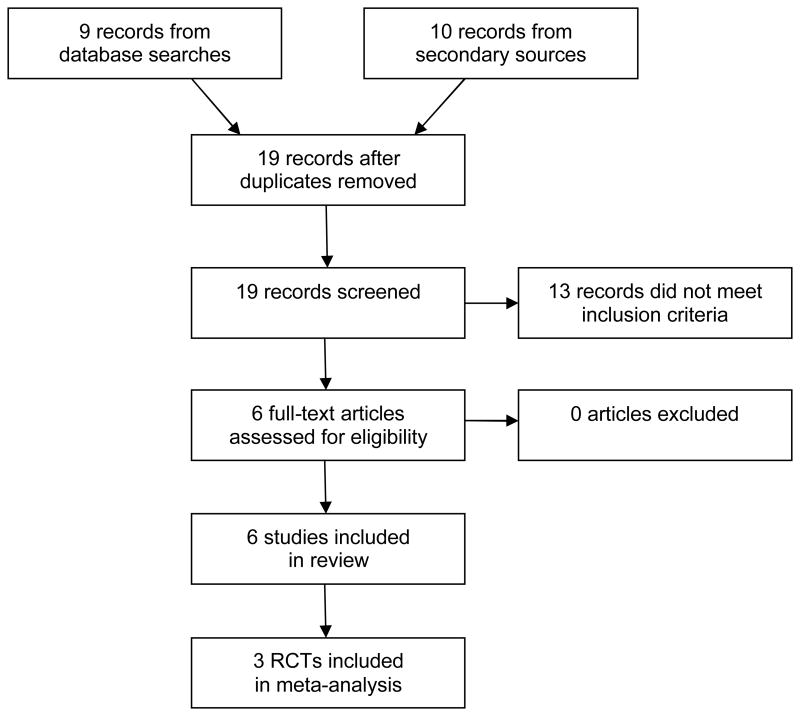
A 2008 systematic review published by Chalwin and colleagues (14), a review prepared for NICE (15), and a review by Steltzer and colleagues (16), came to similar conclusions about the available evidence on this technology: only two randomized trials of ECMO for patients with ARF have been published. We retrieved those articles (17-18) along with a newly published RCT (19). The latter study was in progress at the time Chalwin's review was published. Three other comparison studies of ECMO in patients with ARF were also cited in the reviews (20-22). All of these studies combined patients with different etiologies for ARF, and none reported influenza as a specific cause. The results of our search strategy are summarized in Figure 1.
Figure 1. Flow-Diagram of Search Strategy Results For our Systematic Review of Extracorporeal Membrane Oxygenation for Acute Respiratory Failure.
The searches for primary studies yielded 9 hits; title and abstract screening found that all of them were either commentaries or reports of individual cases. During our search, a highly publicized case series report which was not identified by our systematic review was published on 68 patients with ARF resulting from confirmed or suspected H1N1 flu that were treated with ECMO in Australia or New Zealand (4). In light of our specific focus on influenza-associated ARF, and the observation that published clinical data on this specific indication were scarce, we include these data in our results. Further detailed results of the literature searches, including database hits, articles retrieved, and articles included are presented in the Appendix.
Clinical Evidence
There has been only one published study evaluating ECMO use in ARF due to influenza. This case series from Australia and New Zealand compared 68 patients with novel influenza A (H1N1) and seasonal influenza A treated with ECMO to 133 patients who were given mechanical ventilation (4). The median duration of mechanical ventilation before initiating ECMO was 2 days and the degree of lung injury was severe, as evidenced by severe hypoxemia (median lowest partial pressure of oxygen/fraction of inspired oxygen ratio of 56) and the need for at least one rescue therapy in 81% of the ECMO group. Mortality in the ECMO group was higher than in the non-ECMO group [14/61 (23%) vs.17/133 (13%), p=0.06]. However, patients were selected for ECMO based on the severity of their condition rather than randomly, and patients in the ECMO group included patients transferred from other hospitals to a hospital with ECMO while patients in the non-ECMO group were not transferred. Mortality in this series of ECMO patients was less than the mortality of ECMO patients in other published studies (see Table 1), but this again is not evidence that ECMO is more suitable for these patients than for other patients, because differences in mortality could have resulted from differences in severity of the underlying disease or from other causes. The case series is useful primarily for estimating potential utilization of ECMO for patients with H1N1 infection. The authors of the study conclude that the incidence of H1N1 cases requiring ECMO during the antipodean winter was 2.6 per million population.
Table 1. Studies of ECMO in Patients with Adult Respiratory Failure.
| Article | Study type Quality | Patients | Infectious disease cases | Mortality (influenza cases) | Mortality (all cases) |
|---|---|---|---|---|---|
| Randomized controlled trials | |||||
| CESAR 2009 (19) (multicenter study) | RCT 6 |
†ECMO: 90 Usual care: 90 |
ARDS etiology for ECMO patients: pneumonia 56/90, trauma 5/90, other 29/90 | Not reported | With ECMO: 33/90 (37%) Usual: 45/90 (50%) p = 0.07 RR 0.73 [0.52-1.03] |
| Morris 1994 (18) | RCT 5 | ECMO: 21 Ventilator only: 19 |
ARDS etiology for all patients: pneumonia 24/40, pulmonary embolism 7/40, trauma 6/40, other 11/40 | Not reported | ECMO 14/21 (67%) Vent. only: 11/19 (58%) p = 0.8 RR 1.15 [0.71-1.88] |
| Zapol 1979 (17) (multicenter study) | RCT 4 | ECMO + vent.: 48 Ventilator only: 42 | ARDS etiology for ECMO patients: pneumonia 28/42, pulmonary embolism 3/42, trauma 2/42, other (including sepsis) 9/42 | Not reported | ECMO 38/42 (90%) Vent. only: 44/48 (92%) p = 0.84 RR 0.99 [0.87-1.12] |
| Other studies with comparison | |||||
| Beiderlinden 2006 (22) | Cohort | ECMO: 32 Usual care: 118 |
Patients with community acquired pneumonia (17 ECMO, 30 conservative) reported separately | Not reported | ECMO: 15/32 (47%) Usual: 34/118 (29%) p = 0.06 |
| Mols 2000 (21) | Cohort | ECMO: 62 Non-ECMO: 183 |
ARDS etiology for ECMO patients: pneumonia 36/62, trauma 15/62, sepsis 5/62, other causes 6/62. | Not reported | ECMO 28/62 (45%) Non-ECMO: 71/183 (39%) p = NS |
| Lewandowski 1997 (20) | Cohort | ECMO: 49 Non-ECMO: 73 |
ARDS etiology for ECMO patients: pneumonia 18/49, trauma 13/49, sepsis 5/49, aspiration 7/49, other causes 6/49. | Not reported | ECMO: 22/49 (45%) Non-ECMO: 8/73 (11%) p < 0.0001 |
Study quality rated on nine-point scale: see Appendix.
RR: risk ratio [95% confidence interval]
ECMO available to this group and used if low-pressure ventilation was not sufficient: ECMO actually used in 68 of the 90 patients randomized to ECMO group.
Because of the limited data regarding ECMO use in ARF due to influenza, we broadened the scope of our analysis to include clinical trials and controlled studies for ECMO use in ARF regardless of etiology. Three randomized trials and three cohort studies met the expanded inclusion criteria (Table 1). All of them combined patients with different etiologies for ARF, and none reported influenza or viral pneumonia as a specific cause of ARF. The cohort studies cannot be used to determine the comparative effectiveness of ECMO in ARDS because differences in outcomes could have been the result of differences between patients treated with ECMO and patients treated without ECMO. Only the randomized trials attempted to control for disease severity and other patient variables. The quality of the randomized trials was rated relatively low (4 to 6 points on our nine-point scale), primarily because of the inability to do these trials in a double-blind manner.
Meta-analysis of the three randomized trials found moderate, statistically significant heterogeneity in their reported risk ratios for mortality with and without ECMO (chi-square test p = 0.09, I2 = 58%). This means that differences in patient populations or methods between the studies affected their results (not unexpected, considering the trials were published over a span of 30 years) and that a summary effect size derived from these published results may not reflect the true effect of ECMO on patient mortality. The summary risk ratio found by the meta-analysis was 0.93 [95% confidence interval (CI): 0.71 – 1.22].
The most recent study, the CESAR trial (19), found a reduction in mortality in patients randomized to early consideration of ECMO. Of 766 screened patients, 90 patients were randomly allocated for consideration of the use of ECMO at a single-center, 68 of whom received ECMO support, and 90 patients were randomized to receive conventional management in their conventional treatment center. The primary outcome was death or severe disability measured at 6 months post-randomization. The relative risk of death or severe disability at 6 months in the ECMO group versus control was 0.69, with a wide 95% confidence interval of 0.05 to 0.97; the relative risk of death at 6 months in the ECMO group was 0.73 (95% CI: 0.52 – 1.03).
Discussion
This systematic review of ECMO for ARF reveals insufficient evidence to recommend this modality in managing all patients whose disease is refractory to conventional management. Additionally, whether ECMO should be considered the preferred salvage therapy for severe H1N1-associated respiratory failure remains unknown. Nonetheless, ECMO use has increased in response to the H1N1 pandemic as a potential means to save young lives which may otherwise be lost (9).
We found that significant heterogeneity existed among the three randomized trials of ECMO for ARF. An important limitation of our study is that we were unable to test for sources of heterogeneity. Heterogeneity of reported effects can have a variety of causes, including differences in patient characteristics across studies, differences in how the procedure was performed, differences in reporting, and bias. Unfortunately, isolating a singular cause for the source of observed heterogeneity to permit controlling for the variable identified, such as in a meta-regression, requires a substantially greater number of relevant studies than were identified in this study. However, it is instructive to note that these trials were conducted over a 30 year interval. The changes in trial design, use of background therapies, and case mixes over this interval are informative in and of themselves and provide the context in which to best view the results of these trials.
In the 1979 RCT (17), less than 10% of subjects in both the ECMO and conventional management arms survived. The pioneers who led this multi-center study were criticized for including inexperienced centers. In addition, the investigators were limited by ECMO technology at the time and the benefits of effectively resting the lung had not been clearly demonstrated. ECMO was in its infancy and relied exclusively on veno-arterial (VA) ECMO, which was fraught with severe bleeding complications given the need for excessive anti-coagulant doses. It was also not known at the time that initiation of ECMO after prolonged mechanical ventilation was associated with worse outcomes; patients were randomized to ECMO only after an average of 9.6 days of mechanical ventilation.
Fifteen years later, Morris and colleagues conducted the second ECMO RCT (18). This second RCT utilized VV ECMO exclusively, based on the rationale that VV ECMO may be associated with less organ dysfunction by preserving pulsatile flow and fewer technical complications given the ability to avoid disrupting the arterial system and the availability of improved cannula technology for VV ECMO. Despite improvements in survival for ECMO-treated subjects as compared to the 1979 trial, ECMO did not confer a survival benefit compared to ventilation strategies alone and was associated with significant transfusion requirements. ECMO proponents have criticized this trial for its small size (n=40) and design, as the study was limited to one center and the intervention was coupled to pressure-controlled inverse ratio ventilation, a novel ventilatory strategy at the time of the study. Further, despite participating in the original NIH trial and enlisting the guidance of international experts, this RCT was also criticized for being conducted in a center without significant ECMO experience.
Most recently, following advances in lung protective ventilation (23) and evidence that overall mortality is declining in severe ARF (24), the CESAR investigators conducted a multi-center trial in which patients were transferred to a specialist center in the UK for consideration of early ECMO versus conventional ventilation at the patients' original centers (19). This study found that referral to a single, experienced ECMO center for consideration of ECMO significantly improved the probability of surviving without disability.
There are several important lessons to learn from the published studies. First, outcomes appear to be improved when VV ECMO is used, rather than VA (4-5, 7, 17-19). Admittedly, outcomes may appear better as a result of selection bias, and no evidence from trials exist to support that outcomes are better with VV ECMO. Second, outcomes differ by respiratory failure etiology, and previous studies have suggested that ECMO use for ARF due to viral pneumonia, compared to other etiologies, is associated with a significant survival benefit, with survival rates ranging from 55% to 86% (5-8, 25-26). Recent evidence suggests that H1N1-associated ARF may be associated with improved survival among those in whom ECMO is initiated (4) and the preliminary data from the international H1N1 registry supports this notion (9). Potential explanations for this survival benefit include case-mix (typically, younger patients with fewer comorbidities), increased utilization of VV ECMO, and the potentially reversible nature of influenza-associated ARF. Third, to maximize the benefit of ECMO, the initiation of ECMO needs to be coupled immediately to a lung protective ventilation strategy (19). Fourth, given the known associations between higher patient volume and improved outcomes among patients with respiratory failure (27), it remains difficult to disentangle the potential benefits of ECMO from those associated with receiving care at high-volume adult centers. Further, outcomes may differ by experience level of the ECMO center with more experienced ECMO teams achieving better outcomes (28).
There are several additional limitations to our study. First, although an experienced analyst conducted the search, study screening and data abstraction was not performed in duplicate. Second, there was insufficient data to allow testing for publication bias. Third, although there were no language restrictions on the primary literature search, our search for reviews was limited to the English language.
Conclusions
In summary, there is insufficient evidence to support a general recommendation for ECMO use among patients with respiratory failure due to influenza. However, this therapy might be considered within the context of other salvage therapies for respiratory failure. For example, if hypoxemia (e.g., PaO2 < 55 mmHg) persists despite optimal mechanical ventilation and use of other less invasive rescue therapies (inhaled nitric oxide/prostacyclin, prone positioning, neuromuscular blockade, corticosteroids) (29), there may be benefit to considering ECMO. It is worth noting that these less invasive rescue therapies, which are often used before ECMO (4), may improve oxygenation, yet have no demonstrable mortality benefit either. For clinicians at hospitals that do not have an ECMO program, it would be advisable to establish institutional guidelines to identify ECMO-eligible patients in a timely manner and to establish a relationship with an ECMO-capable institution to facilitate safe inter-hospital transport of these potentially salvageable patients and to be familiar with the general guidelines for ECLS cases and H1N1 cases in particular provided by ELSO (30). Guidelines will need to account for the fact that deterioration is rapid in some etiologies of respiratory failure, such as H1N1 (4). Important questions remain regarding patient selection and determinants of improved outcomes for ECMO and its use in H1N1 in particular. In light of the scarcity of trained physicians, nurses, and perfusionists, as well as other resources required for ECMO use, future studies are needed to define the optimal use of this potentially life-saving intervention.
Supplementary Material
Acknowledgments
Financial support for this work: The study was supported in part by K08 HS018406-01 (SDH), National Institutes of Health, Bethesda, MD, and by Departmental funds, Center for Evidence-Based Practice, University of Pennsylvania Health System.
References
- 1.Zilberberg MD, Luippold RS, Sulsky S, et al. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008 Mar;36(3):724–30. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009 Nov 4;302(17):1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009 Nov 4;302(17):1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 4.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009 Nov 4;302(17):1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 5.Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004 Oct;240(4):595–605. doi: 10.1097/01.sla.0000141159.90676.2d. discussion 605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad SA, Rycus PT, Dalton H. Extracorporeal life support registry report 2004. ASAIO J. 2005 Jan-Feb;51(1):4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen ME, Woo YJ, Sager JS, et al. Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J. 2009 Jan-Feb;55(1):47–52. doi: 10.1097/MAT.0b013e3181901ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehra D, Goldstein AM, Doody DP, et al. Extracorporeal membrane oxygenation for nonneonatal acute respiratory failure: The Massachusetts general hospital experience from 1990 to 2008. Arch Surg. 2009 May;144(5):427–32. doi: 10.1001/archsurg.2009.45. discussion 432. [DOI] [PubMed] [Google Scholar]
- 9.H1N1 ELCS registry. 2010 Jan 25; http://www.elso.med.umich.edu/H1N1.htm.
- 10.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers TC, Smith H, Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981 May;2(1):31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. London: The Cochrane Collaboration; 2008. version 5.0.1. [Google Scholar]
- 13.Higgins JPT: Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalwin RP, Moran JL, Graham PL. The role of extracorporeal membrane oxygenation for treatment of the adult respiratory distress syndrome: Review and quantitative analysis. Anaesth Intensive Care. 2008 Mar;36(2):152–61. doi: 10.1177/0310057X0803600203. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Clinical Excellence. London: Dec, 2002. Interventional procedure overview of extracorporeal membrane oxygenation in adults. http://www.nice.org.uk/nicemedia/pdf/ip/029overview.pdf. [Google Scholar]
- 16.Steltzer H, Krafft P, Fridich P, et al. Severity and outcome of ARDS: the present place of extracorporeal lung assist (ECLA) Int J Artif Organs. 1995 Oct;18(10):607–10. [PubMed] [Google Scholar]
- 17.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979 Nov 16;242(20):2193–6. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 18.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 19.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure. Lancet. 2009;374(9698):1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 20.Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997 Aug;23(8):819–35. doi: 10.1007/s001340050418. [DOI] [PubMed] [Google Scholar]
- 21.Mols G, Loop T, Geiger K, et al. Extracorporeal membrane oxygenation: A ten-year experience. Am J Surg. 2000 Aug;180(2):144–54. doi: 10.1016/s0002-9610(00)00432-3. [DOI] [PubMed] [Google Scholar]
- 22.Beiderlinden M, Eikermann M, Boes T, et al. Treatment of severe acute respiratory distress syndrome: Role of extracorporeal gas exchange. Intensive Care Med. 2006 Oct;32(10):1627–31. doi: 10.1007/s00134-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 23.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. the acute respiratory distress syndrome network. N Engl J Med. 2000 May 4;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 24.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008 May;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 25.Peek GJ, Moore HM, Moore N, et al. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997 Sep;112(3):759–64. doi: 10.1378/chest.112.3.759. [DOI] [PubMed] [Google Scholar]
- 26.Kolla S, Awad SS, Rich PB, et al. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997 Oct;226(4):544–64. doi: 10.1097/00000658-199710000-00015. discussion 565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006 Jul 6;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez TE, Costarino AT, Helfaer MA, et al. The relationship between center extracorporeal membrane oxygenation (ECMO) volume and patient outcomes for neonatal pulmonary ECMO. Crit Care Med. 1999;27(12 Suppl):A120. [Google Scholar]
- 29.Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza: A virus-associated acute respiratory distress syndrome: Response to combination oseltamivir and prolonged corticosteroid treatment. Chest. 2009;136(4 Supp):47S. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ELSO guidelines for cardiopulmonary extracorporeal life support. Extracorporeal Life Support Organization; Ann Arbor, MI: Apr, 2009. Version 1:1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.