Abstract
The pontine parabrachial nucleus (PBN) receives substantial descending input from higher order forebrain regions that exerts inhibitory and excitatory influences on taste-evoked responses. Somatostatin (Sst) and corticotrophin releasing hormone (Crh) reporter mice were used in conjunction with injection of the retrograde tracer CTb-488 into the caudal PBN to determine the extent to which Sst and Crh cell types contribute to the descending pathways originating in the lateral hypothalamus (LH), central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and insular cortex (IC). Five to 7 days following injections, the animals were euthanized and tissue sections prepared for confocal microscopy. Crh cell types in each forebrain site except IC project to the PBN with the greatest percentage originating in the BNST. For Sst cell types, the largest percentage of double-labeled cells was found in the CeA followed by the BNST. Few retrogradely labeled cells in the LH coexpressed Sst, whereas no double-labeled cells were observed in IC. The present results suggest that Sst and Crh cell types are a substantial component of the descending pathways from the amygdala and/or BNST to the PBN and are positioned to exert neuromodulatory effects on central taste processing.
Key words: amygdala, bed nucleus, cortex, hypothalamus, parabrachial, taste
Introduction
The pontine parabrachial nucleus (PBN) receives information about nutritional status from the hypothalamus, as well as gustatory and vagal-derived sensory signals from the nucleus of the solitary tract and integrates these neural signals to bidirectionally influence feeding (Aponte et al. 2011; Atasoy et al. 2012; Wu et al. 2012; Carter et al. 2013; Weiss et al. 2013). The PBN also receives substantial descending input from higher order forebrain regions (Moga et al. 1990a; Tokita et al. 2009; Zhang et al. 2011), which likely plays a role in neural processing of these feeding-related signals. One of the feeding-related signals processed in the PBN and modulated by centrifugal inputs is gustatory information from the oral cavity (Norgren 1974; Lundy and Norgren 2001, 2004; Li and Cho 2006; Tokita et al. 2009).
Prior studies in rats have shown that several neuropeptide cell types in the lateral hypothalamus (LH), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST) including somatostatin, neurotensin, corticotrophin releasing factor, cholecystokinin, enkephalin, and substance P project to the PBN (Veening et al. 1984; Moga and Gray 1985; Moga et al. 1989, 1990a). Using electrophysiologically guided injections of a retrograde tracer, we previously showed that at least two of these peptides, somatostatin and corticotrophin releasing factor, were expressed in CeA and BNST cells that innervated the gustatory responsive region of the PBN (Panguluri et al. 2009). Thus, somatostatin and corticotrophin releasing factor are well positioned to exert neuromodulatory effects on central taste processing. The caveat being that the majority of previous neurophysiological and anatomical studies were conducted using rats.
The mouse is becoming an increasing important model for the study of the gustatory system. The current state of knowledge indicates striking similarity in the anatomical connectivity of the PBN and ventral forebrain in mice compared with rats and hamsters. That is, neurons in the mouse PBN are reciprocally connected to the CeA, BNST, LH, and insular cortex (IC; Tokita et al. 2009, 2010). The neurochemical cell types that comprise these centrifugal pathways have not yet been elucidated. This study used transgenic mouse strains to compare the degree with which somatostatin and corticotrophin releasing hormone cell types of LH, CeA, BNST, and IC origin project to caudal regions of the PBN, the area where neurons responsive to taste stimulation of the anterior tongue are concentrated (Perrotto and Scott 1976; Nishijo and Norgren 1997; Tokita and Boughter 2012; Tokita et al, 2012).
Experimental procedures
Subjects
Two strains of mice, Sst-cre and Crh-cre (Jackson Laboratories, Ssttm2.1(cre)Zjh/J and Crhtm1(cre)Zjh/J, respectively), were bred with floxed-TdTomato mice (Jackson Laboratories, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) to generate two reporter mouse lines that expressed TdTomato in Sst and Crh cell types (Sst/Tdtomato and Crh/TdTomato lines). Three male and three female mice from each reporter line (total 6×2 lines = 12) weighing 18–23g were used in this study. The animals were maintained in a temperature-controlled colony room on a 12-h light/dark cycle and allowed free access to normal rodent chow and distilled water. All procedures conformed to National Institutes of Health guidelines and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Surgery
The mice were anesthetized with an intraperitoneal injection of Ketamine/Xylazine mixture [(100mg/kg (K)/10mg/kg (X)]. If needed, an additional dose of Ketamine (50mg/kg) was administered to continue a deep level of anesthesia. The animals were placed on a feedback-controlled heating pad, and rectal temperature was monitored to maintain body temperature at 37±1°C. Animals were secured in a stereotaxic instrument and the skull was exposed with a midline incision then leveled with reference to bregma and lambda cranial sutures. A small hole was drilled through the bone overlying the cerebellum to allow access to the parabrachial nucleus. The analgesic buprenex (0.1mg/kg) was administered prior to wound incision and again for at least 2 days post-surgery.
Retrograde tracer injection
The caudal PBN was located using the following stereotaxic coordinates relative to bregma, −5.4 posterior, −1.2 lateral, and −2.9 ventral. Injections were performed using a 10-µL nanofil syringe (34-g beveled needle, World Precision Instruments) mounted in a microprocessor-controlled injector (UltraMicroPump III, World Precision Instruments) attached to the stereotaxic instrument. The syringe was first front-filled with light mineral oil followed by a 0.2% solution of cholera toxin subunit B (CTb, Alexa Fluor 488 conjugate, Life Technologies) in 0.1M phosphate-buffered saline. The microprocessor was set to deliver 75 nL of CTb at a rate of 25 nL/min, and the syringe retracted 5min post-injection. Five to 6 days following CTb injection, the animals were administered a lethal dose of Nembutal (150mg/kg) and perfused through the ascending aorta with 8ml of 4% paraformaldehyde with 4% sucrose in 0.1M phosphate buffer (Electron Microscopy Sciences). The brains were removed, blocked just rostral to the PBN, and post-fixed overnight at 4°C in the same fixative. Coronal (50 µm) sections were cut using a freezing microtome.
Data analysis
Cell bodies positive for CTb and TdTomato (fluorescein isothiocyanate; excitation filter: 490nm; barrier filter: 550nm; Cy-3; excitation filter: 520–554nm; barrier filter: 580nm) in the IC, CeA, BNST, and LH were identified using sequential scanning with an Olympus confocal microscope. In every other section, the number of fluorescent positive cells was calculated for each forebrain site and used for statistical analyses. The color segmentation function in Image-Pro Plus software was used to separate and count retrogradely labeled, peptide positive, and double-labeled neurons (Panguluri et al. 2009; Zhang et al. 2011). Briefly, confocal images were opened in Image-Pro Plus and invert contrast applied, which changed the black background to white, the green color of retrogradely labeled cells to pink, the red color of TdTomato cells to turquoise, and double-labeled cells to dark blue/purple. The threshold for counting a cell as singly or double labeled was set to >10 adjacent pixels exhibiting the same color. For each neurochemical, separate one-way analysis of variances were used to compare differences between forebrain sites resulting from caudal PBN injections (SPSS 17.0). The results of Shapiro–Wilk and Levene tests indicated that the data satisfied assumptions of normality and equality of variance, respectively. In some instances, post hoc analyses (least significant difference) were used to determine the source of statistically significant differences. The results are presented as mean ± SE. A value of P < 0.05 was considered statistically significant.
Results
Injection sites
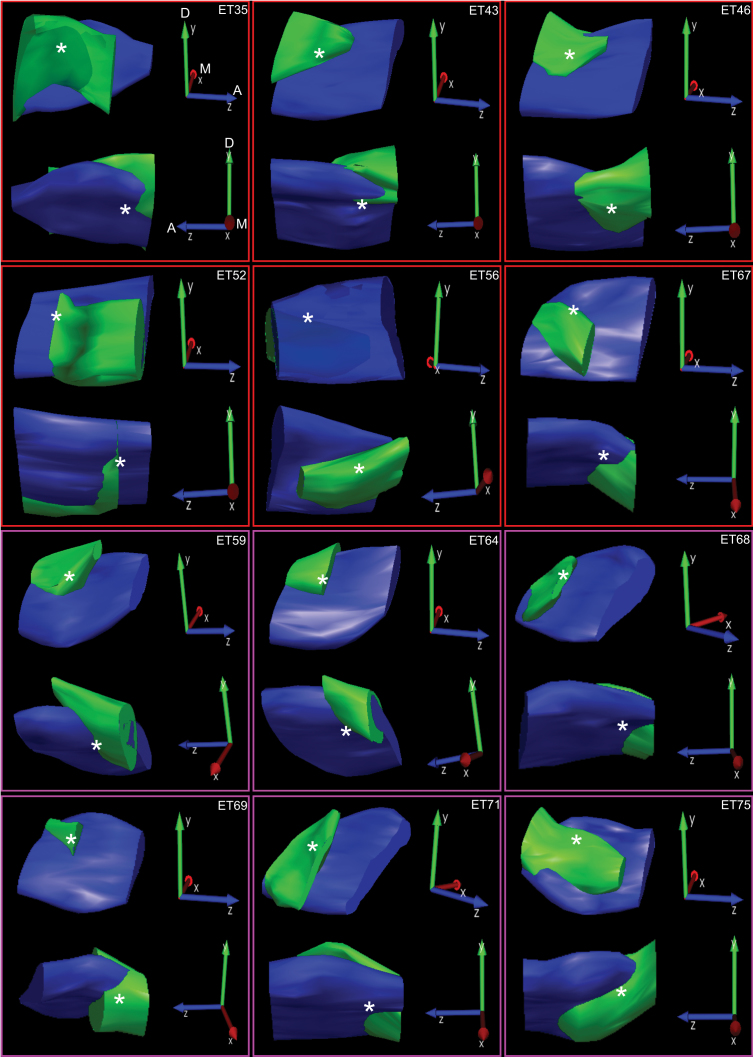
Figure 1 shows photomicrograph examples of CTb-488 injected into the caudal PBN. Images such as these were used to create accurate reconstructions of the location and extent of tracer injections (Figure 2). Each panel contains a medial and lateral view of a Neurolucida 3D reconstruction of CTb-488 injected into the PBN of an individual mouse. To maximize visualization of the CTb-488 injections, the orientations vary somewhat across cases as indicated by the 3-vector axis in each panel. Each injection targeted predominately the medial, ventral lateral, and waist portions of the caudal PBN with minimal spread into the rostral regions. The asterisks in each panel denote the approximate location of the “waist area” in which gustatory responsive neurons are typically encountered (Tokita and Boughter 2012; Tokita et al. 2012). As an example, the 2D images in Figure 1 were used to create the 3D representations shown in the bottom middle panel of Figure 2 (i.e., animal ear tag 71 [ET-71]).
Figure 1.
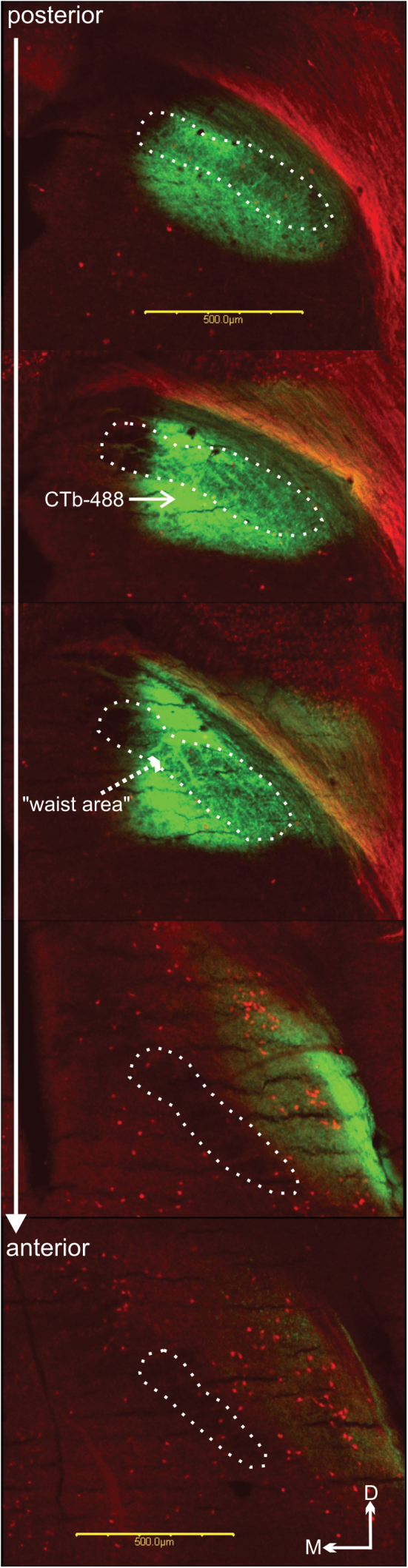
Representative photomicrograph images for ear tag-71 (ET-71) showing CT-488 tracer injection (solid arrow) into the caudal region of the PBN. The approximate boundary of the superior cerebellar peduncle is outlined by white dots. Scale bar equals 500 µm.
Figure 2.
Neurolucida reconstructions of CTb-488 injections into the PBN of Sst/TdTomato mice (ETs 35, 43, 46, 52, 56, and 67) and Crh/TdTomato mice (ETs 59, 64, 68, 69, 71, and 75). The darker solid in each panel represents the contour traced around the superior cerebellar peduncle and the lighter solid the contour traced around the CTb-488 fluorescence. Two 3D representations are shown for each animal; a lateral view (top image in each panel) and a medial view (bottom image in each panel) of the injection. The orientation of the 3D axis shown to the right of each image is as follows: Y arrow points dorsal, Z arrow points anterior, and X arrow points medial.
Distribution of Crh, Sst, and CTb positive neurons
Because the distributions of forebrain-PBN projecting neurons were similar to those described in earlier tracing studies derived mostly from rats, the present findings are only briefly summarized (Moga and Gray 1985; Moga et al. 1989, 1990b; Saggu and Lundy 2008; Kang and Lundy 2009; Tokita et al. 2009). Retrogradely labeled neurons were observed in the LH, CeA, BNST, and IC following injections in the caudal regions of PBN. The IC was identified as the area from bregma to approximately 0.6mm anterior and directly lateral to the claustrum. The BNST was identified as the area approximately 0.3mm anterior and 0.1mm posterior to bregma, directly medial to the internal capsule above the anterior commissure. The CeA was identified as the area approximately 0.7 to 1.9mm posterior to bregma, ventral to the striatum, medial to the basolateral nucleus of the amygdala, and lateral to the optic tract. The LH was identified as the area approximately 1.8 to 2.3mm posterior to bregma, sandwiched between the internal capsule lateral and the fornix medial. Subsequent analyses of cell counts exclude the IC because no evidence was found for coexpression of CTb-488 and Crh or Sst, despite the fact that these peptidergic cell types were intermingled with CTb-labeled projection neurons in IC.
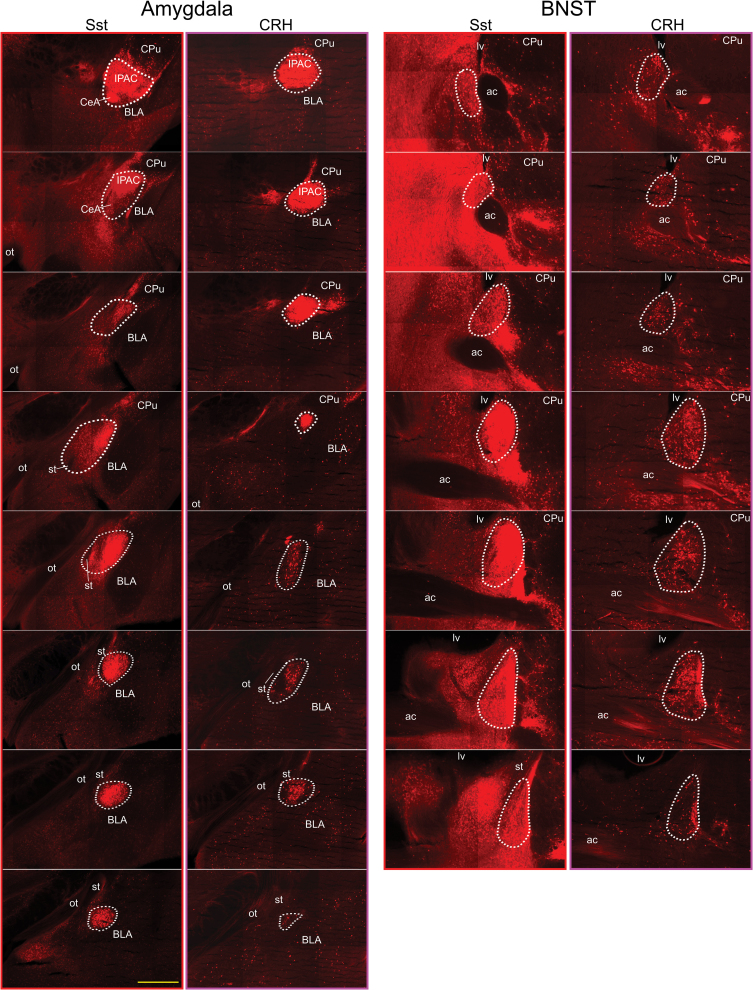
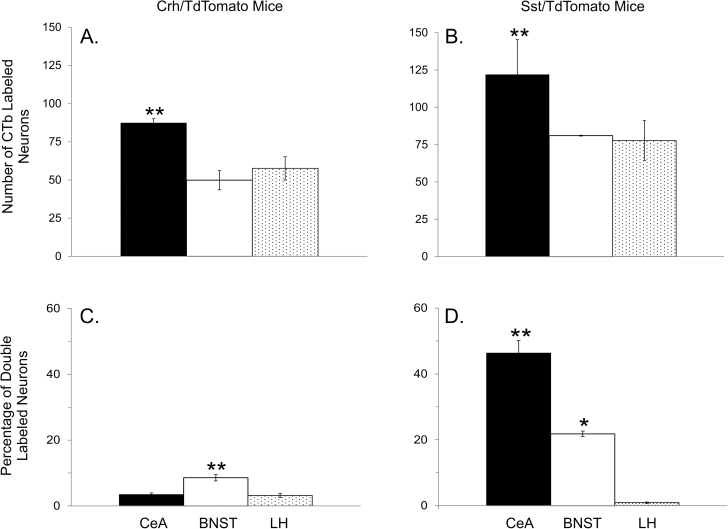
Figure 3 shows photomicrograph examples of TdTomato reporter expression in the CeA and BNST of Sst and Crh mice. Tissue is arranged from rostral to caudal and corresponds to the sections from which cell counts were obtained. Visual inspection indicates a clear difference between Sst and Crh cell types where Sst cells are more densely packed in both forebrain nuclei. In the CeA, CTb-488-labeled cells were found throughout the rostrocaudal extent, whereas in the BNST they were more densely packed around the midline crossing of the anterior commissure with few cells observed in the most rostral sections. In the LH (not shown), CTb-488 cells were scattered between internal capsule lateral and the fornix medial with retrogradely labeled cells, concentrating around the ventral tip of the internal capsule posteriorly. Statistically significant differences were observed in the number of CTb-labeled cells between forebrain areas (Sst/TdTomato mice, F 2,12 = 5.1, P = 0.02; Crh/TdTomato mice, F 2,16 = 6.2, P = 0.01). More neurons were retrogradely labeled in the CeA following tracer injections into the caudal PBN compared with the LH and BNST (Figures 4A and B; P’s ≤ 0.04), which were not different from one another (P’s ≥ 0.4).
Figure 3.
Fluorescent montage images (×10) showing TdTomato expression in the amygdala (left panels) and BNST (right panels) of an Sst/TdTomato mouse and Crh/TdTomato mouse. Tissue sections are arranged from anterior (top) to posterior (bottom) and correspond to the levels from which cell counts were obtained. Medial is to the left and dorsal to the top. The core of fluorescent reporter expression within the CeA and BNST is outlined with white dots. ac, anterior commissure; BLA, basolateral nucleus of the amygdala; CPu, caudate/putamen; IPAC, interstitial nucleus of the posterior limb of the anterior commissure; lv, lateral ventricle; ot, optic tract; st, stria terminalis. Scale bar equals 500 µm.
Figure 4.
Graphs A and B show, respectively, the per section average of retrogradely labeled neurons in the CeA, BNST and LH following injections of CTb-488 into the caudal PBN of Crh/TdTomato and Sst/TdTomato mice. Graphs C and D show, respectively, the per section average percentage of retrogradely labeled neurons that coexpressed Crh or Sst. *, significantly different from LH. **, significantly different from other two brain areas.
Double-labeled neurons
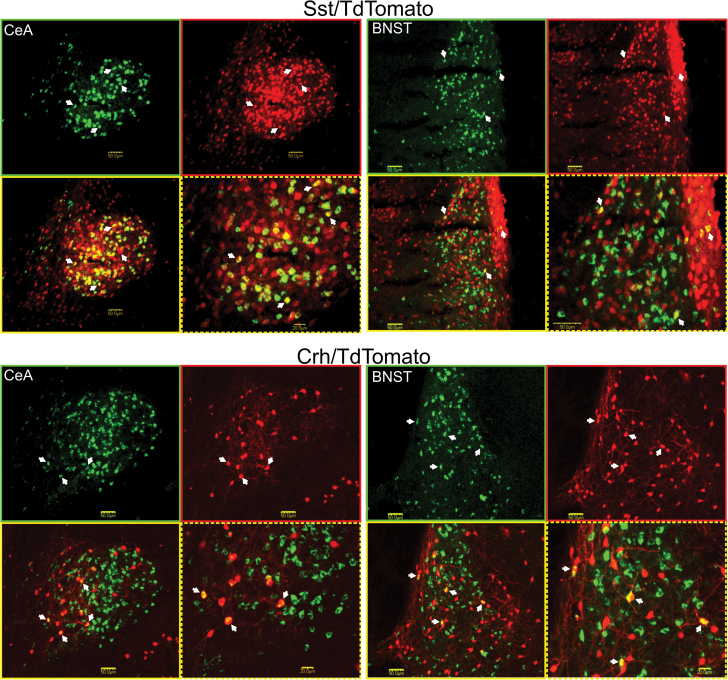
A significant main effect for forebrain site was observed for the percentage of CTb-labeled cells that coexpressed Crh (F 2,16 = 20.0, P < 0.01) and Sst (F 2,12 = 63.5, P < 0.01). The BNST contained a greater percentage of CTb cells that coexpress Crh compared with the CeA and LH (P’s < 0.01), which were not statistically different from one another (Figure 4C, P > 0.5). Across animals, the average number of PBN projection cells that coexpressed Crh was 27.4±6.3 in the BNST, 22.8±3.5 in the CeA and 8.3±1.7 in the LH. In contrast to Crh, the order of greatest percentage of CTb cells that coexpressed Sst was CeA > BNST > LH (Figure 4D, P’s < 0.01). Across animals, the average number of PBN projection cells that coexpressed Sst was 457.8±70.6 in the CeA, 75±10.4 in the BNST and 3.5±0.6 in the LH. Figure 5 shows photomicrograph examples of single- and double-labeled forebrain neurons. The amygdala images from Sst and Crh mice correspond to the posterior part of the CeA (e.g., roughly the 7th section from the top in Figure 3), whereas those for the BNST correspond to the level at which the anterior commissure crosses the midline (e.g., roughly the 5th section from the top in Figure 3). It was at these anatomical levels in which the bulk of double labeled cells were observed. In Sst mice, the percentage of CTb positive cells in the three most caudal sections of the CeA that coexpressed Sst was 70.7±5.8% compared with 33.5±7.5% in the three most rostral sections. In the BNST of Crh mice, the percentage of CTb positive cells that coexpressed Crh was 13.5±2.3% in the three mid-rostrocaudal sections compared with 5.4±1.1% and 3.5±1.8% in the two most caudal and rostral sections, respectively. These data are consistent with previous studies in rats where peptide expressing cells in the CeA that project to the PBN were concentrated in the caudal regions (Panguluri et al. 2009; Moga and Gray 1985; Veening et al. 1984). Our results also indicate spatial organization of BNST-to-PBN peptidergic cells.
Figure 5.
Representative photomicrographs of neurons projecting to the caudal PBN and TdTomato fluorescence in the CeA and BNST of an Sst/TdTomato mouse (top panels) and Crh/TdTomato mouse (bottom panels). In each panel, the image at top left shows CTb-488 retrogradely labeled neurons only, top right TdTomato positive peptidergic cells only, bottom left the merged confocal images, and bottom right higher magnification of the merged images. Single headed arrows point to neurons positive for CTb-488 and Crh or CTb-488 and Sst. A scale bar is shown in each image.
Discussion
The objective of the present experiments was to further our knowledge of the mouse central gustatory system by delineating neurochemical pathways from the forebrain to the caudal PBN. Our results extend prior investigations by showing that corticotrophin releasing hormone and somatostatin cell types in certain forebrain regions are a major source of descending input to caudal regions of the PBN that receive gustatory orosensory signals.
In general, our data are in good agreement with a prior study in mice examining the afferent connections of the PBN (Tokita et al. 2009). Similar to this study, Tokita and colleagues placed retrograde tracer injections centered on the caudal waist region of the PBN and observed labeled neurons in the IC, LH, CeA, and BNST. Neurons projecting to the PBN were found almost exclusively ipsilateral to the injection site in the BNST and CeA, but bilateral with ipsilateral dominance in the IC and LH. Both the present and previous studies indicate that the CeA contained the largest number of neurons projecting to the caudal PBN compared with the other forebrain regions. Together, these results are consistent with previous studies in rats that placed retrograde tracer injections into the caudal gustatory responsive region of the PBN under electrophysiological guidance (Kang and Lundy 2009; Panguluri et al. 2009).
Prior studies investigating descending peptidergic pathways to the PBN in rats have reported somewhat discrepant results in particular concerning coexpression in the CeA. For example, the percentage of retrogradely labeled cells in the CeA that coexpressed Sst-ir ranged from 6% to 50% (Veening et al. 1984; Moga and Gray 1985; Panguluri et al. 2009), whereas those coexpressing Crh-ir ranged from 11% to 66% (Moga and Gray 1985; Panguluri et al. 2009). For the LH, 9–21% of retrogradely labeled cells were found to be immunoreactive for Crh, but only 1—4% were immunoreactive for Sst (Moga et al. 1990b; Panguluri et al. 2009). Finally, 14–20% of retrogradely labeled cells in the BNST were Crh-ir, whereas 4–11% were Sst-ir (Moga et al. 1989; Panguluri et al. 2009). Some of this inconsistency might be related to the size of retrograde tracer injections into the PBN and, thus, the region of the PBN targeted.
A previous study from our laboratory using electrophysiology to guide small injections of retrograde tracer that targeted either the caudal gustatory responsive or the rostral non-gustatory responsive regions of the PBN in rats reported difference in terms of peptide coexpression (Panguluri et al. 2009). Compared with caudal PBN injections, injections into the rostral PBN produced a significantly greater percentage of cells that coexpressed retrograde tracer and Crh-ir or Sst-ir in the BNST and CeA. The largest discrepancy between our previous data set in rat, and the present mouse data set relates to peptide coexpression in the CeA and IC. The present results indicate that a far greater of percentage of Sst cell types in the CeA project to the caudal PBN in mice (46.3±3.8%) compared with that observed in rats (6.2±1.1%). Moreover, a small percentage of Crh cell types in the IC of rats projected to the caudal PBN, but not in the present mouse study. These inconsistencies might represent species differences or differences in experimental approaches such as different retrograde tracers, tracer injection techniques, and/or approaches to reveal peptidergic expression.
Although each of the circuits investigated in this study are known to participate in ingestive behavior (Roth et al. 1973; Schwartz and Teitelbaum 1974; Roldan and Bures 1994; Zardetto-Smith et al. 1994; Bielavska and Roldan 1996; Caulliez et al. 1996; Lamprecht et al. 1997; Currie et al. 2001), the precise role(s) of specific descending peptidergic inputs is not yet defined. Previous electrophysiological studies demonstrate that stimulation or inactivation of the IC, BNST, CeA, and LH produces inhibitory and/or excitatory effects on PBN taste cells (Di Lorenzo and Monroe 1992; Lundy and Norgren 2001, 2004; Li et al. 2005; Li and Cho 2006). Thus, Crh and/or Sst forebrain-PBN pathways, in particular those arising from the BNST and CeA, might be involved in mediating these neurophysiological changes thought to play a role in the elaboration of gustatory preference/aversion and, consequently, ingestive behavior (Shimura et al. 1997a, 1997b; Tokita et al. 2004; Grossman et al. 2008; Li et al. 2013; Moran and Katz 2014). Centrally administered Crh and its homolog urocortin have been shown to diminish intake in a variety of species including rodent, whereas Sst administration augmented intake (Parrott 1990; Heinrichs et al. 1993; Feifel and Vaccarino 1994; Spina et al. 1996; Jones et al. 1998; Ciccocioppo et al. 2003; Fekete et al. 2007; Stengel et al. 2010a, 2010b). Furthermore, injections of Crh into the lateral PBN inhibits sodium chloride intake in sodium-depleted rats, whereas injections of a Crh receptor antagonist had the opposite effect, increasing sodium chloride intake (De Castro et al. 2006). To the best of our knowledge the action(s) of Crh and Sst on gustatory neurons in the PBN has not been determined; however, in several other brain regions, the influence of Crh on neural activity is predominately excitatory (Lowry et al. 2000; Blank et al. 2003; Kash et al. 2008; Ugolini et al. 2008), whereas that for Sst is inhibitory (Saleh and Cechetto 1993; Saleh and Cechetto 1995; Jacquin et al. 1988; Chieng and Christie 2010; Connor et al. 2004).
Perspective
Sensory systems play a fundamental role in allowing us to perceive and appreciate the world around us. Information processing in sensory circuits is not fixed but modifiable at every level of the pathway. Such neuromodulation enables flexibility in neural circuits and, thus, adaptive behavior in the face of changing conditions. Within the gustatory system, flexibility in taste-guided behavior involves neural communication between specific forebrain and hindbrain gustatory nuclei to extract meaning from the sensory stream that can promote or discourage consumption. Although this functional association between hindbrain and forebrain taste areas involved in eating has been clear for decades, the neuromodulatory substances and cellular mechanisms that mediate input/output interactions remain ill-defined. The use of transgenic mice in which cre recombinase expression is driven by specific promoters provides a unique and powerful tool for future investigations aimed at delineating the contribution of specific neurochemical pathways to gustatory sensory processing. The results of this study provide a first step by identifying two specific peptidergic pathways of forebrain origin that are well positioned to influence taste processing in the PBN, a hindbrain nucleus critical for adaptive taste-guided behavior.
Funding
The project described was supported by Kentucky Science and Engineering Foundation (KSEF-2392-RDE-014); Kentucky Spinal Cord Injury Research Center National Institutes of Health/National Institute of General Medical Sciences (P30 GM103507); University of Louisville School of Medicine Bridge Award.
References
- Aponte Y, Atasoy D, Sternson SM. 2011. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 14(3):351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. 2012. Deconstruction of a neural circuit for hunger. Nature. 488(7410):172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielavska E, Roldan G. 1996. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 81(1–2):25–31 [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. 2003. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 23(2):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. 2013. Genetic identification of a neural circuit that suppresses appetite. Nature. 503(7474):111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulliez R, Meile MJ, Nicolaidis S. 1996. A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Res. 729(2):234–245 [PubMed] [Google Scholar]
- Chieng B, Christie MJ. 2010. Somatostatin and nociceptin inhibit neurons in the central nucleus of amygdala that project to the periaqueductal grey. Neuropharmacology. 59(6):425–430 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. 2003. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 23(28):9445–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Bagley EE, Mitchell VA, Ingram SL, Christie MJ, Humphrey PP, Vaughan CW. 2004. Cellular actions of somatostatin on rat periaqueductal grey neurons in vitro. Br J Pharmacol. 142(8):1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. 2001. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 916(1–2):222–228 [DOI] [PubMed] [Google Scholar]
- De Castro e S, Fregoneze JB, Johnson AK. 2006. Corticotropin-releasing hormone in the lateral parabrachial nucleus inhibits sodium appetite in rats. Am. J. Physiol Regul. Integr. Comp Physiol. 290: R1136–R1141 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. 1992. Corticofugal input to taste-responsive units in the parabrachial pons. Brain Res Bull. 29(6):925–930 [DOI] [PubMed] [Google Scholar]
- Feifel D, Vaccarino FJ. 1994. Growth hormone-regulatory peptides (GHRH and somatostatin) and feeding: a model for the integration of central and peripheral function. Neurosci Biobehav Rev. 18(3):421–433 [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP. 2007. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 32(5):1052–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. 2008. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 28(11):2864–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. 1993. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 611(1):18–24 [DOI] [PubMed] [Google Scholar]
- Jacquin T, Champagnat J, Madamba S, Denavit-Saubié M, Siggins GR. 1988. Somatostatin depresses excitability in neurons of the solitary tract complex through hyperpolarization and augmentation of IM, a non-inactivating voltage-dependent outward current blocked by muscarinic agonists. Proc Natl Acad Sci U S A. 85(3):948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. 1998. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology (Berl). 138(2):124–132 [DOI] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. 2009. Terminal field specificity of forebrain efferent axons to brainstem gustatory nuclei. Brain Res. 1248:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. 2008. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 28(51):13856–13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. 1997. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 17(21):8443–8450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Cho YK. 2006. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol. 291(4):R914–R926 [DOI] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. 2005. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol. 93(3):1183–1196 [DOI] [PubMed] [Google Scholar]
- Li JX, Yoshida T, Monk KJ, Katz DB. 2013. Lateral hypothalamus contains two types of palatability-related taste responses with distinct dynamics. J Neurosci. 33(22):9462–9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. 2000. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 20(20):7728–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. 2001. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol. 85(2):770–783 [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. 2004. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol. 91(3):1143–1157 [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. 1985. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 241(3):275–284 [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. 1990a. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 295(4):624–661 [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. 1989. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 283(3):315–332 [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. 1990b. Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J Comp Neurol. 295(4):662–682 [DOI] [PubMed] [Google Scholar]
- Moran A, Katz DB. 2014. Sensory cortical population dynamics uniquely track behavior across learning and extinction. J Neurosci. 34(4):1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. 1997. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol. 78(5):2254–2268 [DOI] [PubMed] [Google Scholar]
- Norgren R. 1974. Gustatory afferents to ventral forebrain. Brain Res. 81(2):285–295 [DOI] [PubMed] [Google Scholar]
- Panguluri S, Saggu S, Lundy R. 2009. Comparison of somatostatin and corticotrophin-releasing hormone immunoreactivity in forebrain neurons projecting to taste-responsive and non-responsive regions of the parabrachial nucleus in rat. Brain Res. 1298:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott RF. 1990. Central administration of corticotropin releasing factor in the pig: effects on operant feeding, drinking and plasma cortisol. Physiol Behav. 47(3):519–524 [DOI] [PubMed] [Google Scholar]
- Perrotto RS, Scott TR. 1976. Gustatory neural coding in the pons. Brain Res. 110(2):283–300 [DOI] [PubMed] [Google Scholar]
- Roldan G, Bures J. 1994. Tetrodotoxin blockade of amygdala overlapping with poisoning impairs acquisition of conditioned taste aversion in rats. Behav Brain Res. 65(2):213–219 [DOI] [PubMed] [Google Scholar]
- Roth SR, Schwartz M, Teitelbaum P. 1973. Failure of recovered lateral hypothalamic rats to learn specific food aversions. J Comp Physiol Psychol. 83(2):184–197 [DOI] [PubMed] [Google Scholar]
- Saggu S, Lundy RF. 2008. Forebrain neurons that project to the gustatory parabrachial nucleus in rat lack glutamic acid decarboxylase. Am J Physiol Regul Integr Comp Physiol. 294(1):R52–R57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh TM, Cechetto DF. 1993. Peptides in the parabrachial nucleus modulate visceral input to the thalamus. Am J Physiol. 264(4 Pt 2):R668–R675 [DOI] [PubMed] [Google Scholar]
- Saleh TM, Cechetto DF. 1995. Neurochemical interactions in the parabrachial nucleus mediating visceral inputs to visceral thalamic neurons. Am J Physiol. 268(3 Pt 2):R786–R795 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Teitelbaum P. 1974. Dissociation between learning and remembering in rats with lesions in the lateral hypothalamus. J Comp Physiol Psychol. 87(3):384–398 [DOI] [PubMed] [Google Scholar]
- Shimura T, Komori M, Yamamoto T. 1997a. Acute sodium deficiency reduces gustatory responsiveness to NaCl in the parabrachial nucleus of rats. Neurosci Lett. 236(1):33–36 [DOI] [PubMed] [Google Scholar]
- Shimura T, Tanaka H, Yamamoto T. 1997b. Salient responsiveness of parabrachial neurons to the conditioned stimulus after the acquisition of taste aversion learning in rats. Neuroscience. 81(1):239–247 [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. 1996. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 273(5281):1561–1564 [DOI] [PubMed] [Google Scholar]
- Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. 2010a. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 151(9):4224–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Tache Y. 2010b. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol. 61(4):399–407 [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Boughter JD., Jr. 2012. Sweet-bitter and umami-bitter taste interactions in single parabrachial neurons in C57BL/6J mice. J Neurophysiol. 108(8):2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr. 2009. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 161(2):475–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr. 2010. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience. 171(1):351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Karádi Z, Shimura T, Yamamoto T. 2004. Centrifugal inputs modulate taste aversion learning associated parabrachial neuronal activities. J Neurophysiol. 92(1):265–279 [DOI] [PubMed] [Google Scholar]
- Tokita K, Yamamoto T, Boughter JD., Jr 2012. Gustatory neural responses to umami stimuli in the parabrachial nucleus of C57BL/6J mice. J Neurophysiol. 107(6):1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Sokal DM, Arban R, Large CH. 2008. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Front Behav Neurosci. 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. 1984. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 303(2):337–357 [DOI] [PubMed] [Google Scholar]
- Weiss MS, Victor JD, Di Lorenzo PM. 2013. Taste coding in the parabrachial nucleus of the pons in awake, freely licking rats and comparison with the nucleus of the solitary tract. J Neurophysiol. 111(8):1655–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. 2012. Deciphering a neuronal circuit that mediates appetite. Nature. 483(7391):594–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Beltz TG, Johnson AK. 1994. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 645(1–2):123–134 [DOI] [PubMed] [Google Scholar]
- Zhang C, Kang Y, Lundy RF. 2011. Terminal field specificity of forebrain efferent axons to the pontine parabrachial nucleus and medullary reticular formation. Brain Res. 1368:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]