Abstract
Background
Many patients scheduled for elective surgery are referred for a preoperative medical consultation. Only limited data are available on factors associated with preoperative consultations. The authors hypothesized that surgical specialty contributes to variation in referrals for preoperative consultations.
Methods
This is a cohort study using data from Group Health Cooperative, an integrated healthcare system. The authors included 13,673 patients undergoing a variety of common procedures—primarily low-risk surgeries—representing six surgical specialties, in 2005–2006. The authors identified consultations by family physicians, general internists, pulmonologists, or cardiologists in the 42 days preceding surgery. Multivariable logistic regression was used to estimate the association between surgical specialty and consultation, adjusting for potential confounders including the revised cardiac risk index, age, gender, Deyo comorbidity index, number of prescription medications, and 11 medication classes.
Results
The authors found that 3,063 (22%) of all patients had preoperative consultations, with significant variation by surgical specialty. Patients having ophthalmologic, orthopedic, or urologic surgery were more likely to have consultations compared with those having general surgery—adjusted odds ratios (95% CI) of 3.8 (3.3–4.2), 1.5 (1.3–1.7), and 2.3 (1.8–2.8), respectively. Preoperative consultations were more common in patients with lower revised cardiac risk scores.
Conclusion
There is substantial practice variation among surgical specialties with regard to the use of preoperative consultations in this integrated healthcare system. Given the large number of consultations provided for patients with low cardiac risk and for patients presenting for low-risk surgeries, their indications, the financial burden, and cost-effectiveness of consultations deserve further study.
The fragmented and economically costly U.S. healthcare system presents potential opportunities for improvements in quality and efficiency in healthcare delivery. An estimated 20–34% of healthcare dollars are spent on ineffective measures, so identification and reduction of these costs are now of particular interest.1-5 Accordingly, there is a growing recognition that improvements are needed in U.S. health care to improve quality and patient experience, and reduce costs, as proposed in the Triple Aim6 strategy.
Within the realm of perioperative medicine, preoperative medical consultation of patients undergoing low-risk surgery may warrant closer evaluation in light of the Triple Aim strategy. Several previous studies focusing on patients with comorbidities undergoing major surgery did not demonstrate any associated improvement in outcomes from preoperative consultations.7-9 Consequently, there is reason to believe that preoperative consultations for relatively healthy patients having low-risk surgery may be a practice with unproven health benefit. Indeed, no current practice guideline recommends that such patients be routinely referred for consultation.10,11
Prior research on preoperative medical consultation has generally focused on patients with comorbidities undergoing major surgery.7-9,12-16 In these previous reports, frequency of consultations for patients undergoing intermediate- to high-risk surgery (sometimes referred to as major surgery) has ranged from 10 to 40%. Some investigators have also found that whereas increased age and comorbidities did predict referral for preoperative consultation, increased surgical risk did not.9 Specifically, surgical procedures with inherently lower perioperative risks (e.g., major joint replacement) had similarly frequent consultations as procedures with much higher risks (e.g., major vascular surgery). In contrast to the previous work on major surgery, there is a paucity of research on preoperative consultations among patients undergoing low-risk surgery, although low-risk surgeries are more common.17 The total cost of frequent consultations for such low-risk surgeries may be substantial, potentially exceeding the costs associated with common preoperative tests such as chest x-rays, electrocardiograms, and laboratory studies.18
To begin to examine patterns of preoperative medical consultations among patients undergoing common, predominantly low-risk surgical procedures, we conducted a cohort study of such patients in an integrated healthcare system.19 Our objectives were to describe the frequency and determinants of preoperative consultations in this population. We also specifically sought to evaluate the association of surgical specialty after accounting for age, burden of comorbidity, and operative risk.
Materials and Methods
The Group Health Cooperative (GHC) and the Veterans Affairs Puget Sound Healthcare System Institutional Review Boards (Seattle, WA) approved the study, and waived the requirement of informed consent. This cohort study used linked administrative and clinical data from GHC, an integrated healthcare insurance and delivery system in the Pacific Northwest. GHC insures approximately 675,000 participants across Washington State. Characteristics of GHC databases and their advantages and limitations for health services research have been described previously.20
Clinical Setting
There were no preanesthesia or preoperative clinics where patients were routinely seen. GHC did not have a policy addressing which patients should be referred for preoperative consultations. Therefore, preoperative consultations were initiated at the surgeons’ discretion. Nurse practitioners were available to do history and physical examinations when requested by surgeons, but these history and physical examinations were not identified in this study.
Assembly of Cohort
Using administrative data, we identified all adults aged 18 and older who had one of 21 inpatient or outpatient surgical procedures performed by six different surgical specialties in 2005 or 2006. The surgical procedures were chosen because they represented low-, intermediate-, and high-risk surgeries that are commonly performed in the United States11 (see Appendix for list of included procedures and their frequencies in the study cohort). We identified patients based on the first occurrence of a Current Procedural Terminology code reflecting one of the aforementioned selected procedures during the study period. The main outcome of interest, the occurrence of preoperative medical consultation, was identified by the presence of codes for moderate to high level preoperative consultations (outpatient consultations Current Procedural Terminology codes 99243, 99244, 99245 and inpatient consultations Current Procedural Terminology codes 99253, 99254, 99255) that were provided by family physicians, general internists, pulmonologists, or cardiologists. We also included office visits (new patient Current Procedural Terminology codes 99203, 99204, 99205, and established patient 99213, 99214, 99215) if they were associated with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code v72.81–v72.84 indicating a preoperative evaluation. For a visit to be defined as a preoperative consultation, it had to occur within 42 days before surgery. Although we assumed that the vast majority of preoperative consultations occurred within 28 days before surgery, we used a more conservative time window of 42 days to ensure that we captured all visits associated with an elective surgical procedure.
Using diagnostic codes present within 365 days before surgery, we calculated both the revised cardiac risk index (RCRI),21 using the method previously described by Lindenauer et al.,22 and the Deyo comorbidity index.23 Briefly, the RCRI is calculated by adding one point for the presence of each of the following: History of ischemic heart disease, history of cerebrovascular disease, history of congestive heart failure, chronic renal insufficiency, diabetes, and high-risk surgery. Consistent with the original study that derived the RCRI, we defined high-risk surgery as intraperitoneal, intrathoracic, and suprainguinal vascular procedures.21 Patients with 0, 1, 2, or ≥3 factors were assigned to classes I, II, III, or IV, respectively, as was described in the original report.21 The Deyo comorbidity index was used as an ordinal variable with categories 0, 1, 2, and 3 representing an index score of 0, 1, 2, and ≥3. Demographic information included age and gender. To identify medications that patients were receiving before their preoperative medical consultation, we searched the automated pharmacy database for records of filled prescriptions between 6 months and up to 43 days prior to index surgery. Medication classes included analgesics, aspirin, nonaspirin antiplatelet agents, oral anticoagulants, angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, β blockers, cardiac medications,** calcium channel blockers, other antihypertensives, insulin, oral hypoglycemic agents, antiinfectives, anticonvulsants, lipid-lowering agents, antineoplastics, α blockers, diuretics, psychotherapeutics, thyroid medications, and uric acid agents. We also counted the total number of medication classes that had been filled. Based on their relevance to perioperative medical care, we included 11 individual medication classes in our primary analysis, namely, analgesics, aspirin, nonaspirin antiplatelet agents, oral anticoagulants, angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, β blockers, cardiac medications, calcium channel blockers, other antihypertensives, insulin, and oral hypoglycemic agents.
Statistical Analysis
Consultations were summarized using frequency distributions by surgical specialty and by day preceding surgery up to 42 days prior to index surgery. In bivariate analyses, we tested the association of the occurrence of preoperative consultation (outcome variable) with age, comorbidity, surgery risk category, and referring surgical specialty (predictors). We fit a multivariable logistic regression model to estimate the association between preoperative consultation (outcome variable) and surgical specialty (main predictor of interest), with adjustment for the other explanatory variables and potential confounders, including RCRI, age, gender, Deyo comorbidity index, total number of prescription medications, and 11 different classes of medications. We performed a priori planned secondary analyses in the subgroup of patients undergoing low-risk surgical procedures to evaluate whether this subgroup of patients influenced the results. In these exploratory analyses, we excluded cataract surgery from the subgroup of patients referred by ophthalmology.
Cataract surgery dominated the ophthalmologic procedures, so we sought to explore if the increase in preoperative consultations for ophthalmologic surgery was driven by cataract surgery alone. A two-sided α level of 0.05 was considered for statistical significance. All statistical analyses were performed using Stata 12 (Stata Corporation, College Station, TX).
Results
Study Population
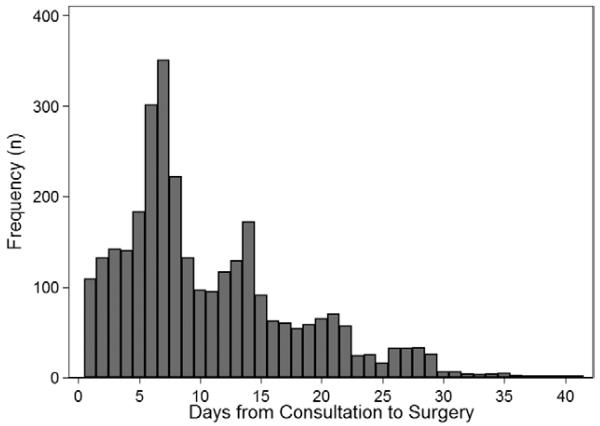
A total of 13,673 patients were identified, and the final sample consisted of 13,670 patients with complete data who underwent one of the 21 selected surgical procedures (see Appendix). The mean age was 63 years and 60% of patients were female. Table 1 shows the cohort characteristics and bivariate associations comparing patients undergoing preoperative consultation or not. Overall, 3,063 (22%) patients underwent preoperative consultation. The distribution of preoperative consultations showed peaks on weekly intervals before surgery (days 7 and 14, see fig. 1). The median time interval from consultation to surgery was 8 days, with an interquartile range of 5–14 days (i.e., 75% of consultations occurred within 14 days of surgery). Patients who were provided a preoperative medical consultation in the 42 days preceding the surgical procedure were older, more likely to be male, and more likely to have a low underlying cardiac risk (RCRI 1) compared with patients who did not have a preoperative consultation. In addition, patients seen in consultation had a slightly higher proportion of ischemic heart disease and diabetes, and were more likely to have filled prescriptions for aspirin, oral anticoagulants, angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, β-blockers, cardiac medications, calcium channel blockers, other antihypertensives, and insulin but less likely to have filled a prescription for analgesics.
Table 1.
Characteristics of Patients Receiving and Not Receiving Preoperative Consultation
| Consultation, n = 3,063 | No Consultation, n = 10,607 | P Value‡ | |
|---|---|---|---|
| Age, mean (SD) | 66.8 (13.0) | 61.4 (14.6) | <0.01 |
| Female, n (%) | 1,818 (54.5) | 6,384 (61.6) | <0.01 |
| Surgical specialty, n (%) | <0.01 | ||
| General | 424 (13.9) | 2,737 (25.8) | |
| Ophthalmology | 1,713 (55.9) | 2,933 (27.7) | |
| Gynecology | 19 (0.6) | 1,123 (10.6) | |
| Orthopedics | 712 (23.3) | 3,098 (29.2) | |
| Urology | 169 (5.5) | 484 (4.6) | |
| Vascular | 26 (0.9) | 232 (2.2) | |
| RCRI, n (%)* | |||
| I | 1,947 (63.6) | 6,245 (58.9) | <0.01 |
| II | 681 (22.2) | 2,861 (27.0) | |
| III | 278 (9.1) | 971 (9.2) | |
| IV | 157 (5.1) | 530 (5.0) | |
| Comorbidities, n (%) | |||
| Ischemic heart disease | 519 (16.9) | 1,501 (14.2) | <0.01 |
| Congestive heart failure | 251 (8.2) | 785 (7.4) | 0.14 |
| Cerebrovascular disease | 213 (7.0) | 797 (7.5) | 0.30 |
| Diabetes mellitus | 614 (20.1) | 1,907 (18.0) | 0.01 |
| Chronic renal insufficiency | 108 (3.5) | 315 (3.0) | 0.12 |
| High-risk surgery, n (%) | 61 (2.0) | 1,294 (12.2) | <0.01 |
| Low-risk surgery, n (%) | 2,406(78.6) | 6,184(58.4) | <0.01 |
| Deyo comorbidity index, n (%)† | |||
| 0 | 1,684 (55.0) | 5,929 (55.9) | <0.01 |
| 1 | 595 (19.4) | 1,910 (18.0) | |
| 2 | 450 (14.7) | 1.588 (15.0) | |
| 3 | 334 (10.9) | 1,180 (11.1) | |
| Number of drugs, median (IQR) |
2(1–4) | 2(1–4) | 0.03§ |
| Medication classes, n (%) | |||
| Analgesics | 791 (25.8) | 3,211 (30.3) | <0.01 |
| Aspirin | 366 (12.0) | 980 (9.2) | <0.01 |
| Nonasp antiplt agents | 36 (1.2) | 153 (1.4) | 0.27 |
| Oral anticoagulants | 175 (5.8) | 447 (4.2) | <0.01 |
| ACE inhibitors or ARBs | 914 (29.8) | 2,665 (25.1) | <0.01 |
| β blockers | 803 (26.2) | 2,183 (20.6) | <0.01 |
| Cardiac medications | 276 (9.0) | 709 (6.7) | <0.01 |
| Calcium channel blockers | 317 (10.4) | 868 (8.2) | <0.01 |
| Other antihypertensives | 203 (6.6) | 540 (5.1) | <0.01 |
| Insulin | 185 (6.0) | 499 (4.7) | <0.01 |
| Oral diabetic agents | 285 (9.3) | 910 (8.6) | 0.21 |
Patients with 0, 1, 2, or 3 or more factors were assigned to classes I, II, III, or IV, respectively.
The Deyo comorbidity index was used as a categorical variable, scores of 0, 1, 2, and ≥3, are represented by categories 0, 1, 2, and 3.
P values computed using two-sample t test or chi-square test comparing patients with consultation versus patients without consultations.
P value computed using Wilcoxon rank sum test.
ACE inhibitors = angiotensin converting enzyme inhibitors; ARBs = angiotensin II receptor antagonists; IQR = interquartile range; Nonasp antiplt agents = nonaspirin antiplatelet agents; RCRI = revised cardiac risk index.
Fig. 1.
Frequency distribution of preoperative consultations in the 42 days preceding the index surgery, showing a bimodal distribution with peaks on preoperative days 7 and 14.
Characteristics of Consultations and Association with Surgical Specialty
Family physicians provided the majority of consultations (table 2). Level 3 and 4 preoperative consultations were the most common, 50 and 47%, respectively. Level 5 visits represented only 2.5% of all consultations. Referrals for preoperative consultation varied significantly by specialty, with ophthalmologic, urologic, and orthopedic surgeries being associated with the highest proportion of preoperative consultations (table 2).
Table 2.
Distribution of Consultations by Surgical Specialty
| Surgical Specialty |
||||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | General (n = 3,161) |
Eye (n = 4,646) |
Gynecology (n = 1,142) |
Orthopedics (n = 3,810) |
Urology (n = 653) |
Vascular (n = 258) |
Total (n = 13,670) |
P Value* |
| All consultations | 424 | 1,713 | 19 | 712 | 169 | 26 | 3,063 | – |
| RCRI† | ||||||||
| RCRI I, n/ntot (%) | 303/2,156 (14.1) |
995/2,589 (38.4) |
1/252 (0.4) |
544/2,805 (19.4) |
104/390 (26.7) |
0/0 (0.0) |
1,947/8,192 (23.8) |
<0.01 |
| RCRI II, n/ntot (%) | 81/648 (12.5) |
416/1,169 (35.6) |
15/766 (2.0) |
114/704 (16.2) |
48/184 (26.1) |
7/71 (9.9) |
681/3,542 (19.2) |
<0.01 |
| RCRI III, n/ntot (%) | 26/240 (10.8) |
186/543 (34.3) |
3/105 (2.9) |
38/222 (17.1) |
15/59 (25.2) |
10/80 (12.5) |
278/1,249 (22.3) |
<0.01 |
| RCRI IV, n/ntot (%) | 14/117 (12.0) |
116/345 (33.6) |
0/19 (0.0) |
16/79 (20.3) |
2/20 (10.0) |
9/107 (8.4) |
157/687 (22.9) |
<0.01 |
| Consultant’s specialty‡ | ||||||||
| Family practice, n (%) |
353 (83.3) | 1,550 (90.5) |
10 (52.6) | 503 (70.7) | 126 (74.6) | 20 (76.9) | 2,562 (83.6) |
– |
| Internal medicine, n (%) |
46 (10.9) | 87 (5.1) | 6 (31.6) | 148 (20.8) | 32 (18.9) | 5 (19.2) | 324 (10.6) | – |
| Cardiology, n (%) | 25 (5.9) | 74 (4.3) | 3 (15.8) | 61 (8.6) | 11 (6.5) | 1 (3.9) | 175 (5.7) | – |
| Pulmonary, n (%) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | – |
Chi-square test of homogeneity.
Patients with 0, 1, 2, or 3 or more factors were assigned to classes I, II, III, or IV, respectively.
Column percentages may not add to 100 because of rounding errors.
RCRI = revised cardiac risk index
Multivariable Regression
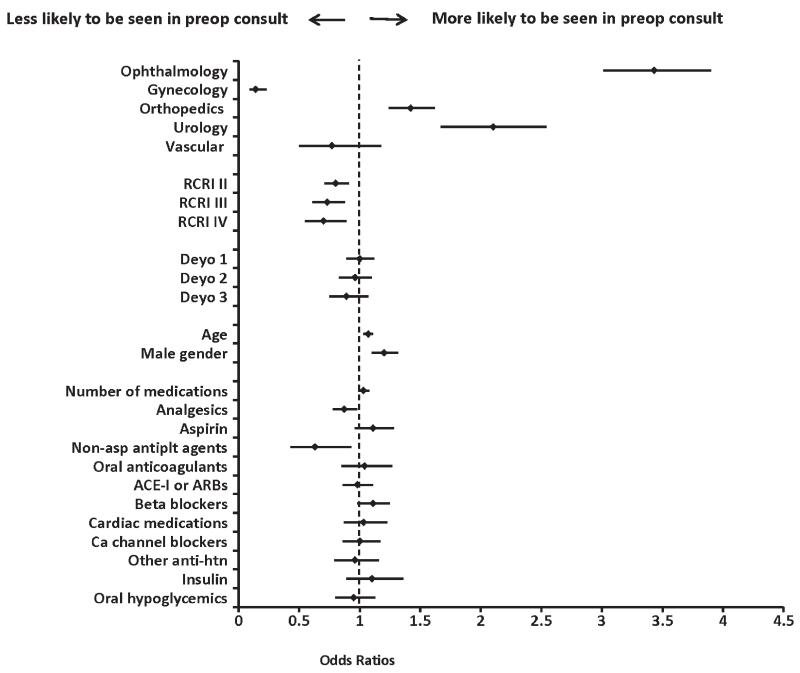
In adjusted models, patients undergoing ophthalmologic, orthopedic, and urologic surgeries were more likely to be seen in consultation than those undergoing general surgery (referent group, table 3). In the multivariable model, older age and male gender were associated with preoperative consultation. Notably, the total burden of preoperative comorbidity was not associated with the likelihood of consultation, while an increased perioperative cardiac risk (i.e., higher RCRI class) was paradoxically associated with a lower adjusted odds ratio of consultation. Aside from analgesics and nonaspirin antiplatelet agents, which were inversely associated with preoperative consultation, in multivariable analyses the prescription of other classes of medications and the total number of prescription medications were not independently associated with the likelihood of preoperative consultation (fig. 2).
Table 3.
Crude and Adjusted Odds Ratios for the Association of Surgical Specialty and Preoperative Consultation in the Entire Study Population and in Patients Undergoing Low-risk Surgery
| Entire Population (N = 13,670) | Low-risk Surgery (N = 8,590)* | |
|---|---|---|
| Surgical specialty | Crude estimates (95% CI) | |
| General | Reference | Reference |
| Ophthalmology | 3.8 (3.3, 4.2) | 3.1 (2.7, 3.6) |
| Gynecology | 0.1 (0.1, 0.2) | 0.02 (0.003, 0.1) |
| Orthopedics | 1.5 (1.3, 1.7) | 1.6 (1.3, 1.9) |
| Urology | 2.3 (1.8, 2.8) | 1.3 (.97, 1.8) |
| Vascular | 0.7 (0.5, 1.1) | NA |
| Surgical specialty | Adjusted estimates (95% CI) | |
| General | Reference | Reference |
| Ophthalmology | 3.4 (3.0, 3.9) | 2.6 (2.3, 3.1) |
| Gynecology | 0.1 (0.1, 0.2) | 0.03 (0.004, 0.2) |
| Orthopedics | 1.4 (1.2, 1.6) | 1.7 (1.4, 2.0) |
| Urology | 2.1 (1.7, 2.5) | 1.4 (1.0, 1.9) |
| Vascular | 0.8 (0.5, 1.2) | NA |
| Model covariates | ||
| RCRI class II | 0.8 (0.7, 0.9) | 0.9 (0.7, 1.0) |
| RCRI class III | 0.7 (0.6, 0.9) | 0.8 (0.6, 1.0) |
| RCRI class IV | 0.7 (0.5, 0.9) | 0.8 (0.6, 1.0) |
| Age (10 years) | 1.1 (1.0, 1.1) | 1.1 (1.1, 1.2) |
| Male gender | 1.2 (1.1, 1.3) | 1.1 (1.0, 1.3) |
| Deyo comorbidity index | ||
| 1 | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| 2 | 1.0 (0.8, 1.1) | 0.9 (0.8, 1.0) |
| ≥3 | 0.9 0.8, 1.1) | 0.8 (0.6, 0.9) |
| Number of medications | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.1) |
| Analgesics | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) |
| Aspirin | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.4) |
| Nonasp antiplt agents | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) |
| Oral anticoagulants | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.4) |
| ACE-I or ARBs | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) |
| β blockers | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.4) |
| Cardiac medications | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) |
| Ca channel blockers | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) |
| Other anti-htn | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.3) |
| Insulin | 1.1 (0.9, 1.4) | 1.1 (0.8, 1.4) |
| Oral hypoglycemic | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.2) |
Estimates are odds ratios with 95% CIs rounded to the nearest one decimal. Patients with RCRI scores of 0, 1, 2, or 3 or more were assigned to classes I, II, III, or IV, respectively; referent category was class I. The referent for the Deyo comorbidity index was 0.
Includes only low-risk surgeries: lymph node biopsy/removal, inguinal hernia repair, mastectomy, cataract removal, ptosis repair, tubal ligation, knee arthroscopy, and lithotripsy.
ACE-I = angiotensin converting enzyme inhibitors; ARBs = angiotensin II receptor antagonists; Ca channel blockers = calcium channel blockers; NA = not applicable (vascular surgery did not perform low-risk procedures); Nonasp antiplt agents = nonaspirin antiplatelet agents; Other anti-htn = other antihypertensive medications; RCRI = revised cardiac risk index.
Fig. 2.
Forest plot displaying the adjusted odds ratios and 95% CIs for predictors of referral for preoperative consultation. The adjusted model included all of the variables displayed. The referent category for surgical specialty was general surgery. Revised cardiac risk index (RCRI): patients with 0, 1, 2, or 3 or more factors were assigned to classes I, II, III, or IV, respectively; referent category was class I. The Deyo comorbidity index was used as a categorical variable, scores of 0, 1, 2, and ≥3 are represented by categories 0, 1, 2, and 3. The referent category was 0. The estimate for age is for 10-yr difference. ACE-I = angiotensin converting enzyme inhibitors; Ca channel blockers = calcium channel blockers; Deyo = Deyo comorbidity index; Nonasp antiplt agents = nonaspirin antiplatelet agents; Other anti-htn = other antihypertensive medications.
Subgroup Analyses
In secondary analyses, when we fitted the same adjusted model restricting to a subset of patients undergoing low-risk surgeries only, our findings were similar to those in the full cohort (table 3). The heterogeneity of the odds ratios across the surgical specialties was of similar magnitude and the odds ratios remained statistically significantly different compared with general surgery (referent).
There was a large number of cataract surgery patients in our sample (n = 4,315, Appendix). However, after excluding patients undergoing cataract surgery, surgical specialty remained an independent predictor of consultation, with no change in the relative rankings of the six specialties (data not shown).
Discussion
In this cohort study of participants from an integrated healthcare system, we found that surgical specialty is a strong predictor of referral for preoperative consultation, independent of a priori selected explanatory variables (i.e., age, comorbidity burden, and surgical risk) and other potential confounders. Surprisingly, the proportion of patients referred for preoperative consultation was highest for ophthalmologic surgery. In addition, referrals for preoperative consultation did not appear to be more frequent in patients with higher RCRI; indeed, the highest likelihood of consultation was in patients with the lowest predicted cardiac risk. This finding is not consistent with previous reports of major surgery where patients were selectively referred for preoperative medical consultations because of medical problems.14,24 A selective approach based on medical problems was recommended by the authors of a cost-benefit evaluation of preoperative and postoperative medical evaluation25 and is consistent with the updated American Society of Anesthesiologists Practice advisory for preanesthesia evaluation.10
The finding that ophthalmologic surgery was associated with higher use of preoperative consultation was unexpected, particularly since ophthalmologic procedures are typically very low risk and have been reported to be associated with few medical and anesthesia complications.26 However, our findings were robust and not driven solely by the high prevalence of cataract surgery patients. Notably, our observation that lower-risk surgical procedures often have substantially higher frequency of consultations than higher risk procedures was unchanged after excluding cataract surgery patients. It is not clear which considerations triggered the referral of a high proportion of patients with low cardiac risk and low to intermediate surgical risk (as defined in the American College of Cardiology-American Heart Association guidelines)11 for preoperative consultation. These consultations do provide the opportunity to improve documentation of comorbid disease, perform risk stratification, optimize factors associated with preexisting medical conditions, and initiate interventions intended to decrease perioperative risk (such as β-blockers).8,9,16,27,28 However, these potential reasons do not pertain to patients without comorbidities undergoing low-risk surgery. One possibility is that while all surgeons are trained to obtain a history and perform a physical examination, some highly specialized providers may only concentrate on a detailed, focused examination and not prioritize to personally evaluate coexisting medical comorbidities. It is possible that requesting a preoperative consultation from a primary care provider increases the efficiency from some surgeons’ perspective because it allows a surgeon to spend less time on the general medical evaluation and focus more time and effort on the evaluation for and performance of the surgical procedures themselves. It is also possible that preoperative consultation is used to establish a primary care provider relationship in patients without significant comorbidities who are not otherwise seen on a regular basis. Future studies are needed to ascertain whether this is an important determining factor and, if so, the cost consequences from a health system perspective. Requesting consultations may be viewed by some surgical specialties as protection from a medicolegal perspective, which is an ever-present concern in the U.S. healthcare system. Medicolegal factors seem likely to contribute to the overall frequency of consultation, as has been suggested by a previous report.28 However, these factors are unlikely to explain the variation among surgical specialties. Again, future studies are needed to assess to what extent this consideration is an important factor, and to determine other potential explanations for these practice variations.
We observed a practice pattern of frequent referral for preoperative consultations of patients with low medical and/or surgical risk. In addition to evaluating potential underlying reasons for the identified predictors of preoperative medical consultation, it is also important to consider the cost consequences and other potential implications of referring patients for additional care before surgery. A recent population-based cohort study showed that preoperative medical consultation was not associated with improved postoperative outcomes after major surgeries.8 Such studies, including our study, raise the important question of whether referrals for preoperative consultation are costeffective or beneficial. For preoperative consultations to be cost-effective, it will be important to demonstrate improved outcomes such as reduced perioperative medical and surgical complications, decreased hospitalizations or length of stay, or improved recovery or higher quality of life. Presumably, such benefits would be most likely in patients presenting with high medical risk or high surgical risk, or both. Since perioperative complications associated with surgeries other than major surgery are relatively rare events, it will be useful to study outcomes in large samples and different healthcare systems.29
Previous studies have focused on major surgery or highrisk surgery. We included vascular surgery because this type of surgery has been included in several prior studies on preoperative evaluation and risk minimization. We believe that this uniquely high-risk type of surgery may have unique patterns of use of preoperative resources, including medical consultations.
The consequences of preoperative consultations from a health economics perspective may be substantial. In 1984, the cost of preoperative consultations for low-risk inpatient procedures was conservatively estimated at $1 billion.25 Medicare reimbursement rates approximately reflect the relative resource use for various perioperative interventions. The 2010 reimbursements for level 3 and 4 consultations were $130 and $183, respectively, while the reimbursement for electrocardiograms, complete blood counts, basic metabolic panel, and coagulation studies were $22, $9, $9, and $14, respectively.†† Therefore, it may be more important to focus on the rational use of preoperative consultation than preoperative testing, although to date the latter has received more attention than the former. A recent pilot study suggests that the cost to the Medicare program of preoperative consultations for patients with low RCRI undergoing lowrisk procedures is indeed greater than the cost of common preoperative tests.18
Limitations
Although integrated healthcare systems are common and over one in four Americans receive their care in these systems,‡‡ our cohort study is derived from a single healthcare system that may have unique referral and practice patterns. However, our finding of important variation across surgical specialties with regard to preoperative consultation is consistent with investigations in other settings.9,30 Nonetheless, further research is needed to determine if these findings are generalizable to other health plans and health systems across the United States.
This study is based on administrative data, which may be affected by coding errors within these databases or misclassification of preoperative consultation. The observation of peak visits at 1 and 2 weeks preoperatively, and very low occurrence of visits preceding day 28 preoperatively, suggests that the majority of these visits were associated with a planned surgery (low misclassification of unrelated visits as preoperative consultations). The use of a large administrative dataset allows a big-picture view of practice patterns to help direct future study to understand the causes and implications of such patterns.
It is possible that preoperative consultations resulted in delays in surgery beyond 42 days or cancellation of surgery. Differential misclassification could potentially bias toward underrepresenting impactful and important preoperative consultations and overemphasizing the potentially unnecessary preoperative consultations that did not delay or cancel surgery. We believe this bias is small, because we included a long preoperative window (42 days) to allow time for delays. Even if this potential bias exists and results in underrepresentation of the use of preoperative consultations in higher risk patients, our data remain important in that they show a high rate of preoperative consultation in low-risk surgery and low-risk patients. Finally, we have used a conservative method for calculation of the RCRI, which would tend to underestimate the proportion of patients without cardiac risk (RCRI class I).
Conclusions
In summary, this cohort study in a single healthcare system found that surgical specialties varied substantially with regard to the use of preoperative consultation, and that a large number of consultations are being provided to patients with low medical and/or surgical risk. We believe that these findings need to be tested in other health plans and other health systems to determine if they are generalizable. Furthermore, outcomes studies to evaluate the impact and guide the optimal use of these preoperative consultations are needed.
What We Already Know about This Topic
Limited data are available on factors associated with preoperative consultations
The investigators thus tested the hypothesis that surgical specialty contributes to variation in referrals for preoperative consultations
What This Article Tells Us That Is New
Among 13,673 patients in a single health system, patients having ophthalmologic, orthopedic, or urologic surgery were more likely to have consultations compared with those having general surgery—adjusted odds ratios (95% CI) of 3.8 (3.3–4.2), 1.5 (1.3–1.7), and 2.3 (1.8–2.8), respectively
There is substantial practice variation among surgical specialties with regard to the use of preoperative consultations that does not appear to be based on underlying risk
Acknowledgments
The authors are grateful for the outstanding assistance provided by our project managers Kelly Ehrlich, M.S., Aaron Scrol, M.A., and David Cammon, B.A., and our computer specialist Bob Harrison, B.A., all with Group Health Research Institute (GHRI), Seattle, Washington. The authors also thank Stephen lavine, M.D., Assistant Medical Director, Perioperative and Central Hospital Services, Group Health Physicians; Amanda lee, M.D., Service line Chief, Consultative Internal Medicine, Group Health Physicians; James Ralston, M.D., M.P.H., Associate Investigator; and Judith Nielsen, M.D., Assistant Medical Director, Specialty Business Processes, Facilities, and Quality, Group Health Physicians, all with GHRI, for their comments on an early draft of the manuscript.
Supported by grant T32GM086270, National Institutes of Health, Bethesda, Maryland (to Dr. Thilen). This material is based on work supported by the U.S. Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Center of Excellence; VA Puget Sound Health Care Systems, Seattle, Washington. Supported by a Clinician Scientist Award from the Canadian Institutes of Health Research (Ottawa, Ontario, Canada), and a Merit Award from the Department of Anesthesia at the University of Toronto, Toronto, Ontario, Canada (to Dr. Wijeysundera). The authors were also supported by Group Health Research Institute, Seattle, Washington, who waived part of the indirect costs. Without this support this project may not have been possible.
Appendix. List of Surgical Procedures
| Surgical Procedure, n (%) | Total Cohort, n = 13,670 | Consultation, n = 3,063 | No Consultation, n = 10,607 |
|---|---|---|---|
| General | |||
| Colon resectionH | 352 (2.6) | 21 (0.7) | 331 (3.1) |
| MastectomyL | 514 (3.8) | 49 (1.6) | 465 (4.4) |
| Node biopsy/removalL | 248 (1.8) | 53 (1.7) | 195 (1.8) |
| Laparoscopic cholecystectomyl | 1,211 (8.9) | 150 (4.9) | 1,061 (10.0) |
| Inguinal hernia repairL | 836 (6.1) | 151 (4.9) | 685 (6.5) |
| Eye | |||
| Cataract removalL | 4,315 (31.6) | 1,609 (52.5) | 2,706 (25.5) |
| Vitrectomyl | 186 (1.4) | 78 (2.6) | 108 (1.0) |
| Ptosis repairL | 145 (1.1) | 26 (0.9) | 119 (1.1) |
| Gynecology | |||
| HysterectomyH | 887 (6.5) | 18 (0.6) | 869 (8.2) |
| Tubal ligationL | 255 (1.9) | 1 (0.03) | 254 (2.4) |
| Orthopedics | |||
| Hip arthroplastyl | 719 (5.3) | 113 (3.7) | 606 (5.7) |
| Knee arthroplastyl | 1,109 (8.1) | 141 (4.6) | 968 (9.3) |
| Knee arthroscopyL | 1,982 (14.5) | 458 (15.0) | 1,524 (14.4) |
| Urology | |||
| Radical prostatectomyH | 45 (0.3) | 14 (0.5) | 31 (0.3) |
| Lap radical prostatectomyl | 52 (0.4) | 26 (0.9) | 26 (0.3) |
| Transurethral prostatectomyl | 261 (1.9) | 70 (2.3) | 191 (1.8) |
| LithotripsyL | 295 (2.2) | 59 (1.9) | 236 (2.2) |
| Vascular | |||
| AAA open repairH | 27 (0.02) | 6 (0.2) | 21 (0.2) |
| AAA endovascular repairH | 2 (0.01) | 0 (0) | 2 (0.02) |
| Carotid endarterectomyl | 187 (1.4) | 18 (0.6) | 169 (1.6) |
| Femoro-popliteal bypassH | 42 (0.3) | 2 (0.1) | 40 (0.4) |
Denotes high-risk surgery,
denotes intermediate-risk surgery,
denotes low-risk surgery.
AAA = abdominal aortic aneurysm.
Footnotes
Cardiac medications include class IA, class IC, and class III antiarrhythmic agents, digitalis glycosides, and vasodilator nitrates.
The Medicare Physician Fee Schedule Database (MPFSDB). Available at: www.cms.gov. Accessed February 21, 2012.
U.S. Census Bureau. U.S. Census data on HMO enrollment. Available at: www.census.gov.compendia/statab. Accessed February 21, 2012.
Health Services Research and Development, VA Puget Sound Health Care System, Seattle, Washington; Group Health Research Institute, Seattle, Washington; and Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, Washington.
Meetings at which work has been presented: Preliminary data were presented at the 5th annual meeting of the Society for Perioperative Assessment and Quality Improvement, March 3, 2011, Miami Beach, Florida, and at the annual meeting of the American Society of Anesthesiologists, October 16, 2011, Chicago, Illinois.
References
- 1.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–6. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 2.Bloche MG. Beyond the “R word”? Medicine’s new frugality. N Engl J Med. 2012;366:1951–3. doi: 10.1056/NEJMp1203521. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn BM. Movement to promote good stewardship of medical resources gains momentum. JAMA. 2012;307:895–902-3. doi: 10.1001/jama.2012.218. [DOI] [PubMed] [Google Scholar]
- 4.Qaseem A, Alguire P, Dallas P, Feinberg LE, Fitzgerald FT, Horwitch C, Humphrey L, LeBlond R, Moyer D, Wiese JG, Weinberger S. Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med. 2012;156:147–9. doi: 10.7326/0003-4819-156-2-201201170-00011. [DOI] [PubMed] [Google Scholar]
- 5.Boat TF, Chao SM, O’Neill PH. From waste to value in health care. JAMA. 2008;299:568–71. doi: 10.1001/jama.299.5.568. [DOI] [PubMed] [Google Scholar]
- 6.Berwick DM, Nolan TW, Whittington J. The triple aim: Care, health, and cost. Health Aff (Millwood) 2008;27:759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach AD, Rasic MA, Sehgal N, Ide B, Stone B, Maselli J. Opportunity missed: Medical consultation, resource use, and quality of care of patients undergoing major surgery. Arch Intern Med. 2007;167:2338–44. doi: 10.1001/archinte.167.21.2338. [DOI] [PubMed] [Google Scholar]
- 8.Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med. 2010;170:1365–74. doi: 10.1001/archinternmed.2010.204. [DOI] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: A population-based study. Anesthesiology. 2012;116:25–34. doi: 10.1097/ALN.0b013e31823cfc03. [DOI] [PubMed] [Google Scholar]
- 10.Apfelbaum JL, Connis RT, Nickinovich DG, Pasternak LR, Arens JF, Caplan RA, Fleisher LA, Flowerdew R, Gold BS, Mayhew JF, Rice LJ, Roizen MF, Twersky RS. Practice advisory for preanesthesia evaluation: An updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522–38. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 11.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine, Society for Vascular Surgery: 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54:e13–e118. [Google Scholar]
- 12.Maggio C, Bonzano A, Conte E, Libertucci D, Panarelli M, Bobbio M, Pintor PP. Preoperative evaluation in non-cardiac surgery: Cardiac risk assessment. Qual Assur Health Care. 1992;4:217–24. doi: 10.1093/oxfordjournals.intqhc.a036722. [DOI] [PubMed] [Google Scholar]
- 13.Pupa LE, Jr, Coventry JA, Hanley JF, Carpenter JL. Factors affecting compliance for general medicine consultations to non-internists. Am J Med. 1986;81:508–14. doi: 10.1016/0002-9343(86)90307-4. [DOI] [PubMed] [Google Scholar]
- 14.Golden WE, Lavender RC. Preoperative cardiac consultations in a teaching hospital. South Med J. 1989;82:292–5. doi: 10.1097/00007611-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mollema R, Berger P, Girbes AR. The value of peri-operative consultation on a general surgical ward by the internist. Neth J Med. 2000;56:7–11. doi: 10.1016/s0300-2977(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 16.Devereaux PJ, Ghali WA, Gibson NE, Skjodt NM, Ford DC, Quan H, Guyatt GH. Physicians’ recommendations for patients who undergo noncardiac surgery. Clin Invest Med. 2000;23:116–23. [PubMed] [Google Scholar]
- 17.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009:1–25. [PubMed] [Google Scholar]
- 18.Thilen S, Treggiari M, Weaver E. An Opportunity for Anesthesiologists to Add Value by Controlling Preoperative Resources. Presented at: the ASA Practice Management Meeting; Orlando, FL. January 27, 2012; abstract no PM21. [Google Scholar]
- 19.Shortell SM, Gillies RR, Anderson DA. The new world of managed care: Creating organized delivery systems. Health Aff (Millwood) 1994;13:46–64. doi: 10.1377/hlthaff.13.5.46. [DOI] [PubMed] [Google Scholar]
- 20.Fishman PA, Wagner EH. Managed care data and public health: The experience of Group Health Cooperative of Puget Sound. Annu Rev Public Health. 1998;19:477–91. doi: 10.1146/annurev.publhealth.19.1.477. [DOI] [PubMed] [Google Scholar]
- 21.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 22.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Kleinman B, Czinn E, Shah K, Sobotka PA, Rao TK. The value to the anesthesia-surgical care team of the preoperative cardiac consultation. J Cardiothorac Anesth. 1989;3:682–7. doi: 10.1016/s0888-6296(89)94472-4. [DOI] [PubMed] [Google Scholar]
- 25.Gluck R, Muñoz E, Wise L. Preoperative and postoperative medical evaluation of surgical patients. Am J Surg. 1988;155:730–4. doi: 10.1016/s0002-9610(88)80031-x. [DOI] [PubMed] [Google Scholar]
- 26.Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, Petty BG, Steinberg EP. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med. 2000;342:168–75. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 27.Pausjenssen L, Ward HA, Card SE. An internist’s role in perioperative medicine: A survey of surgeons’ opinions. BMC Fam Pract. 2008;9:4. doi: 10.1186/1471-2296-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz RI, Barnhart JM, Ho G, Hersch D, Dayan SS, Keehn L. A survey on the intended purposes and perceived utility of preoperative cardiology consultations. Anesth Analg. 1998;87:830–6. doi: 10.1097/00000539-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Devereaux PJ, Chan MT, Eisenach J, Schricker T, Sessler DI. The need for large clinical studies in perioperative medicine. Anesthesiology. 2012;116:1169–75. doi: 10.1097/ALN.0b013e31825037bc. [DOI] [PubMed] [Google Scholar]
- 30.Bugar JM, Ghali WA, Lemaire JB, Quan H. Canadian Perioperative Research Network: Utilization of a preoperative assessment clinic in a tertiary care centre. Clin Invest Med. 2002;25:11–8. [PubMed] [Google Scholar]