Abstract
Background
Conversion disorder is characterized by unexplained neurological symptoms presumed related to psychological issues. The main hypotheses to explain conversion paralysis, characterized by a lack of movement, include impairments in either motor intention or disruption of motor execution, and further, that hyperactive self-monitoring, limbic processing or top-down regulation from higher order frontal regions may interfere with motor execution. We have recently shown that conversion disorder with positive abnormal or excessive motor symptoms was associated with greater amygdala activity to arousing stimuli along with greater functional connectivity between the amgydala and supplementary motor area. Here we studied patients with such symptoms focusing on motor initiation.
Methods
Subjects performed either an internally or externally generated two-button action selection task in a functional MRI study.
Results
Eleven conversion disorder patients without major depression and 11 age- and gender-matched normal volunteers were assessed. During both internally and externally generated movement, conversion disorder patients relative to normal volunteers had lower left supplementary motor area (SMA) (implicated in motor initiation) and higher right amygdala, left anterior insula and bilateral posterior cingulate activity (implicated in assigning emotional salience). These findings were confirmed in a subgroup analysis of patients with tremor symptoms. During internally versus externally generated action in CD patients, the left SMA had lower functional connectivity with bilateral dorsolateral prefrontal cortices.
Conclusion
We propose a theory in which previously mapped conversion motor representations may in an arousing context hijack the voluntary action selection system which is both hypoactive and functionally disconnected from prefrontal top-down regulation.
Keywords: conversion disorder, action selection, motor initiation, supplementary motor area, psychogenic movement disorder
Introduction
Conversion disorder, or unexplained neurological symptoms related to a psychological cause, is associated with high morbidity and financial burden1, 2 and is poorly understood. In this study, we focused on conversion disorder with positive motor symptoms (CD) such as tremor, dystonia and gait disorders. Two fundamental questions underpin the mechanisms of this disorder: how is the symptom generated and why is the symptom experienced as involuntary? We recently addressed an aspect of the first question. In response to arousing relative to neutral stimuli, CD patients have greater amygdala activity along with greater amygdala-supplementary motor area functional connectivity compared to healthy volunteers3. This finding, consistent with the observation that such patients have greater startle reflexes to arousing stimuli4, suggest that CD patients may have greater neural responses to arousal, along with a greater interaction between neural regions associated with arousal and motor control. Thus, an upstream mechanism may be influencing motor activity. We also indirectly addressed the second question by suggesting that the involuntary nature of conversion tremor may be related to abnormal generation of the sensory prediction during conversion tremor5. This current study sought to address the first question of generation of the conversion movement by focusing on motor initiation.
Previous functional imaging studies in conversion motor disorders have studied conversion paralysis characterized by movement absence, the mechanisms of which are likely going to differ from that of aberrant or excessive movement. The main hypotheses to explain conversion paralysis include either impairments in the generation of motor intention or conceptualization6–8 or that motor intention is intact but execution is disrupted9, 10. Furthermore, impairments in self-monitoring9, 11, 12, limbic processing10, 13 or top-down regulation from higher order frontal regions10, 14, 15 have been proposed to inhibit motor execution. Our study focuses on symptoms of aberrant or excessive movements. The generation of positive conversion motor symptoms may be characterized by abnormalities either in the process of selection or inhibition of the conversion motor representation during action selection or abnormal representation of the conversion movement programme possibly through implicit learning processes. Upstream inputs such as emotion, arousal or hyperactive self-monitoring may modulate these processes. The involuntary movements associated with positive conversion motor symptoms are believed to use the voluntary motor network based in part on the following observations16. First, conversion movements such as myoclonus are preceded by the Bereitschaftspotential, consistent with voluntary movements. Given the origin of the Bereitschaftspotential from regions including the premotor cortex, supplementary motor area and primary motor cortex, it is likely the conversion movements involve these same regions17. Second, the clinical sign of entrainment, which describes the observation that the frequency of conversion tremor entrains to the same frequency of any voluntary rhythmic movement made by patient, is a cardinal and diagnostic feature of conversion tremor. This interference can include an alteration of tremor frequency as well as full entrainment. In contrast, entrainment is not a feature of involuntary tremor from neurological disorders such as Parkinson’s disease or essential tremor. This observation suggests that the generators involved in voluntary movement and conversion tremor are highly interactive and likely utilize the same central oscillatory mechanism and similar pathways16. Since the same motor networks may be implicated in conversion movement symptoms and voluntary movement, we sought to understand whether regions associated with motor initiation, particularly the supplementary motor area, premotor cortex and primary motor cortex, were affected during voluntary action selection in CD patients compared to healthy volunteers. We reasoned that the process of voluntarily initiating an internally generated as compared to an externally generated response might engage similar motor preparatory systems utilized during the internal generation of involuntary conversion movements. Thus, we hypothesized that CD would be associated with abnormal activity in motor initiation regions during preparation for an internally generated relative to an externally generated action selection as compared to healthy volunteers. We secondarily hypothesized that CD would also be associated with abnormal activity in regions associated with self-monitoring or limbic activity during an internally generated relative to an externally generated action selection.
Methods
Patients
The patients with CD were recruited from the Human Motor Control Section clinic at the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH). Patients were included if they had “clinically definite” psychogenic movement disorder, a form of CD, as defined by Fahn and Williams diagnostic criteria18 (confirmed by two neurologists) (one neurologist was always MH) and a diagnosis of conversion disorder [Diagnostic and Statistical Manual of Mental Disorders, Version IV-TR (DSM IV)] (confirmed by a psychiatrist VV), lack of movement at rest and greater than 19 years old. Exclusion criteria included a current major depression, antidepressants or anticonvulsant medications, a serious neurological or medical illness or history of traumatic brain injury. Age- and gender-matched medication-free healthy volunteers were recruited from the NIH database. Subjects were assessed for psychiatric disorders using the, Structured Clinical Interview for the Diagnosis of DSM IV psychiatric diagnoses and completed the Beck Depression Inventory (BDI). The study was approved by the NIH IRB and all subjects gave informed consent.
Action selection task
Subjects were scanned while performing the action selection task (Figure 1A). During the two second selection phase, subjects saw either a directional cue (an arrow pointing left or right) to prompt an externally determined action selection (E) or a neutral cue (an upward pointing arrow) indicating internally determined action selection (I). Subjects were instructed to either “prepare to press the button in the specified direction of the arrow” to the E cue or “independently choose which of the two buttons to press” to the I cue. Subjects pressed the button during the choice phase (red cross, 1 second) using their right hand with a Lumina response box. Subjects practiced for 5 minutes and were monitored visually to ensure accurate task performance. The task was coded in e-PRIME.
Figure 1. Action selection task.
(A) Task. White arrow stimuli indicate internally generated (I) choice between one of two button responses or left or right cued externally generated (E) selection. The button press occurs at the red fixation cross, followed by a white fixation cross (F). (B) Selection effect of I-E. The SPM images represent the Selection effect of the mixed measures ANOVA comparing I-F and E-F as within-subject factors and conversion disorder patients and normal subjects as between-subject factors. VCA=vertical anterior commissure line, SMA=supplementary motor area. The SPM images are shown at P<0.001 uncorrected Cluster size >10.
Shannon’s Diversity Index for choice randomness
To determine the randomness of selection for the I responses we calculated Shannon’s diversity index (D) [D = 1/(pL*pL + pR*pR)] for each individual where pL is the probability of selecting the left button and pR is the probability of selecting the right button. The E-value, or measure of choice randomness, was calculated [E-value = D/Dmax] where Dmax reflects the total number of outcomes, with a pL and pR equal to 0.5. In this case, Dmax=2 and the E-value ranged from 0 to 1 with 1 reflecting perfectly random selection.
Statistical Analysis
The ages, BDI, choice randomness scores and reaction times (RT) were compared between groups using a two-sample t-test.
Imaging
MRI scans were obtained with a General Electric 1.5 Tesla scanner (8 channel head coil). Forty-two axial slices (slice thickness=2mm, gap 1mm) were acquired using T2*-weighted echo planar images at a temporal resolution of 3 seconds, echo time 33 msec, flip angle 90, matrix 64 × 64 with interleaved acquisition. The first four echo planar image volumes were discarded from analysis as dummy scans to allow for magnetization to reach steady state. To minimize head motion, the head was secured with an elastic bandage and foam padding. The imaging data was processed using SPM5 (www.fil.ion.ucl.ac.uk/spm) and adjusted for slice timing, realigned to the first image of the first run, normalized to the Montreal Neurological Institute atlas and smoothed using an 8mm Gaussian kernel.
For the imaging analysis, we modeled the selection phase, choice and the Fixation phase using the General Linear Model. To control for residual movement artifacts, head motion parameters derived from the realignment corrections were entered as regressors of no interest. Statistical parametric maps were generated from the linear contrasts between the hemodynamic response function parameter estimates. A random-effects group analysis was conducted on contrast images from the individual analyses. We used a repeated measures ANOVA with Selection (I-Fixation and E-Fixation contrasts) as a within-subjects factor and patient Group as a between-subjects factor with RT, BDI and choice randomness as covariates. For the hypotheses, focal activations were considered significant at P<0.05 FDR whole brain corrected 19.
Given a previous study demonstrating greater functional connectivity between higher order and lower order motor control regions in conversion paralysis15, on an exploratory basis, we also conducted psychophysiological interaction analyses focusing on these hypotheses. Psychophysiological interaction activations were considered significant at the cluster threshold of >40 contiguous voxels P<0.001 uncorrected.
Results
Patient characteristics and behavioral results
Eleven patients [mean age in years old: 52.5 (SD 9.0); females=7] and eleven controls [mean age in years old: 56.5 (SD 11.1); females=7] (t=1.01, p=0.71) were tested. Nine patients had tremor symptoms (4 bilateral upper extremities; 5 had involvement of all 4 extremities); one patient had right upper extremity dystonia; one patient had truncal dystonia; 4 patients had gait abnormalities. Three patients had a history of depression in remission; three had generalized anxiety disorder; one had panic attacks without panic disorder; nine had psychosocial stressors. Two patients were on benzodiazepines (lorazepam) for sleep or movement control, which were held on the night before imaging. Depression scores were significantly higher in CD patients [13.63 (SD=10.06)] relative to controls [4.00 (SD=3.74)] (t=2.98, df=21, p=0.007). RT was not significantly different between CD patients [mean RT in milliseconds I: 436.75 (SD 98.51); E: 417.39 (SD 95.32)] and controls [I: 394.06 (SD 78.03); E: 407.44 (SD 87.87)] [main effects: F(1,21)<0.51, p>0.48. Choice randomness was not different between CD patients [0.93 (SD 0.15)] and controls [0.94 (SD 0.09)] (t=0.21, df=21, p=0.21). There were no correlations between any of the behavioral outcomes of BDI, RT or choice randomness (Pearson correlation coefficient = −0.122 – 0.07; p>0.05).
Imaging Results
In the imaging analysis, as expected, there was a main Selection effect with greater I – E activity in motor initiation regions including bilateral supplementary motor area (SMA), left posterior parietal and right dorsal premotor cortex and right lateral prefrontal cortices along with the left anterior insular/inferior frontal cortex (Table 1) (Figure 1B)20–23. There was a main Group effect: relative to controls, CD patients had lower activity in the left SMA (Figure 2) and greater activity in the right amygdala, left anterior insula and bilateral posterior cingulate (Figure 3). There was no Group by Selection interaction effects. The analysis was repeated with only the tremor patients (N=9) which demonstrated decreased left SMA and increased right amygdala, left anterior insula and bilateral posterior cingulate activity but at a lower threshold (uncorrected P<0.001). In the psychophysiological interaction analysis, during I – E in CD patients, the left SMA had lower connectivity with bilateral dorsolateral prefrontal cortices (dlpfc) (BA 9/46) (Figure 4).
Table 1.
Main and interaction effects of action selection in conversion motor patients and normal volunteers.
| Effect | Contrast | Region | BA | x y z (mm)* | Z | Cluster size | FWE P-value | FDR P-value |
|---|---|---|---|---|---|---|---|---|
| Group | CD-NV | Bilateral posterior cingulate/culmen/parahipp gyrus | 19/23/29/30/31 | R: 10 −50 10 10 −48 24 L: −6 −52 8 |
5.27 | 480 | 0.01 | 0.005 |
| LAIC/inferior pfc | 13/47 | −34 22 −4 | 4.79 | 139 | 0.04 | 0.01 | ||
| R superior temporal | 22 | 54 −46 10 | 4.46 | 55 | 0.15 | 0.01 | ||
| L superior temporal | 21/22 | −62 −50 4 | 4.31 | 141 | 0.30 | 0.02 | ||
| R amygdala/parahipp gyrus | 34 | 20 −2 −14 | 4.25 | 52 | 0.25 | 0.02 | ||
| NV-CD | L thalamus/globus pallidus/posterior putamen | −20 −20 0 | 5.20 | 90 | 0.01 | 0.006 | ||
| L SMA | 6 | −8 −4 74 | 4.21 | 33 | 0.20 | 0.02 | ||
| Selection | I-E | LAIC/inferior pfc | 13/45/47 | −44 20 0 | 5.55 | 101 | 0.005 | 0.002 |
| L posterior parietal cx | 7/39/40 | −36 −56 38 | 4.88 | 351 | 0.03 | 0.006 | ||
| Bilateral SMA | 6/8 | 2 14 54 | 4.33 | 199 | 0.05 | 0.01 | ||
| R premotor cx | 6/8 | 36 0 62 | 4.21 | 50 | 0.15 | 0.03 | ||
| R ventrolateral pfc | 42 48 −4 | 4.18 | 65 | 0.36 | 0.04 | |||
| E-I | Bilateral posterior cingulate | 7/23/31 | −6 −56 18 6 −48 24 | 4.81 | 310 | 0.03 | 0.005 |
Peak local maxima in Montreal Neurological Institute coordinates
Abbreviations: BA=Brodmann area, FWE=family wise error whole brain corrected, FDR=false discovery rate whole brain corrected, uncorr=uncorrected, CD=conversion disorder patients, NV=normal volunteers, I=internally generated, E=externally generated, R=right, L=left, SMA=supplementary motor area, AIC=anterior insular cortex, pfc=prefrontal cortex, cx=cortex
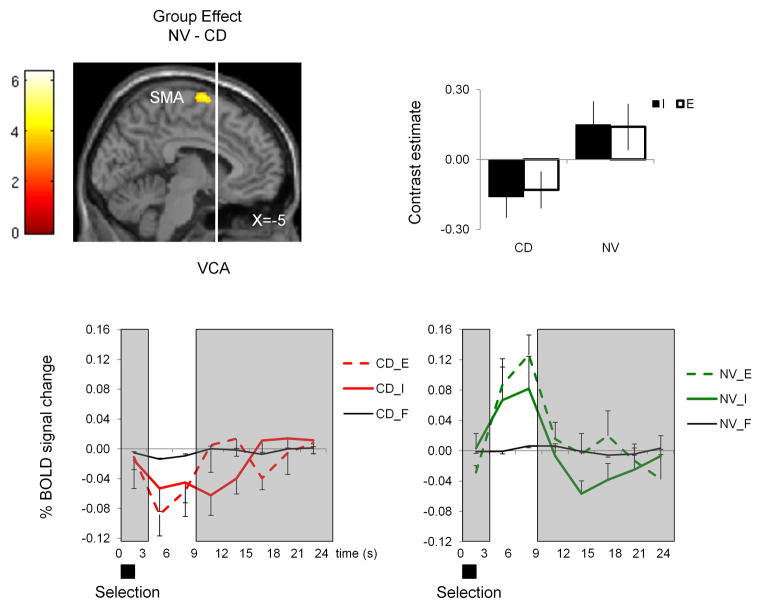
Figure 2. Group effect.
The SPM image represents the Group effect of the mixed measures ANOVA comparing internally generated versus fixation (I–F) and externally generated versus fixation (E–F) as within-subject factors and conversion disorder patients (CD) and normal subjects (NV) as between-subject factors. The SPM image shows decreased activity of the left supplementary motor area (SMA) in CD patients relative to NV during I-E. The contrast estimate and time course analysis for I and E in CD patients and NV are also shown for the left SMA. The time course analysis using the Finite Impulse Response function of MarsBar (marsbar.sourceforge.net) was time-locked to the two-second cue and the areas in white indicate the cue-related peak hemodynamic response function. The SPM image is shown at P<0.001 uncorrected Cluster size >10. S=seconds. Error bars represent standard deviation.
Figure 3. Group effect.
The SPM images represent the Group effect of the mixed measures ANOVA comparing internally generated versus fixation (I–F) and externally generated versus fixation (E–F) as within-subject factors and conversion disorder patients (CD) and normal subjects (NV) as between-subject factors. The SPM image shows increased activity in CD patients relative to NV during I-E. The contrast estimate for I and E in CD patients and NV are also shown. The SPM image is shown at P<0.001 uncorrected Cluster size >10. Error bars represent standard deviation.
Figure 4. Connectivity map of left supplementary motor area.

The SPM images and contrast estimates show functional connectivity between the left supplementary motor area (Seed) and decreased connectivity with bilateral lateral prefrontal cortices (BA 9/46) during internally generated (I) versus externally generated (E) action selection in conversion disorder (CD) patients relative to normal subjects (NV). Left lateral prefrontal cortex (x y z = −44 42 30 mm, Z=4.07, Cluster size=148, P<0.0001 uncorrected) and right lateral prefrontal cortex (x y z = 48 42 24 mm, Z=4.73, Cluster size=154, P<0.0001 uncorrected) The SPM image is shown at P<0.001 uncorrected Cluster size >10. Error bars represent standard deviation.
Discussion
In this study, we asked whether motor preparatory and limbic pathways were abnormally engaged during action selection in CD patients. CD patients relative to healthy subjects had lower activity in a region associated with motor preparation and inhibition, the left SMA, and greater activity in regions associated with emotional processing including the right amygdala, left anterior insula and bilateral posterior cingulate. We show that any form of motor initiation (i.e. both internally and externally generated) are likely relevant in defining an impairment of action selection in CD patients. In an exploratory analysis, CD patients relative to healthy volunteers, had lower functional connectivity between the dlpfc and left SMA during internal compared to external selection suggesting potentially impaired top-down regulation from higher order regions to guide action selection.
The supplementary motor area and prefrontal regulation
We recently demonstrated that CD patients have greater amygdala activity to arousing stimuli along with greater functional connectivity between the right amygdala and SMA3. Here we demonstrate that during motor initiation, CD patients have lower SMA activity thus implicating the SMA as a potential nodal point of motor impairment in CD patients with excessive motor activity. The SMA is a major source of input to the corticospinal tract24 and is reciprocally connected to the primary motor cortex25 and basal ganglia26. The supplementary motor complex is implicated in self-initiated action27, 28. In primate studies, the SMA is active during both internally generated and externally cued movements29 and is one source of the Bereitschaftspotential or ‘readiness potential’, a slowly increasing negative potential that precedes movement onset30 thus emphasizing its role in motor initiation. The supplementary motor complex has also been implicated in non-conscious motor inhibition31 and in the sense of agency32, or the experience that one is in control of one’s actions.
Although our main findings generalized to action selection in CD patients, the connectivity map observations conducted on an exploratory basis demonstrated lower functional connectivity of the SMA with bilateral dlpfc (BA 9/46) in CD patients during internally relative to externally generated actions. Multiple sources of evidence suggest a role for the dlpfc in higher order guidance of internally generated action selection. The dlpfc is activated during the selection between movements20, 23, 33 and the dlpfc and parietal cortex are believed to be involved in selecting the action-target representation23, 34 and biasing the competing representations encoded in the premotor cortex35, 36. The hypothesis of impaired top-down regulation has been previously raised in the conversion paralysis literature. Tiihonen et al. demonstrated in one patient with conversion paralysis and paresthesia that median nerve stimulation was associated with lower activity of the somatosensory parietal cortex along with greater activity of the lateral prefrontal cortex14. The authors suggested that greater prefrontal activity might play a top-down role in inhibition of the parietal cortex. De Lange et al. have recently shown greater functional connectivity between the dlpfc and sensorimotor regions during an imagery task in conversion paralysis characterized by lack of movement15. In contrast, our study in CD characterized by excessive movements demonstrates the opposite suggesting a potential disconnection in top-down motor control to guide internally generated action selection. The psychophysiological connectivity analyses do not provide the direction of causality; hence the role of the interactions is speculative. Taken together, we suggest that CD is associated with lower activity in a region implicated in motor control and suggest a potential impairment in top-down regulation from regions associated with higher motor control.
Limbic regions
During both I and E, CD patients had greater activity in regions associated with assigning salience including the right amygdala, left anterior insula and bilateral posterior cingulate. Our findings suggest a potential motor-limbic network that may influence motor action selection in CD patients. The amygdala increases in activity to both negative and positive stimuli with greater activity to negative relative to positive stimuli37. The anterior insula is implicated in the subjective representation of both internal bodily cues (temperature, gastric distention and pain) and feeling states38. The ventral posterior cingulate is proposed to act to evaluate the inputs of emotional objects and past events for self-relevance 39. A recent study has demonstrated greater posterior cingulate and primary motor cortex functional connectivity during externally cued motor initiation in conversion paralysis and suggested a role for internal states in influencing motor execution9. Put together, the greater activity of the amygdala, anterior insula and posterior cingulate in CD patients may be related to aberrant assignment of external or internal stimuli, states or memories as self-relevant or salient. In CD patients, these states may be more likely to facilitate the execution of specific motor representations (i.e. the conversion motor representation) or interfere with the general process of action selection via limbic-mesial frontal interactions.
Study limitations
We did not observe any behavioural differences consistent with the very simple nature of the task. This also means the neural findings are specific to motor preparation rather than secondary to any behavioural differences. Tasks with greater complexity are indicated to assess for behavioural differences in motor preparation. We had selected patients without conversion movements at rest and did not observe movements during the practice trial outside of the scanner. However, we did not monitor for conversion movements during motor preparation. An alternate and intriguing explanation may be that the abnormal motor preparatory activity represents either conversion movement preparation or inhibition. We argue that the findings are unlikely to represent conversion movement preparation or inhibition given our previous study comparing conversion tremor and mimicked voluntary movements40. In this previous study, the conversion movement would entail conversion movement preparation and the mimicked movements may also involve inhibition of conversion movements. We did not observe any differences in SMA activity suggesting that our findings in this current study may be specific to voluntary movement preparation. Although this study included different presentations of positive conversion motor presentations, only tremor patients were of sufficient sample size to conduct sub-analyses. Thus, whether the findings can be generalized beyond tremor symptoms remains to be established. That the study focuses only on positive conversion motor symptoms and hence does not include other conversion symptoms also limits generalizability.
Conclusion
We demonstrate that action selection in CD patients with aberrant or excessive motor symptoms is characterized by lower SMA activity, a region critical to motor initiation, and by greater activity in regions engaged in assigning salience. Our findings further suggest a potential impairment of prefrontal top-down regulation of motor control to guide action selection. We have previously shown that conversion tremor as compared to mimicked tremor is associated with lower temporoparietal junction activity, possibly representing a mismatch between motor prediction and outcome. We had suggested that this may represent aberrant conversion motor prediction or expectation40. We had also shown that arousing stimuli increases amygdala activity and increases amygdala-SMA functional connectivity in CD patients41. Here, we propose a theory in which previously mapped conversion motor representations may hijack the voluntary action selection system. During voluntary motor initiation, the action selection system is abnormally hypoactive and regions involved in assigning salience are abnormally hyperactive. In the context of arousing stimuli, the amygdala-supplementary motor complex is aberrantly engaged41, and without prefrontal top-down control, may facilitate the expression of salient previously learned and mapped conversion motor representations. The aberrant conversion motor prediction may conflict with intended motor prediction resulting in a mismatch between prediction and outcome and hence the sense of involuntariness. This theory may explain the expression of aberrant involuntary conversion motor symptoms occurring in an arousing context. Thus, a patient under stress, rather than their intended movement of reaching for a cup for instance, may experience an involuntary action such as tremor.
Acknowledgments
Funding source for study: Intramural National Institutes of Health
This study was funded by and conducted at the intramural National Institute of Neurological Disorders and Stroke, National Institutes of Health
Footnotes
Author roles:
Valerie Voon:
Research Project: A. Conception, B. Organization, C. Execution
Statistical Analysis: A. Design, B. Execution
Manuscript Preparation: A. Writing of first draft, B. Review and critique
Christina Brezing:
Research Project: A. Conception, B. Organization, C. Execution
Statistical Analysis: A. Design, B. Execution
Manuscript Preparation: A. Writing of first draft, B. Review and critique
Cecile Gallea:
Statistical Analysis: B. Execution
Manuscript Preparation: B. Review and critique
Mark Hallett
Research Project: A. Conception
Statistical Analysis: A. Design
Manuscript Preparation: B. Review and critique
Disclosure
Dr. Voon has not had any activities to disclose for the last two years.
Dr. Brezing has not had any activities to disclose for the last two years.
Dr. Gallea has not had any activities to disclose for the last two years.
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds came from the US Army via the Henry Jackson Foundation, Ariston Pharmaceutical Company via a Cooperative Research and Development Agreement (CRADA) with NIH, and the Kinetics Foundation via a Clinical Trials Agreement (CTA) with NIH.
Dr. Hallett serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation and Chair of the Medical Advisory Board of the International Essential Tremor Foundation. He serves on editorial advisory boards for Clinical Neurophysiology, Brain, Acta Neurologica Scandinavica, Journal of Clinical Neurophysiology, Italian Journal of Neurological Sciences, Medical Problems of Performing Artists, Annals of Neurology, Neurology and Clinical Neurophysiology, The Cerebellum, NeuroRx, Current Trends in Neurology, Faculty of 1000 Medicine, Brain Stimulation, Journal of Movement Disorders (Korea), and is Editor-in-Chief of World Neurology. He receives publishing royalties from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, Massachusetts Medical Society, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia University, the Parkinson and Aging Research Foundation, University of Maryland, University of Wisconsin, State of New York, and University of Navara.
References
- 1.Carson AJ, Best S, Postma K, Stone J, Warlow C, Sharpe M. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897–900. doi: 10.1136/jnnp.74.7.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass C, Peveler R, House A. Somatoform disorders: severe psychiatric illnesses neglected by psychiatrists. Br J Psychiatry. 2001;179:11–14. doi: 10.1192/bjp.179.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seignourel PJ, Miller K, Kellison I, et al. Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov Disord. 2007;22:1265–1271. doi: 10.1002/mds.21451. [DOI] [PubMed] [Google Scholar]
- 5.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgmer M, Konrad C, Jansen A, et al. Abnormal brain activation during movement observation in patients with conversion paralysis. Neuroimage. 2006;29:1336–1343. doi: 10.1016/j.neuroimage.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–1244. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- 8.Roelofs K, van Galen GP, Keijsers GP, Hoogduin CA. Motor initiation and execution in patients with conversion paralysis. Acta Psychol (Amst) 2002;110:21–34. doi: 10.1016/s0001-6918(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 9.Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- 11.de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45:2051–2058. doi: 10.1016/j.neuropsychologia.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 12.de Lange FP, Roelofs K, Toni I. Motor imagery: a window into the mechanisms and alterations of the motor system. Cortex. 2008;44:494–506. doi: 10.1016/j.cortex.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- 14.Tiihonen J, Kuikka J, Viinamaki H, Lehtonen J, Partanen J. Altered cerebral blood flow during hysterical paresthesia. Biol Psychiatry. 1995;37:134–135. doi: 10.1016/0006-3223(94)00230-Z. [DOI] [PubMed] [Google Scholar]
- 15.de Lange FP, Toni I, Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Hallett M. Physiology of psychogenic movement disorders. J Clin Neurosci. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231–257. [PubMed] [Google Scholar]
- 19.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 20.Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 21.Hyder F, Phelps EA, Wiggins CJ, Labar KS, Blamire AM, Shulman RG. “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc Natl Acad Sci U S A. 1997;94:6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading hidden intentions in the human brain. Curr Biol. 2007;17:323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 23.Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- 24.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticospinal projections from mesial frontal and cingulate areas in the monkey. Neuroreport. 1994;5:2545–2548. doi: 10.1097/00001756-199412000-00035. [DOI] [PubMed] [Google Scholar]
- 26.Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- 27.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 28.Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one’s own performance. Trends Cogn Sci. 2010;14:16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romo R, Schultz W. Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp Brain Res. 1987;67:656–662. doi: 10.1007/BF00247297. [DOI] [PubMed] [Google Scholar]
- 30.Deecke L, Kornhuber HH. An electrical sign of participation of the mesial ‘supplementary’ motor cortex in human voluntary finger movement. Brain Res. 1978;159:473–476. doi: 10.1016/0006-8993(78)90561-9. [DOI] [PubMed] [Google Scholar]
- 31.Sumner P, Nachev P, Morris P, et al. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore JW, Ruge D, Wenke D, Rothwell J, Haggard P. Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci. 2010;277:2503–2509. doi: 10.1098/rspb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J Neurophysiol. 1990;64:164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- 36.Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 37.Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 38.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 39.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]