Abstract
Background
Progression of Parkinson’s disease (PD) is characterized by motor deficits, which eventually respond less to dopaminergic therapy and, thus, pose a therapeutic challenge. Deep brain stimulation has proven efficacy, but carries risks and is not possible in all patients. Non-invasive brain stimulation has shown promising results and may provide a therapeutic alternative.
Objective
To investigate the efficacy of transcranial direct current stimulation (tDCS) in the treatment of PD
Design
Randomized, double blind, sham-controlled study.
Setting
Research institution
Methods
We investigated efficacy of anodal tDCS applied to the motor and prefrontal cortices in 8 sessions over 2.5 weeks. Assessment over a 3-month period included timed tests of gait (primary outcome measure) and bradykinesia in the upper extremities, UPDRS, Serial Reaction Time Task, Beck Depression Inventory, Health Survey and self-assessment of mobility.
Results
Twenty-five PD patients were investigated, 13 receiving tDCS and 12 sham stimulation. TDCS improved gait by some measures for a short time and improved bradykinesia in both the on- and off-states for longer than 3 months. Changes in UPDRS, reaction time, physical and mental well-being, and self-assessed mobility did not differ between tDCS and sham intervention.
Conclusion
TDCS of the motor and prefrontal cortices may have therapeutic potential in PD, but better stimulation parameters need to be established to make the technique clinically viable.
Keywords: transcranial direct current stimulation (tDCS), non-invasive brain stimulation, therapeutic study, Parkinson’s disease
Background
The progression of Parkinson’s disease (PD) is therapeutically challenging. In the early stages of PD, motor deficits respond to dopaminergic therapy. This response diminishes and additional symptoms arise which result from the progressive degeneration affecting non-dopaminergic neuronal systems [1]. One hallmark of this progression is the emergence of difficulties with gait and postural control, which eventually become refractory and critical causes of disability.
Surgical interventions, primarily deep brain stimulation (DBS) of various target nuclei, are the ultimate therapeutic option when conventional therapy fails. Yet, DBS is limited to a small well-defined patient population and carries the risk of serious surgical complications and significant neuropsychiatric side effects. Therapeutic alternatives are needed.
Therapeutic studies of non-invasive brain stimulation, foremost repetitive transcranial magnetic stimulation (rTMS), have yielded promising results in PD. Two meta-analyses concluded there was a modest therapeutic effect of rTMS in motor performance in PD [2;3].
Transcranial direct current stimulation (tDCS) is another mode of non-invasive brain stimulation, whereby a direct current is applied via surface electrodes on the head for a certain time in contrast to the electric impulse induced by the short-lasting magnetic field in TMS. The possibility to modulate cortical excitability [4;5] and to promote motor learning in healthy adults [6] and motor recovery in chronic stroke [7] has raised interest in tDCS as an intervention in PD. An open study reported improvement of gait and bradykinesia in PD [8]. A recent cross-over study found acute motor improvement after a single session [9]. These findings are promising, especially since refractory gait disturbances might be improved. Thus, these effects need to be confirmed in a controlled study and explored to determine if they persist for a longer period to exert a therapeutic benefit.
TDCS carries some advantages over rTMS, including a favorable safety profile, tolerability, easier applicability and cost-effectiveness. Thus, tDCS could potentially complement the therapeutic armamentarium.
In this double-blind, randomized, sham-controlled study, we investigated whether anodal tDCS of the motor and prefrontal cortices improves gait and bradykinesia in PD and whether these effects persist for a longer time. The primary endpoint of interest was improvement of gait in the on-state, chosen to see if any benefit would be beyond current best therapy.
Methods
Study population
Inclusion criteria were patients aged 40 to 80 years with PD according to UK PD Brain Bank criteria in a Hoehn and Yahr (HY) stage of 2 to 4 while “off” medication. Patients had to have slowing of gait defined as a time of 6 seconds or more to walk 10 meters. Patients with severe freezing or unable to walk 10 meters were excluded. Patients were required to be on an optimal medication regimen with a total levodopa equivalent dose (LED) of ≥ 300 mg. Exclusion criteria were significant medical or psychiatric illness, and metal objects or stimulators in the head, which might pose a hazard during tDCS.
A power analysis yielded a sample size of 21 participants per arm providing 80% power with two-sided alpha=0.05 postulating a 20% improvement in gait time in the on-state with tDCS compared to sham (primary outcome). After enrollment of more than 50 % target population, we recalculated the power because of absence of subjective gait improvement. The rather small effect size of 0.256 would have required a sample size of 292. We opted to terminate the study. Thus, in this study, we prospectively investigated twenty five patients with mild to moderate PD (9 women, mean age 63.9 ± 8.7 years, range 49–77 years, all right-handed; HY stage mean 2.4 ± 0.2 in “on” and 2.8 ± 0.4 in “off” medication). Demographic and clinical findings of patients in the tDCS (n=13) and sham (n=12) intervention groups were comparable (see Table 1).
Table 1.
Demographic and clinical findings (± standard deviation) in the patients with Parkinson’s disease (PD) receiving tDCS (n=13) or sham (n=12).
| Sham (n=12) | tDCS (n=13) | p value | |
|---|---|---|---|
| Age (y) | 64.2 ± 8.8 | 63.6 ± 9.0 | 0.88* |
| Gender (women) | 5 (41.7%) | 4 (30.8%) | 0.57† |
| Age at onset (y) | 55.1 ± 8.7 | 53.5 ± 11.5 | 0.70§ |
| Duration of disease (y) | 9.1 ± 3.3 | 10.6 ± 7.1 | 0.49§ |
| Hoehn-Yahr on | 2.4 ± 0.2 | 2.5 ± 0.1 | 0.47§ |
| Hoehn-Yahr off | 2.9 ± 0.4 | 2.7 ± 0.3 | 0.50§ |
| LED (total, mg) | 1287.7 ± 808.8 | 1024.3 ± 541.5 | 0.35§ |
| Tremor (present) | 8 (66.7%) | 6 (46.2%) | 0.30† |
| Freezing (present) | 11 (91.7%) | 10 (76.9%) | 0.32† |
| Fluctuations (present) | 10 (83.3%) | 10 (76.9%) | 0.69† |
| Dyskinesias (present) | 7 (58.3%) | 8 (61.5%) | 0.87† |
| Falls (present) | 6 (50%) | 10 (76.9%) | 0.16† |
LED = Levodopa equivalent dosage,
independent t-Test,
Mann-Whitney U-Test,
Pearson χ2-Test
The NIH Institutional Review Board approved the study and the early termination. We obtained written informed consent from all study participants. This study was publicly registered (ClinicalTrial.gov: NCT00082342).
TDCS intervention
TDCS was applied in 8 sessions within 2–1/2 weeks (Monday, Wednesday and Friday) when “on” medication. Patients were at rest without concurrent cognitive or motor task. A battery-driven stimulator, Phoresor II Model PM850 (IOMED), delivered the tDCS through electrodes (saline-soaked sponges). The device is FDA-approved for trans-dermal ionto-phoretic drug delivery and for the purpose of tDCS considered no significant risk. We randomly assigned patients to a real or sham group according to a computer-generated number with equal probability. All were naïve to tDCS. In the tDCS treatment group, anodal tDCS (2mA) was delivered for 20 min through a large “3.5″× 7″ Rubber Pad W/Sponge Insert” electrode (surface 97.5 cm2; current density 0.021 mA/cm2) that we placed symmetrically either over the pre- and motor (electrode’s center 8 mm anterior to Cz) or prefrontal cortices (forehead above eyebrows). We stimulated a single target area during one session and alternated the anode’s position between sessions (starting with the motor area) so that each target area was stimulated 4 times. Cathodes (25 cm2 each) were positioned over the mastoids. These specific montages and approximate spatial distribution of the current density during tDCS were reported elsewhere [10]. In sham tDCS, we placed anode and cathode (each 9 cm2) 1 cm apart over the forehead and DC (1mA) applied for 1–2 min, which was short-circuited through the skin creating the same temporary “tingling” sensation without effects on the brain. We placed two additional electrodes inversely over the mastoids, not connected to the stimulator. We set up the stimulating apparatus out of sight of the patients and blinded investigators. In both sham and tDCS, the current was ramped up over 10 sec and similarly decreased. A temperature sensor in the first three patients demonstrated that skin temperature did not increase during stimulation.
Clinical assessment
Baseline and follow-up evaluations were performed before and 24 hrs, 1 and 3 months after the last tDCS intervention session. Primary outcome measures were the change in the timed test of gait in the on- and off-state 24 hrs after the intervention period compared to baseline. Secondary measures included changes 1 and 3 months after intervention completed. We assessed gait by measuring the time to walk 10 meters. Patients were instructed to walk at a fast pace without taking the risk of falling wearing the same shoes and using assistive devices consistently if needed. We timed gait from initiation while standing and in the same conditions (location, lighting, etc.). Secondary outcome measures included bradykinesia assessed in the hands and arms. We measured the time to perform the following sequence ten times: 1) hand-closing (squeezing a ball) and -opening – 2) elbow-flexion – 3) hand-closing and -opening – 4) elbow–extension. This is similar to a previously studied sequential task with elbow flexion and hand closure shown to correlate with bradykinesia [11]. Before baseline assessment, patients practiced until performance appeared not to get faster and, then, abstained from further practice to minimize learning effects. We chose timed tests because they are more sensitive for detecting changes than scores such as UPDRS and are independent from subjective assessment. These motor tests and the UPDRS were assessed in the “best on-” and “practically-defined off-state” by the same blinded raters for the entire study on the same day. “Practically-defined off-state” corresponded to overnight (≥ 12 hr) withdrawal of dopaminergic medication and preceded, therefore, assessment in the “best on-state”, considered by the patients and blinded rater to be the best response to their usual dopaminergic medication. Gait and bradykinesia were also timed before and after each intervention to evaluate acute effects. Additionally, un-blinded investigators performed a short clinical assessment to monitor for the safety of tDCS.
The evaluation included the Beck Depression Inventory (BDI) and a Health Survey (SF-12v2) addressing the subjective perception of health and well-being. Patients appraised their state of mobility by checking boxes of defined states (“on”-condition, “on” with dyskinesias, “off”-condition, “off” with tremor or sleep) for each hour in a log (for 3 days). Visuomotor speed and procedural learning were tested in the Serial Reaction Time Task (SRTT) as described [12].
Statistical Analysis
To compare the two groups on various outcome measures, a two-sample t test or Wilcoxon ranked sum test, whichever was deemed appropriate, was used. Fisher’s exact test was used to assess association between two categorical variables. For longitudinal continuous data, summary statistics (mean, standard deviation) at each time point were reported. Linear mixed effects models (SAS, Proc Mixed, [13]) were used to analyze the serial gait time. The independent variables included group (tDCS or sham), condition (“on” or “off”), time (1 day, 1 month, and 3 months after the intervention), and all the 2- and 3-way interactions of the above three factors. The baseline gait measure was used as a covariate. The intercept and the time variable were treated as the random effects to account for within-subject variability. An unstructured variance-covariance matrix was adopted in the model. Similar approaches were used to analyze other outcome measures such as the sequential hand and arm movement time (the average of the left and right sides of the patient), UPDRS, UPDRS III, UPDRS bradykinesia. For session data on gait and hand and arm movement, treatment differences were assessed with linear mixed effects models, in which post-session measures were the dependent variable, and pre-session measures; session, treatment group, and the interaction of these two were the independent variables. As for the analysis of the learning rate in the SRTT, we used linear mixed effects models with autoregressive (1) covariance structure to take into account the correlations among the 5 measurements (blocks 2–6) per patient. The primary comparisons for this study were the between-group differences in changes from baseline to 1 day after the treatment in gait time in the “on” or “off” condition. A two-sided significance threshold of 0.025 was set to adjust for the multiplicity. All other comparisons obtained from the model-based contrasts were secondary. P-values less than 0.05 were considered significant. Statistical analysis was done using both SPSS (Version 12.0.1) and SAS (Version 9.1).
Results
All 25 patients enrolled completed the study. In a single patient, there were small first-degree burns likely caused by accidentally mal-positioned electrodes over the mastoids partially covering the earlobes with reduced contact surface resulting in an increased current density, which healed completely within 3 days. We observed no other adverse events. All patients experienced occasional “tingling”, which was most commonly of short-duration, but no pain or discomfort. Blinding appeared reliable based on patients’ and blinded raters’ reports. At baseline, primary and secondary outcome measures did not differ between the groups besides a trend toward a lower score of physical health in the sham group (p=0.065) (Tables 1–3).
Table 3.
Secondary outcome measures (± standard deviation) at baseline, 1 day, and at 1 and 3 months after the tDCS and sham intervention
| Secondary outcome measures |
Baseline | 1 day post- intervention |
p value (95% CI) |
1 month post- intervention |
p value (95% CI) |
3 months post- intervention |
p value (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| tDCS sham | ||||||||
| UPDRS | on | 42.5 ± 10.8 39.5 ± 12.8 |
36.9 ± 11.1 32 ± 11.7 |
0.30 (−9.9, 3.1) | 42.5 ± 7.8 36.1 ± 11 |
0.16 (−10.7, 1.87) | 43.4 ± 9.1 35.5 ± 13.8 |
0.31 (−8.2, 2.6) |
| off | 58.4 ± 14.7 53.6 ± 14.5 |
54.1 ± 13.1 50.4 ± 16.2 |
0.98 (−7.8, 7.7) | 56.1 ± 13.2 54.4 ± 12.1 |
0.80 (−8.5, 6.6) | 60.1 ± 13.6 52.4 ± 17 |
0.52 (−10.4, 5.3) | |
| UPDRS (III) | on | 22.2 ± 8.7 17.5 ± 8 |
20.4 ± 7.7 15.6 ± 7.9 |
0.49 (−3.2, 1.5) | 22.7 ± 6.6 18.6 ± 8.1 |
0.38 (−5.3, 2.1) | 23.1 ± 7.8 17.6 ± 8.5 |
0.40 (−5.1, 2.1) |
| off | 34 ± 10 26.5 ± 8.4 |
31.5 ± 7.3 29 ± 11.7 |
0.083 (−1.0, 7.9) | 31.4 ±9.9 29 ± 8.1 |
0.54 (−3.2, 6.1) | 34 ± 10.3 27.1 ± 10.5 |
0.81 (−5.6, 4.4) | |
| UPDRS Bradykinesia* | on | 7.2 ± 2.5 6.8 ± 3.1 |
6.7 ± 3.1 5.8 ± 2.8 |
0.41 (−1.5, 0.6) | 7.7 ± 2.5 7.8 ± 3.3 |
0.91 (−1.8, 1.6) | 7.8 ± 3.1 6.8 ± 3.5 |
0.53 (−2.5, 1.3) |
| off | 12 ± 4.3 9.5 ± 3.9 |
10.7 ± 4.7 10.3 ± 3.9 |
0.039 (0.099, 3.6) | 11.2 ± 4.7 11.4 ± 4.4 |
0.13 (−0.4, 3.4) | 11.8 ± 4.6 10.4 ± 4.5 |
0.40 (−1.2, 2.9) | |
| SRTT | reaction time (ms) | 789 ± 247 755 ± 122 |
731 ± 315 773 ± 254 |
0.72 (−131.5, 188.7) | 657 ± 153 777 ± 284 |
0.92 (−156.7, 148.9) | 703 ± 299 753 ± 142 |
0.13 (−35.4, 272.5) |
| error rate (%) | 17.6 ± 19.6 9.3 ± 12.8 |
17.7 ± 23.3 14.1 ± 18.3 |
0.27 (−0.04, 0.11) | 14.1 ± 17.7 18.5 ± 23.3 |
0.18 (−0.02, 0.12) | 26.1 ± 28.3 11.2 ± 12.5 |
0.52 (−0.07, 0.13) | |
| BDI | 10.4 ± 11.3 7 ± 4.2 |
7.5 ± 6.8 5.5 ± 3.8 |
0.48 (−2.2, 1.0) | 6.5 ± 4.1 7.7 ± 6.5 |
0.19 (−1.5, 7.3) | 8.0 ± 6.4 5.3 ± 5.1 |
0.49 (−4.7, 2.3) | |
| Health survey (SF-12v2) | physical health mental health | 44.3 ± 7.2 38.1 ± 8.7 50.6 ± 9.7 48.7 ± 10.5 |
42.0 ± 7.9 41.0 ± 7.9 54.1 ± 7.0 52.5 ± 9.7 |
0.15 (−1.3, 7.9) 0.84 (−6.1, 5.0) |
39 ± 8.9 41.1 ± 10.2 53.9 ± 10.0 52.7 ± 9.4 |
0.10 (−1.0, 11.1) 0.93 (−5.0, 5.5) |
39.5 ± 8.7 38.5 ± 10.2 53.6 ± 8.3 50.2 ± 12.4 |
0.28 (−2.7, 9.1) 0.49 (−9.1, 4.5) |
| Self-assessment | sleep (hrs) | 6.6 ± 0.1 6.6 ± 0.1 |
6.9 ± 0.1 6.8 ± 0.1 |
0.48 (−0.14, 0.07) | 6.4 ± 0.1 6.4 ± 0.1 |
0.93 (−0.08, 0.07) | 6.7 ± 0.2 6.6 ± 0.1 |
0.25 (−0.22, 0.06) |
| on (hrs) | 11.0 ± 0.3 11 ± 0.2 |
11.3 ± 0.3 11.4 ± 0.2 |
0.08 (−0.02, 0.28) | 10.9 ± 0.1 10.9 ± 0.1 |
0.84 (−0.09, 0.11) | 12.0 ± 0.1 12.1 ± 0.1 |
0.12 (−0.02, 0.16) | |
| dyskinesias (hrs) | 2.4 ± 0.1 2.5 ± 0.1 |
3.2 ± 0.2 3.2 ± 0.1 |
0.90 (−0.11, 0.10) | 2.8 ± 0.1 2.9 ± 0.1 |
0.40 (−0.04, 0.10) | 2.1 ±0.1 2.1 ± 0.1 |
0.89 (−0.8, 0.10) | |
| off (hrs) | 3.2 ± 0.3 3.2 ± 0.2 |
2.5 ± 0.3 2.4 ± 0.3 |
0.32 (−0.29, 0.09) | 3.4 ± 0.1 3.4 ± 0.1 |
0.28 (−0.15, 0.04) | 2.7 ± 0.2 2.7 ± 0.1 |
0.85 (−0.09, 0.08) | |
| off tremor (hrs) | 0.8 ± 0.2 0.8 ± 0.1 |
0.2 ± 0.1 0.1 ± 0.0 |
0.83 (−0.01, 0.02) | 0.4 ± 0.1 0.4 ± 0.1 |
0.72 (−0.06, 0.08) | 0.8 ± 0.1 0.8 ± 0.0 |
0.83 (−0.07, 0.08) | |
Results of tDCS and sham groups are presented in first and second row, respectively. 95% confidence intervals were obtained from the linear mixed effects models. BDI = Beck Depression Inventory
UPDRS Bradykinesia: composite UPDRS bradykinesia score consisting of items 23–25: finger tapping, opening/closing and pro-/supination of the hands.
Gait
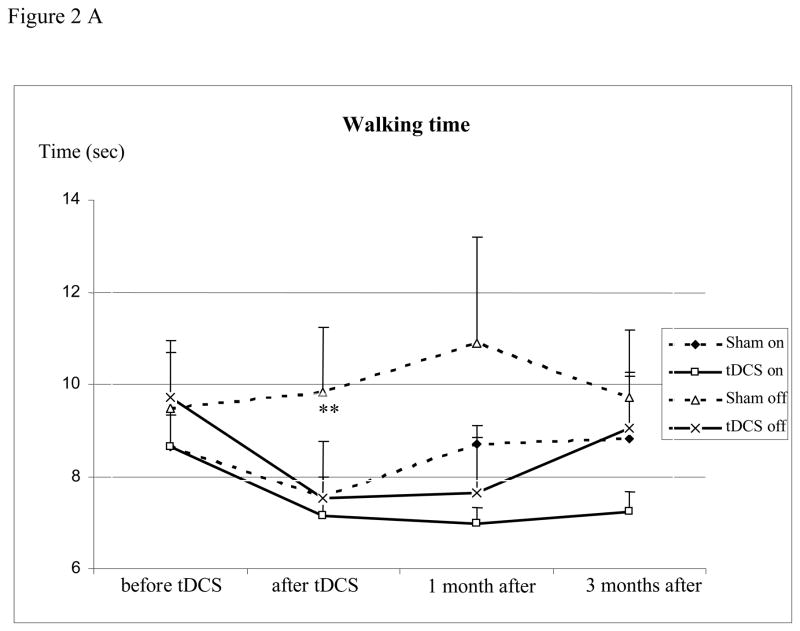
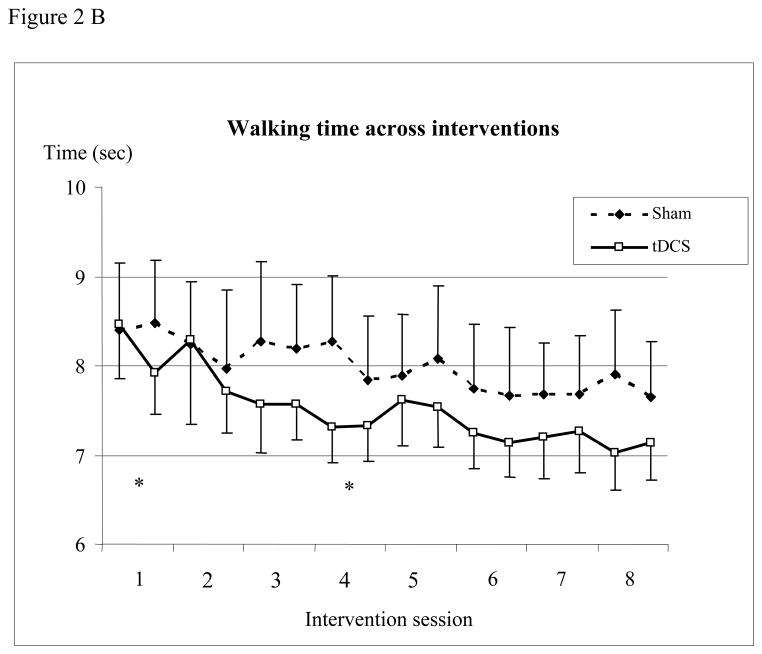
Compared to sham intervention, the decrease in walking time in those receiving tDCS in the “off” condition 1 day after tDCS barely missed significance (−22.6% vs. −19.6%, p=0.03). Since walking times in the off-state alone in one patient (sham stimulation) were extreme outliers (baseline 87.8 sec and a day, at 1 and 3 months after tDCS 46.3, 24.6 and 9.7 sec) and the marked decrease remained inexplicable but for the patient’s declared desire that the intervention be successful, we repeated the analysis of the off-state excluding those measurements, and this analysis indicated a significant decrease of walking time with tDCS (−22.6% vs 3.6%; p=0.002). No differences were seen when “on” (−17.4% vs. −12.7%, p=0.44) or beyond the immediate post-intervention period at 1 or at 3 months thereafter (Table 2, Figure 2A). Comparing post-interventional performance (1 day after the last intervention) with baseline in each group, the decrease in walking time was significant with tDCS and sham when “on” (− 17.4%, p<0.01, and − 12.7%, p=0.03) and with tDCS when “off” (− 22.6%, p<0.01) and remained significant 1 month later in the tDCS group when “on” (− 19.3%, p=0.02). Walking time decreased with tDCS compared to sham intervention when looking at the sessions (Treatment x Time interaction, p = 0.0007, Figure 2B) being significant at the first session (p = 0.014), while the opposite was found in the fourth session (p = 0.011).
Table 2.
Gait and sequential hand and arm movement time (± standard deviation) at baseline, 1 day, and at 1 and 3 months after the tDCS and sham intervention
| Timed tests (sec) | Baseline | 1 day post-inter-vention | p value (95% CI) | 1 month post-inter-vention | p value (95% CI) | 3 months post-inter-vention | p value (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| tDCS sham | ||||||||
| Gait * | on | 8.7 ± 2.4 8.6 ± 2.6 |
7.2 ± 1.6 7.6 ± 1.5 |
0.43 (−1.5, 1.4) | 7.0 ± 1.2 8.7 ± 3.7 |
0.87 (−2.1, 2.5) | 7.2 ± 1.5 8.8 ± 4.2 |
0.57 (−8.0, 4.4) |
| off | 9.7 ± 3.6 9.5 ± 4.1 |
7.5 ± 1.5 9.8 ± 4.7 |
0.0019 (1.0, 4.3) | 7.6 ± 1.4 10.9 ± 7.3 |
0.19 (−1.1, 5.5) | 9.0 ± 4.8 9.7 ± 4.6 |
0.30 (−0.7, 2.3) | |
| Hand and arm movements | on | 12.3±3.5 12.3±4.0 |
8.5 ± 1.8 10.5 ± 2.3 |
0.0021 (0.8, 3.2) | 9.1 ± 2.0 10.5 ± 2.3 |
0.0299 (0.1, 2.2) | 9.0 ± 1.8 10.4±2.5 |
0.0075 (0.4, 2.7) |
| off | 14.5±4.5 14.4±5.3 |
8.9 ± 1.8 11.1 ± 2.6 |
<.0001 (1.3, 3.2) | 9.4 ± 1.9 11.7 ± 3.8 |
0.0147 (0.4, 3.7) | 9.7 ± 2.0 11.6±4.0 |
0.0238 (0.3, 3.6) | |
Gait measurements excluding a patient with extreme outliers. Results of tDCS and sham groups are presented in first and second row, respectively. 95% confidence intervals were obtained from the linear mixed effects models.
Figure 2.
(A) Walking time before, 1 day, and at 1 and 3 months after the intervention (mean ± standard error). The figure shows the decrease in time needed to walk 10 meters in the “on” and “off” condition. Abscissa indicates the time of measurement. Ordinate indicates the walking time. The solid lines indicate the tDCS (n=13) and the dashed lines the sham group (n=11, patient with outliers excluded). Triangles and crosses indicate the “off” (medication) condition and diamonds and squares indicate the “on” condition measurements. (B) Walking time before and after each intervention (mean ± standard error). The figure shows the time needed to walk 10 meters. Abscissa indicates the time of measurement; ordinate indicates the walking time. The solid lines indicate the tDCS (n=13) and the dashed lines the sham group (n=12). Walking time decreased with tDCS compared to sham intervention when looking at the sessions (Treatment x Time interaction, p = 0.0007) being significant at the first session (p = 0.014), while the opposite was found in session 4 (p = 0.011). (* p <0.05; ** p <0.01)
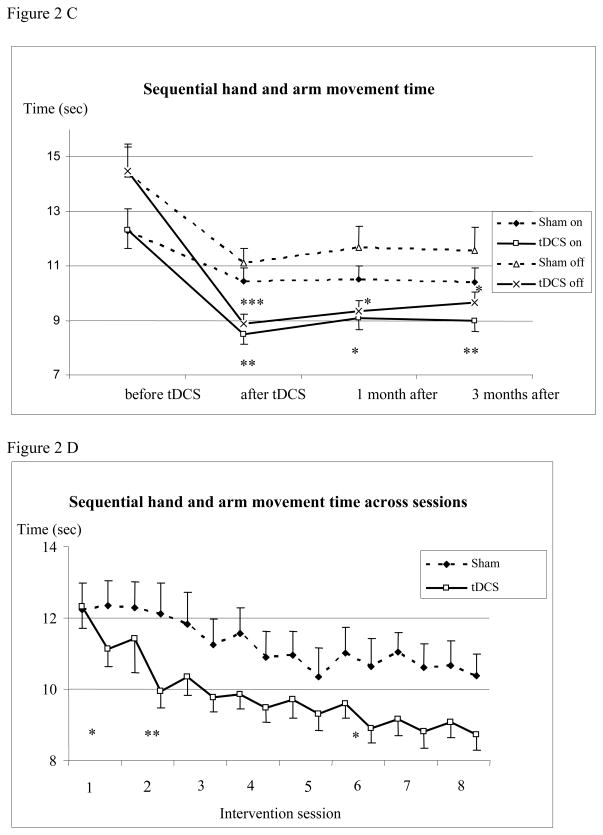
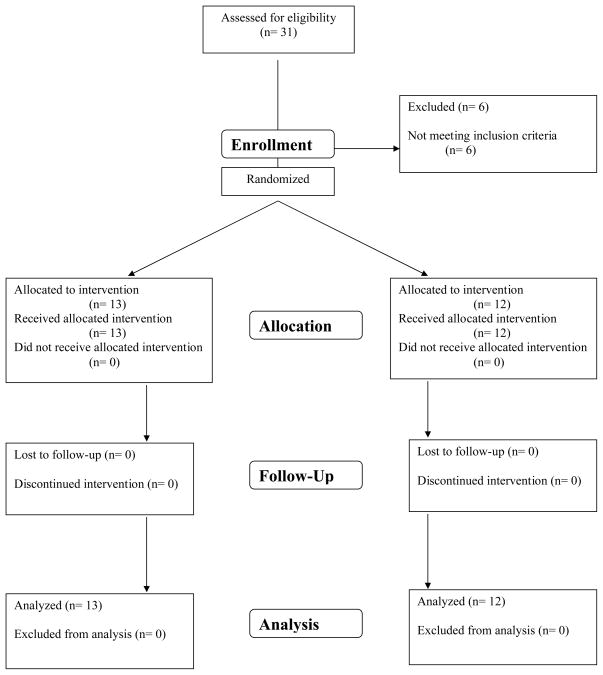
Figure 2(C) Sequential hand and arm movement test before, 1 day, at 1 and 3 months after the intervention (mean ± standard error). The figure shows the decrease of time needed to execute the sequential hand and arm movement test in the “on” and “off” conditions which persist for 3 months after tDCS. Measurements for the left and right hands were pooled. Abscissa indicates the time of measurement. Ordinate indicates the execution time. The solid lines indicate tDCS (n=13) and the dashed lines sham group (n=12). Triangles and crosses indicate the “off” (medication) condition and diamonds and squares indicate the “on” condition measurements. (D) Sequential hand and arm movement test before and after each intervention (mean ± standard error). The figure shows the time needed to execute the sequential hand and arm movement test. Measurements for the left and right hands were pooled. Abscissa indicates the time of measurement; ordinate indicates the execution time. The solid lines indicate tDCS and the dashed lines sham group. Sequential hand and arm movement time decreased with tDCS compared to sham intervention when looking at the sessions (Treatment x Time interaction, p = 0.015) being significant after the first two and sixth session (p = 0.035, 0.005 and 0.034). (* p <0.05; ** p <0.01;*** p <0.0001)
Bradykinesia
Bradykinesia decreased significantly more with tDCS than sham intervention (−28.4% vs. −11%, p=0.002, when “on” and −36.0% vs. −17.8%, p<0.0001, when “off”; Table 2, Figure 2C). Comparing post-interventional performance with baseline in each group, the decrease in bradykinesia was significant in the tDCS and sham group (at all time-points in on and off condition, p<0.001 and p<0.05, respectively, Figure 2C). Bradykinesia decreased with tDCS compared to sham intervention when looking at the sessions (Treatment x Time interaction, p = 0.015; Figure 2D) being significant after the first two and sixth session (p = 0.035, 0.005 and 0.034).
UPDRS
Compared to sham, tDCS had no effects on the total and motor UPDRS scores (Table 3). However, a composite UPDRS bradykinesia score (items 23–25: finger tapping, opening/closing and pro-/supination of the hands) indicated an improvement with tDCS in the immediate post-intervention period in the off-state, but this decrease was not significant in the on-state. Comparing UPDRS scores in each group, there were significant decreases in the total scores when “on” (p<0.05) in both groups and UPDRS III motor scores when “on” in the sham group (p<0.05). There was a trend to a decreased UPDRS III motor scores when “on” and “off” (p=0.07 and p=0.06).
Serial Reaction Time Task
Comparing tDCS and sham groups, there were no significant changes from baseline to any time after the intervention in RT, ER and sequence-specific learning (Table 3). There was no significant block effect during sequence repeating blocks 2–6 for percent change of RT and ER in the tDCS and sham groups at any time point.
Changes from baseline to any post-intervention time in BDI, Health Survey and self-assessment of mobility (log) did not differ between groups (Table 3).
Discussion
The principal finding of this first double-blind, randomized, sham-controlled study is that anodal stimulation of the motor and prefrontal cortices improved upper extremity bradykinesia. The effects on gait are somewhat ambiguous: tDCS increased walking speed in the off-state when excluding the patient with excessive walking times we considered factitious, but statistical significance was barely missed when including this patient and correcting for multiplicity. Gait did not improve when medicated, but session data suggests that tDCS might have had a short-lived beneficial effect.
This study supports findings of efficacy of tDCS on bradykinesia and potentially on gait [8;9] encouraging further research into therapeutic potential. Larger studies could confirm these findings, since the observed effect is small, which emphasizes the need for a more powerful stimulation for clinical impact.
Gait disturbances arise from various pathophysiological mechanisms, which might differ in their response to tDCS. So far, reports of gait improvement with tDCS [8] contrast with reports not showing benefit [9]. Likewise, rTMS improved gait [14;15], but not in all studies [16]. DBS of the pedunculo-pontine nucleus (PPN) [17;18] is reported to improve gait disturbances refractory to conventional therapy. Since the PPN connects with the cortico-striato-thalamo-cortical circuit, its activity could, theoretically, be modulated by cortical stimulation. We evaluated gait by speed alone, but may have missed qualitative gait improvement, which needs to be addressed in future studies.
The improvement of bradykinesia in the best on-state suggests that effects of anodal tDCS may exceed the best response of certain symptoms to dopamine substitution. Thus, tDCS may act on mechanisms eluding dopaminergic medication and complement conventional therapy. There was also an improvement in the sham group. An explanation is motor learning produced by the repeated assessment sessions as we kept patients from further practice otherwise after enrollment. If true, we expect a similar learning effect in the tDCS group, which provides another rationale for controlled studies. Motor learning could explain why the proper effect of tDCS on bradykinesia might be smaller as the composite UPDRS bradykinesia score suggests. TDCS might enhance learning [6], and, thus, combining tDCS and rehabilitation training could carry a potential benefit. The decrease in walking time with sham most plausibly reflects familiarization with the task.
Other than what has been discussed, no superior effects of tDCS could be discerned from sham stimulation. There was improvement in most measures with sham intervention substantiating a placebo effect. The persuasive concept of stimulation “boosting” under-active brain areas may have heightened the expectancy, and emphasizes the importance of controlled studies, which are feasible since blinding appears reliable [19].
The efficacy of tDCS is enhanced when repeated [8] as with rTMS [14], but the number of sessions for the optimal response remains unknown. There might be a larger effect of tDCS within the first sessions particularly for bradykinesia. Yet, potential effects could have been masked by the larger variability in motor performance since assessments before and after the intervention could not control for fluctuations in contrast with assessments in the well-defined best on-state and “practically defined off-state”. Additionally, the placebo effect appeared larger immediately after the intervention.
The mechanisms by which tDCS and other stimulation modalities improve motor performance in PD are not known and they probably differ. The best evidence supporting clinical efficacy of brain stimulation comes from DBS. DBS supposedly interferes with pathological activity and induces changes in activity [20;21] and excitability [22;23] of the motor cortex suggesting a possible mechanism that acts trans-synaptically along cortico-striato-thalamo-cortical circuits. Direct stimulation of the motor cortex in small series support this concept [24], but findings are not uniformly positive and changes in brain activity not always found [25]. High-frequency rTMS with modest efficacy [2;3] increases excitability that presumably correlates with improvement in bradykinesia [14]. No other controlled study explored changes in brain physiology and behavior and their interaction. In contrast to pulsed stimulation, tDCS delivers a continuous current that modulates membrane excitability and induces shifts in cortical excitability without rapid depolarization sufficient to produce an action potential [4;5]. Anodal stimulation increases excitability and, thereby, firing of active neurons [4], and supposedly reverses decreased activity in motor and prefrontal cortices in PD [8;9].
TDCS may cause release of dopamine as does rTMS of prefrontal and motor cortices in the caudate and putamen in healthy [26;27], in PD [28] and even sham rTMS highlighting a possible mechanism of placebo [29]. The widespread activation with anodal tDCS [30] may release dopamine and could be the mechanism for acute improvement [9]. Further evidence for an involvement of dopamine in tDCS effects comes from the observation that anodal tDCS of M1 prolongs the cortical silent period (CSP) [31] shown to reflect dopaminergic action in PD [22;32]. Both DBS [22] and rTMS [33–35] modulate CSP suggesting similar mechanisms on motor cortex excitability.
This persistence of effects implies functional and structural changes in synaptic strength, which constitutes the basic mechanism in plasticity. Pharmacological blocking of N-methyl-D-aspartate (NMDA) receptors prevents long-lasting effects of tDCS on cortical excitability [36;37] suggesting tDCS may recruit NMDA receptor-dependent plasticity, an action thought to depend on dopamine [38]. This role of dopamine in plasticity in PD is demonstrated by the effects of 5 Hz [39] and 1Hz rTMS [40] on cortical excitability in on-, but not off-state. Thus, induction of longer-lasting effects with tDCS might depend on dopamine. This would explain the absence of enduring effects with tDCS when un-medicated [8].
This study suggests a therapeutic potential of tDCS, but stimulation must be more powerful to improve functional status and quality of life along with motor performance.
Supplementary Material
Figure 1.
Flow diagram of patients with Parkinson’s disease (PD) enrolled in this therapeutic study.
Acknowledgments
Acknowledgements to David Prosper for help in the research, Sungyoung Auh for statistical analysis and Devera Schoenberg for skillful editing.
This research was supported by the Intramural Research Program of the NINDS, NIH, and in part by a grant from the USAMRMC (W81XWH-06-1-0534).
Footnotes
Statistical analysis: Xiaobai Li
Disclosures
- D. H. Benninger, M. Lomarev, G. Lopez, E. Wassermann, X. Li and E. Considine have no disclosures
- M. Hallett has received personal compensation or travel expenses for activities with Neurotoxin Institute, John Templeton Foundation, Parkinson’s and Ageing Research Foundation, University of Pennsylvania, Thomas Jefferson University, Baylor College of Medicine, American Academy of Neurology, Medical University of South Carolina, Northshore-Long Island Jewish Hospital, American Clinical Neurophysiology Society, Columbia University, University of Alabama, Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier as an advisory board member, an editor, a writer, or a speaker. Dr. Hallett has received license fee payments from the National Institutes of Health for the H-coil, a type of coil for magnetic stimulation. Dr. Hallett and his wife held stock in Agilent Technologies, Amgen, Amylin Pharmaceuticals, Merck & Co., Monsanto Co New Del, Sanofi Aventis Adr., Coventry Health Care Inc., Sigma Aldrich Corp., Warner Chilcott Ltd., Pfizer Inc, Genentech, Inc., United Health Group, St. Jude Medical, and Eli Lilly & Company.
This study was publicly registered (ClinicalTrial.gov: NCT00082342).
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a non exclusive licence - as government employees - on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JNNP and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in JNNP’s licence.
Contributor Information
Mikhail Lomarev, Email: Mlom2005@hotmail.com.
Grisel Lopez, Email: glopez@mail.nih.gov.
Eric M. Wassermann, Email: wassermanne@ninds.nih.gov.
Xiaobai Li, Email: xiaobai.li@osumc.edu.
Elaine Considine, Email: considineE@ninds.nih.gov.
Mark Hallett, Email: hallettm@ninds.nih.gov.
References
- 1.Braak H, Del Tredici K, Rub U, De Vos RAI, Steur ENHJ, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. Journal of Neurology Neurosurgery and Psychiatry. 2005;76:1614–23. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elahi B, Elahi B, Chen R. Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function-Systematic Review of Controlled Clinical Trials. Movement Disorders. 2009;24:357–63. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology-London. 2000;527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 6.Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience. 2003;15:619–26. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 7.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 8.Lomarev M, Gurtchin FA, Kirsanova GV. Method of parkinsonism treatment. Invention SU 1630836. Bulleten Isobreteny. 1991:N8. [Google Scholar]
- 9.Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MTA, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Movement Disorders. 2006;21:1693–702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- 10.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinical Neurophysiology. 2006;117:1623–9. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Benecke R, Rothwell JC, DICK JPR, Day BL, Marsden CD. Disturbance of sequetial movements in patients with Parkinson’s disease. Brain. 1987;110:361–79. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 12.Benninger D, Lomarev M, Wassermann EM, Lopez G, Fasano R, Houdayer E, Dang N, Hallet M. Safety study of 50 Hz repetitive transcranial magnetic stimulation in patients with Parkinson’s disease. Clinical Neurophysiology. 2009;120:809–15. doi: 10.1016/j.clinph.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for Mixed Models. SAS Publishing; 2006. [Google Scholar]
- 14.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Movement Disorders. 2006;21:325–31. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- 15.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Movement Disorders. 2006;21:2201–5. doi: 10.1002/mds.21089. [DOI] [PubMed] [Google Scholar]
- 16.Rektorova I, Sedlackova S, Telecka S, Hlubocky A, Rektor I. Repetitive transcranial stimulation for freezing of gait in Parkinson’s disease. Movement Disorders. 2007;22:1518–9. doi: 10.1002/mds.21289. [DOI] [PubMed] [Google Scholar]
- 17.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–7. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 18.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 19.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (OCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Davis KD, Taub E, Houle S, Lang AE, Dostrovsky JO, Tasker RR, Lozano AM. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nature Medicine. 1997;3:671–4. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- 21.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Annals of Neurology. 1997;42:283–91. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Garg RR, Lozano AM, Lang AE. Effects of internal globus pallidus stimulation on motor cortex excitability. Neurology. 2001;56:716–23. doi: 10.1212/wnl.56.6.716. [DOI] [PubMed] [Google Scholar]
- 23.Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology. 2002;58:1665–72. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- 24.Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurology. 2007;6:279–86. doi: 10.1016/S1474-4422(07)70056-X. [DOI] [PubMed] [Google Scholar]
- 25.Strafella AP, Lozano AM, Lang AE, Ko JH, Poon YY, Moro E. Subdural motor cortex stimulation in Parkinson’s disease does not modify movement-related rCBF pattern. Movement Disorders. 2007;22:2113–6. doi: 10.1002/mds.21691. [DOI] [PubMed] [Google Scholar]
- 26.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001:21. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–15. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 28.Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[C-11]raclopride PET study. European Journal of Neuroscience. 2005;22:2946–52. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strafella A, Ko JH, Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: the contribution of expectation. NeuroImage. 2006;31:1666–72. doi: 10.1016/j.neuroimage.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? European Journal of Neuroscience. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Experimental Brain Research. 2004;156:439–43. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu AD, Petzinger GM, Lin CHJ, Kung M, Fisher B. Asymmetric corticomotor excitability correlations in early Parkinson’s disease. Movement Disorders. 2007;22:1587–93. doi: 10.1002/mds.21565. [DOI] [PubMed] [Google Scholar]
- 33.Siebner HR, Mentschel C, Auer C, Lehner C, Conrad B. Repetitive transcranial magnetic stimulation causes a short-term increase in the duration of the cortical silent period in patients with Parkinson’s disease. Neuroscience Letters. 2000;284:147–50. doi: 10.1016/s0304-3940(00)00990-3. [DOI] [PubMed] [Google Scholar]
- 34.Gilio F, Curra A, Inghilleri M, Lorenzano C, Manfredi M, Berardelli A. Repetitive magnetic stimulation of cortical motor areas in Parkinson’s disease: Implications for the pathophysiology of cortical function. Movement Disorders. 2002;17:467–73. doi: 10.1002/mds.1255. [DOI] [PubMed] [Google Scholar]
- 35.Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clinical Neurophysiology. 2004;115:2530–41. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 37.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology-London. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. European Journal of Neuroscience. 2006;23:1651–7. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- 39.Mir P, Matsunaga K, Gilio F, Quinn NP, Siebner HR, Rothwell JC. Dopaminergic drugs restore facilitatory premotor-motor interactions in Parkinson disease. Neurology. 2005;64:1906–12. doi: 10.1212/01.WNL.0000163772.56128.A8. [DOI] [PubMed] [Google Scholar]
- 40.Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, Weiller C, Siebner HR, Munchau A. Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease. Brain. 2004;127:2732–46. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.