Abstract
Metabolic syndrome (MS) is commonly associated with left ventricular (LV) diastolic dysfunction and LV hypertrophy. We sought to examine whether preclinical LV diastolic dysfunction can occur independent of LV hypertrophy in MS. We recruited 90 consecutive participants with MS and without cardiovascular disease (mean age 46 years, 78% women), and 26 controls (no risk factors for MS; mean age 43 years, 65% women). Participants underwent echocardiography with tissue Doppler imaging. In age- and sex-adjusted analyses, MS was associated with higher left atrial (LA) diameter, higher LV mass, lower E/A ratio, and lower mean e' (P<0.001 for all). These associations remained significant after further adjusting for blood pressure, anti-hypertensive medication use, and body-mass index. After adjusting for LV mass, MS remained independently associated with higher LA diameter, lower E/A ratio and lower mean e' (P≤0.01 for all). Specifically, subjects with MS had a 1.8 cm/s lower mean e' compared with controls (P=0.01). Notably, differences in mean e' between those with and without MS were more pronounced at younger ages (P for interaction=0.003). In conclusion, MS was associated with preclinical LV diastolic dysfunction independent of LV mass, as reflected by higher LA diameter, lower E/A ratio, and lower mean e'. This suggests that MS can lead to the development of diastolic dysfunction via mechanisms independent of hypertrophy. Differences in diastolic function were more pronounced at younger ages, highlighting the potential importance of early risk factor modification and preventive strategies in MS.
Keywords: Metabolic syndrome, Left ventricular hypertrophy, Diastolic dysfunction
Metabolic Syndrome (MS) has been associated with subclinical changes in cardiac structure and function, including diastolic dysfunction and left ventricular (LV) hypertrophy.1 Previous studies have shown that preclinical LV diastolic dysfunction and LV hypertrophy both are strong risk factors for the future development of clinical heart failure, and specifically increase the risk of heart failure with preserved ejection fraction.2,3 The pathways leading to preclinical LV diastolic dysfunction are diverse, and mechanisms of progression to heart failure poorly understood. In the MS, LV diastolic function and LV hypertrophy appear to worsen in a stepwise fashion with the number of risk factors for MS.1,4 These findings may account in part for the augmented cardiovascular morbidity and mortality that is associated with MS.5 Whether these associations are due to age-related changes, hypertension, or other cardiometabolic effects of MS remains unclear. Further, the true prevalence of preclinical diastolic dysfunction in MS and relation to components of the MS are not well defined. We sought to further characterize cardiac structure and function in subjects with and without MS. Specifically, we hypothesized that MS is associated with preclinical diastolic dysfunction, and that this association can occur independent of the hypertrophy. These findings might lend further insight into potential mechanisms by which MS is associated with the eventual development of heart failure.
Methods
We conducted an observational cross-sectional study of consecutive participants with MS who attended outpatient visits at general cardiology, hypertension, obesity and nutrition clinics at Boston Medical Center. MS was defined as meeting 3 or more of the following criteria: (a) increased waist circumference (≥102 cm in men or ≥88 cm in women); (b) increased fasting triglyceride (≥150 mg/dL); (c) high blood pressure (≥130/85 mmHg) or anti-hypertensive therapy; (d) decreased high-density lipoprotein cholesterol (<40 mg/dL in men or, <50 mg/dL in women); (e) impaired fasting glucose (≥100 mg/dL).6 Controls without MS were recruited at Boston Medical Center, and were defined as meeting none of the 5 criteria for MS. Participants with existing cardiovascular disease (heart failure, LV ejection fraction (LVEF) <50%, coronary artery disease, or valvular heart disease) were excluded from the study.
All participants underwent a comprehensive medical history and physical examination. Resting heart rate, anthropometrics, blood pressure (obtained after 10 min of rest in the sitting position, expressed as the average of 3 consecutive measurements), and fasting blood work were obtained. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg and/or current anti-hypertensive therapy. Severe hypertension was defined as taking 2 or anti-hypertensive medications. Diabetes mellitus was defined as a fasting serum glucose level ≥126 mg/dL and/or current medical therapy with an oral hypoglycemic agent and/or insulin. The study was approved by the Boston University Medical Center Institutional Review Board. All participants provided informed consent prior to study enrollment.
Transthoracic echocardiography was performed with 1–5 MHz transducer and commercial ultrasound system (Philips iE33) by an experienced sonographer. Studies were analyzed off-line using a digital echo interface (Philips XCelera) by a single observer blinded to MS status (NA). Internal dimensions, left ventricle wall thickness, and LVEF (by modified Simpson’s rule) were measured according to published recommendations.7,8
Left atrial (LA) volume was measured in the apical 2-and 4-chamber views and indexed to body-surface area according to published recommendations.7,8 Relative wall thickness (RWT) was calculated as the mean of the end-diastolic posterior and septal walls thicknesses, divided by the LV end-diastolic diameter. LV mass was determined by the cubed method and indexed to height to the power of 2.7 to correct for body habitus, and LV hypertrophy defined as LV mass index > 44 g/m2.7 in women and >48 g/m2.7 in men.8,9 Pulsed-wave Doppler derived transmitral inflow velocities were obtained in the apical 4-chamber view at a sweep speed of 100 mm/s with the sample volume placed at the mitral valve leaflet tips. Measurements included the transmitral early diastolic (E-wave) and atrial (A-wave) velocities to calculate E/A ratio and E-wave deceleration time (DT).7,10 Tissue Doppler imaging was used to obtain LV myocardial velocities in the apical 4- chamber view with a sample volume placed at the medial and lateral mitral annulus. Measurements included medial and lateral early diastolic (e') myocardial velocities, and mean e' was calculated as the average of medial and lateral e'. All echocardiographic measurements were averaged over three consecutive cardiac cycles (when available). Repeated measurements of 10 scans showed an intra-observer coefficient of variation of 0.9–4.7%, and an intra-class correlation coefficient of 91–99% for linear measures.
Baseline clinical characteristics and echocardiographic measures were summarized for participants with and without MS. Between-group differences in baseline measures were assessed using two-sample t-tests or Pearson's chi-squared tests as appropriate. The association of MS and measures of cardiac structure and function was assessed using multivariable linear regression. Hierarchical models were constructed, first adjusting for age and sex, and then further adjusting for systolic blood pressure, the use of anti-hypertensive medications and body-mass index (BMI). Lastly, analyses examining measures of diastolic function that remained associated with MS were further adjusted for LV mass. Because age is known to be a strong determinant of diastolic function,11 we tested for statistical interaction between age and MS.
In exploratory analyses, we examined the association of the total number of MS risk factors and echocardiographic parameters for the subgroup of individuals with MS using one-way ANOVA. We also examined the association of different components of MS and measures of cardiac structure and function in participants with MS. Stepwise multivariable regression models were constructed, using forward selection with retention of variables at a P<0.05, forcing age and sex. Models were also repeated using backward elimination. Analyses were performed using STATA version 10.1 (Stata Corp, College Station, TX, USA) software.
Results
A total of 116 subjects were enrolled in our study, including 90 subjects with MS (mean age 46 years, 78% women), and 26 controls without MS (mean age 43 years, 65% women). Baseline characteristics by group are displayed in Table 1. Overall, subjects with MS had a worse cardiovascular risk factor profile, including higher blood pressure, BMI, and dyslipidemia. Fifty seven percent of subjects with MS met at least four of the established criteria.
Table 1.
Baseline characteristics by metabolic syndrome status
| Variable | MS (n=90) |
Controls (n=26) |
|---|---|---|
| Age (years) | 46 ±10 | 43 ±12 |
| Women | 70 (78%) | 17 (65%) |
| White | 45 (50%)* | 21 (81%) |
| Systolic blood pressure (mm Hg) | 126 (16)* | 109 (12) |
| Diastolic blood pressure (mm Hg) | 79 (11)* | 70 (7) |
| Body-mass index (kg/m2) | 39 (7)* | 24 (3) |
| Triglyceride (mg/dl) | 186 (120)* | 86 (30) |
| High-density lipoprotein cholesterol (mg/dl) | 43 (11)* | 57 (12) |
| Smoker | 11 (12%)* | 0 |
| Diabetes mellitus | 35 (39%)* | 0 |
| Anti-hypertensive medication use | 66 (73%)* | 0 |
| Severe hypertension | 37 (32%)* | 0 |
| Elevated waist circumference | 85 (94%)* | 0 |
| Elevated fasting triglyceride | 57 (63%)* | 0 |
| Low HDL cholesterol | 70 (78%)* | 0 |
| High blood pressure | 80 (89%)* | 0 |
| Impaired fasting glucose | 47 (52%)* | 0 |
| Three MS risk factors | 39 (43%)* | 0 |
| Four MS risk factors | 35 (39%)* | 0 |
| Five MS risk factors | 16 (18%)* | 0 |
Values are means (standard deviation) unless otherwise noted,
P<0.05 for between group comparisons
Echocardiographic measures for control and MS groups are presented in Table 2. While there were small differences in LV dimensions and LVEF between groups, subjects with MS have greater LV mass, with 28% and 24% meeting criteria for concentric remodeling or concentric hypertrophy, respectively8. None of the subjects in the control group had concentric hypertrophy, and two participants had concentric remodeling. Subjects with MS also had worse measures of diastolic function, including higher left atrial diameters, lower E/A ratio and lower mean e'. Specifically, 34% of subjects with MS had a mean e' < 8 cm/s, whereas only 13% of control subjects had a mean e' < 8 cm/s.
Table 2.
Echocardiographic measurements by metabolic syndrome status
| Variable | MS (n=90) |
Controls (n=26) |
P-value |
|---|---|---|---|
| Dimension | |||
| Left atrial dimension (mm) | 37 (4) | 32 (4) | <0.001 |
| Left ventricular end diastolic dimension (mm) | 46 (5) | 47 (4) | 0.25 |
| Left ventricular end systolic dimension (mm) | 30 (4) | 31 (4) | 0.46 |
| Posterior wall thickness (mm) | 9.9 (1.5) | 7.8 (1.0) | <0.001 |
| Interventricular septal thickness (mm) | 10.0 (1.5) | 7.7 (1.1) | <0.001 |
| Relative wall thickness (cm) | 0.44 (0.08) | 0.33 (0.07) | <0.001 |
| Left ventricular mass/height2.7 (g/m2.7) | 39.9 (9.4) | 28.8 (4.8) | <0.001 |
| Left ventricular ejection fraction (%) | 63 (5) | 63 (4) | 0.85 |
| Diastolic parameters | |||
| E (cm/s) | 80 (16) | 74 (15) | 0.14 |
| A (cm/s) | 72 (17) | 48 (13) | <0.001 |
| E/A ratio | 1.1 (0.3) | 1.6 (0.5) | <0.001 |
| Deceleration time (ms) | 203 (44) | 202 (34) | 0.95 |
| Mean e' (cm/s) | 9.0 (2.0) | 11.7 (3.0) | <0.001 |
| E/mean e' | 9.2 (2.4) | 6.6 (1.7) | <0.001 |
Values are means (standard deviation)
In age- and sex- adjusted analyses, MS was associated with echocardiographic features of diastolic dysfunction, including higher LA diameter, higher LV wall thickness, higher LV mass, lower E/A ratio, and lower mean e' (P<0.001 for all, Table 3). These associations of MS and LA diameter, relative wall thickness, E/A ratio, and mean e' appeared to be independent of blood pressure, anti-hypertensive medication use, and BMI (P<0.05 for all; Table 3). After additional adjustment for LV mass, MS remained independently associated with lower E/A ratio (P=0.002) and lower mean e' (P=0.01; Table 3). A total of 32 participants with MS (34%) had a mean e' < 8 cm/s, of whom 15 (47%) had low mean e' in the absence of LV hypertrophy criteria.
Table 3.
The association of metabolic syndrome with echocardiographic measures
| Age- and sex-adjusted | Age-, sex-, blood pressure- and BMI-adjusted |
Age-, sex-, blood pressure-, BMI, and LV mass- adjusted |
||||
|---|---|---|---|---|---|---|
| β estimate (s.e.) | P value | β estimate (s.e.) | P value | β estimate (s.e.) | P value | |
| Left atrial dimension | 5.0 (0.8) | <0.001 | 3.5 (1.4) | 0.01 | 3.5 (1.4) | 0.01 |
| Left ventricular end diastolic dimension | −0.6 (1.1) | 0.55 | ||||
| Left ventricular end systolic dimension | −0.1 (0.9) | 0.89 | ||||
| Posterior wall thickness | 2.2 (0.3) | <0.001 | 0.8 (0.5) | 0.11 | ||
| Interventricular septal thickness | 2.5 (0.3) | <0.001 | 1.1 (0.5) | 0.02 | 1.2 (0.4) | 0.001 |
| Relative wall thickness | 0.10 (0.02) | <0.001 | 0.07 (0.03) | 0.01 | 0.07 (0.03) | 0.01 |
| Left ventricular mass/height2.7 | 11.2 (2.0) | <0.001 | −1.2 (2.8) | 0.66 | ||
| Left ventricular ejection fraction (%) | −0.1 (1.2) | 0.90 | ||||
| E (cm/s) | 6.4 (3.6) | 0.08 | −1.9 (5.6) | 0.73 | ||
| A (cm/s) | 21.0 (3.3) | <0.001 | 12.1 (5.2) | 0.02 | 11.9 (5.2) | 0.02 |
| E/A ratio | −0.4 (0.1) | <0.001 | −0.4 (0.1) | 0.002 | −0.4 (0.1) | 0.002 |
| Deceleration time (ms) | −3.3 (10.2) | 0.75 | ||||
| Mean e' (cm/s) | −2.2 (0.4) | <0.001 | −1.7 (0.7) | 0.015 | −1.8 (0.7) | 0.01 |
| E/mean e‘ | 2.2 (0.5) | <0.001 | 0.8 (0.8) | 0.32 | ||
β estimate represents the change in echocardiographic measure in the presence versus absence of metabolic syndrome.
While LA diameter was significantly higher in MS in multivariable-adjusted models, LA volume when indexed to body-surface area was not significantly associated with MS after accounting for blood pressure and BMI (P>0.05).
In sensitivity analyses including only white subjects (45 MS, 21 controls), primary results were not materially different. Similarly, results did not change appreciably after adjusting for smoking status or diabetes mellitus status.
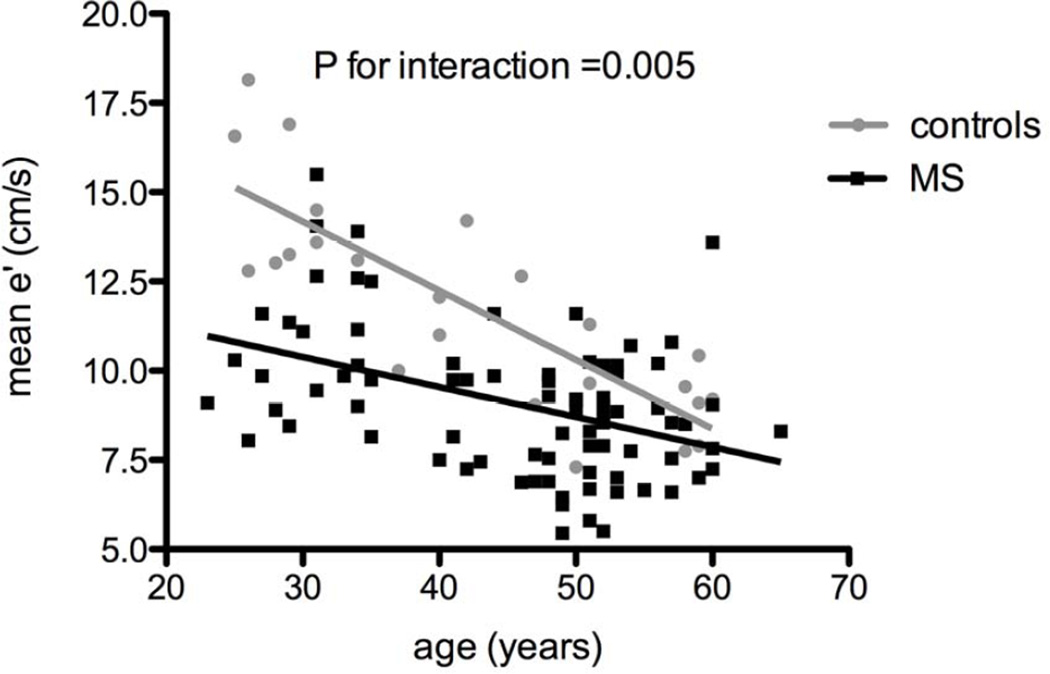
Among the different echocardiographic measures that were found to be significantly associated with MS, age was a significant predictor of A, E/A ratio and mean e'. To further explore whether age is an effect modifier in the associations between MS and diastolic function measures, we tested for an age interaction term. There was a significant interaction between age and MS as predictors of mean e' (P=0.003), suggesting that although MS was associated with lower mean e' at any age, this difference was most pronounced at younger ages (Figure 1). For example, for a man age 30 with a systolic blood pressure of 120 mmHg and BMI of 30, the predicted mean e' was 11.0 cm/s in individuals with MS and 14.3 cm/s in those without MS. In contrast, at age 50 years for the same clinical criteria, the predicted mean e' was 9.3 cm/s with MS, and 10.3 cm/s without MS.
Figure 1. The effect of age on mean e' among subjects with and without MS.
Individuals with MS had lower mean e' compared with those without MS, and this difference was more pronounced at younger ages (P for interaction=0.005). Black squares represent subjects with metabolic syndrome, and gray circles represent controls.
In exploratory analyses, we examined the association of the total number of MS risk factors and echocardiographic parameters within individuals with MS. The number of MS risk factors was associated with E/A ratio (P=0.003) and mean e' (P=0.03), but not other echocardiographic parameters. We further examined the association of individual components of the MS and echocardiographic measures using stepwise selection models. In multivariable analyses, increased waist circumference and lower high-density lipoprotein cholesterol were independently associated with increased LV mass after adjustment for age and sex (P<0.05 for all). Elevated triglycerides were associated with mean e' after adjustment for age and sex.
Discussion
We found that MS was associated with preclinical LV diastolic dysfunction as reflected by higher LA diameter, lower E/A ratio, and lower mean e' in a sample of individuals without existing cardiovascular disease. Notably, this association was independent of other clinical factors commonly associated with diastolic dysfunction, including age, blood pressure, and LV mass.3,10,11 These findings suggest that MS can lead to the development of diastolic dysfunction via mechanisms that are independent of hypertrophy, and potentially lend further insights into the increased cardiovascular risk observed in MS.5,12 Further, we found that age acts as an effect modifier, such that differences in diastolic function in participants with and without MS were more pronounced at younger ages. This may be due to bigger differences in subclinical cardiovascular disease at younger ages, which attenuate with increasing age and the development of other comorbidities. The presence of preclinical diastolic dysfunction at younger ages highlights the importance of future studies focused on early risk factor modification and preventive strategies in MS.
Our results are consistent with prior studies showing an association of MS and increased LV mass.13–15 While elevated blood pressure is one of the important components of MS, and hypertension is known to lead to increases in LV mass,16 we found that the association of MS and increased LV mass was independent of blood pressure. Further, in exploratory analyses, we show that measures of obesity and lower high-density lipoprotein cholesterol influence LV mass in individuals with MS. These results showing the association of metabolic disease and increased LV mass are consistent with prior studies looking at MS or its risk factors that have linked increases in LV mass with dyslipidemia17 and obesity.18
A number of previous studies have demonstrated subclinical cardiac remodeling in MS1,19 or components thereof, including insulin resistance, diabetes, and obesity.20–22 Some previous studies of MS have largely focused on LA dimensions, mitral E/A ratios and E deceleration time as metrics of diastolic function.1,19 We now extend these findings and show an association of MS with lower myocardial relaxation velocity as measured by tissue Doppler. Tissue Doppler indices have previously been shown to be relatively unaffected by changes in loading conditions, particularly in the presence of diastolic dysfunction,7 and thus are advantageous in this patient population, where subclinical diastolic dysfunction is more common.
We specifically show that the association of MS and lower global e' is independent of blood pressure, LV mass, age, and other clinical factors.1 Importantly, while LV hypertrophy may lead to diastolic dysfunction,16 our results suggest that MS can lead to impaired myocardial relaxation independent of changes in LV mass. In this regard, it is noteworthy that nearly half of individuals with MS who had diastolic dysfunction (mean e' < 8 cm/s) did not have LV hypertrophy. Interestingly, previous studies have shown diastolic dysfunction in diabetics to be associated with diffuse myocardial fibrosis, which supports our findings
We found that left atrial dimension was higher in those with MS compared with controls, while there was no difference in left atrial volume even after indexing for body size. This is consistent with the mild degree of diastolic dysfunction seen in this preclinical sample, where definitive left atrial enlargement may not have developed yet. Other studies of preclinical disease have demonstrated changes in tissue Doppler velocities, which may reflect early myocardial changes in the absence of significant left atrial remodeling.23
Higher BMI has previously been found to be associated with worse LV diastolic function independent of LV mass in a population-based study where MS was not specified.24 However, after adjusting for BMI, our results remained robust, and suggest that obesity alone does not account for the association of MS and diastolic dysfunction.
The pathophysiological mechanism by which MS can lead to abnormalities in LV diastolic function is not well understood. In mouse models of diet-induced MS, increased myocardial oxidative stress has been implicated in the development of diastolic dysfunction, and was associated with both hypertrophy and fibrosis of the myocardium.25 Animal models of insulin resistance, hypertension, or dyslipidemia have also implicated the development of cardiac fibrosis, abnormal intracellular calcium handling,25,26 cardiomyocyte lipotoxicity, mitochondrial dysfunction, impaired endothelial blood flow, increased vascular stiffness, and inflammation.27 While mechanistic inferences cannot be drawn from our observational study, these results support the notion that metabolic heart disease can lead to impaired myocardial relaxation in the absence of LVH. Further studies are needed to elucidate potential mechanisms and potential therapeutic targets.
Several limitations deserve mention. First, ours is a cross-sectional observational study, and causal inferences are therefore limited. Second, healthy controls were selected based on the absence of any MS criteria. This resulted by design in baseline differences of clinical characteristics between participants with and without MS. It is therefore possible that residual confounding could in part account for our findings. Lastly, healthy controls in our sample were predominantly white, whereas nearly half of participants with MS were black, an important point since LV hypertrophy,28 and diastolic dysfunction, are more prevalent among blacks. Our sample size was not powered to examine racial differences, however, the primary study results were not materially different in sensitivity analyses including only whites. In our study LV mass was indexed to height to the power of 2.7,9 which has been shown to be more applicable in obese populations,29 however other methods of indexing may have resulted in different findings. Lastly, exploratory analyses examining individual components of MS with echocardiographic traits were limited due to a modest sample size.
Acknowledgments
This work was supported by the National Institutes of Health, including grant number NO1-HV-00239 (Dr. Colucci), and K23-HL116780 (Dr. Ho). Dr. Ho is supported by a Boston University School of Medicine, Department of Medicine Career Investment award.
We thank Aaron Sverdlov and Deborah Siwik for their scientific contribution; to Lalita Khaodhiar, Amanda Powell, and Dong Wook Kim, for their involvement in the recruiting process; and Bryan Doldt and Jean Doricent for their continued efforts in performing the echocardiogram studies. We also want to acknowledge the fundamental work of Lorraine Keane on the recruitment and enrollment parts of this study protocol.
Funding Sources: This work was supported by the National Institutes of Health, including grant number NO1-HV-00239 (Dr. Colucci), and K23-HL116780 (Dr. Ho). Dr. Ho is supported by a Boston University School of Medicine, Department of Medicine Career Investment award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, Davila-Roman VG. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- 2.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 3.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azevedo A, Bettencourt P, Almeida PB, Santos AC, Abreu-Lima C, Hense HW, Barros H. Increasing number of components of the metabolic syndrome and cardiac structural and functional abnormalities--cross-sectional study of the general population. BMC Cardiovasc Disord. 2007;7:17. doi: 10.1186/1471-2261-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F, American Heart A, National Heart L, Blood I. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 11.Munagala VK, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr. 2003;16:1049–1056. doi: 10.1016/S0894-7317(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 12.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA., Jr Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005;112:819–827. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 14.Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 15.de Simone G, Devereux RB, Chinali M, Roman MJ, Lee ET, Resnick HE, Howard BV. Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2009;19:98–104. doi: 10.1016/j.numecd.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 17.Horio T, Miyazato J, Kamide K, Takiuchi S, Kawano Y. Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens. 2003;16:938–944. doi: 10.1016/s0895-7061(03)01015-x. [DOI] [PubMed] [Google Scholar]
- 18.Guerra F, Mancinelli L, Angelini L, Fortunati M, Rappelli A, Dessi-Fulgheri P, Sarzani R. The association of left ventricular hypertrophy with metabolic syndrome is dependent on body mass index in hypertensive overweight or obese patients. PLoS One. 2011;6:e16630. doi: 10.1371/journal.pone.0016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masugata H, Senda S, Goda F, Yoshihara Y, Yoshikawa K, Fujita N, Daikuhara H, Nakamura H, Taoka T, Kohno M. Left ventricular diastolic dysfunction as assessed by echocardiography in metabolic syndrome. Hypertens Res. 2006;29:897–903. doi: 10.1291/hypres.29.897. [DOI] [PubMed] [Google Scholar]
- 20.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 21.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5:349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 22.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992–2997. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 24.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan JP. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med. 1991;325:625–632. doi: 10.1056/NEJM199108293250906. [DOI] [PubMed] [Google Scholar]
- 27.Katz AM, Zile MR. New molecular mechanism in diastolic heart failure. Circulation. 2006;113:1922–1925. doi: 10.1161/CIRCULATIONAHA.106.620765. [DOI] [PubMed] [Google Scholar]
- 28.Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail. 2004;10:15–18. doi: 10.1111/j.1527-5299.2004.02026.x. quiz 21–12. [DOI] [PubMed] [Google Scholar]
- 29.de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB Strong Heart Study I. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]