Abstract
Introduction
Limited data are available regarding the tolerance of anti-epidermal growth factor receptor (EGFR) antibodies among elderly metastatic colorectal cancer (mCRC) patients. We retrospectively reviewed our experience of treating elderly mCRC patients with these agents between 2004 and 2011.
Methods
mCRC patients ≥65 years old treated with anti-EGFR agents were included in this analysis. We recorded demographic and disease characteristics, treatment regimen and duration, KRAS status, and overall survival. Toxicity evaluation included common hematologic and non-hematologic toxicities seen with these agents.
Results
117 patients were included with median age at treatment initiation of 73 years (65–86), 59% male gender, 82% colon primary, and 51% with metastatic disease at presentation. Median time on anti-EGFR treatment was 2.4 months. Older age at treatment initiation was associated with use of anti-EGFR antibody as monotherapy versus combination (p=0.0009). Worse performance status at treatment initiation was associated with a shorter overall survival (p=0.013) and shorter treatment duration (p=0.01). The incidence of hematologic/non-hematologic grade 3 or higher toxicity was 36% and 15% respectively. No association was found between age and presence of ≥ grade 3 toxicities. Longer treatment duration and better performance status at treatment initiation were the only factors associated with higher incidence of grade 3 toxicity.
Conclusions
Our data demonstrate that anti-EGFR antibodies can be used among older mCRC patients, with toxicity profiles similar to those reported in large phase III studies of younger patients. Advanced age was associated with receipt of anti-EGFR agents as monotherapy, but did not impact treatment outcomes in this population.
Keywords: Elderly, Cetuximab, Panitumumab, Adverse Events, Performance status
Introduction
Colorectal cancer (CRC) is generally a diagnosis of older adults, with 40% of diagnoses made over the age of 75 and the median age of diagnosis being 71 years1. Twenty percent of these patients have metastatic disease at the time of diagnosis2. Despite the large number of older mCRC patients seen in clinical practice, the pivotal trials guiding clinical management include small numbers of older patients3. Due to the lack of prospective data to guide treatment decisions, the management of elderly colorectal cancer patients is challenging.
Cetuximab and panitumumab are epidermal growth factor receptor (EGFR) inhibitors that are approved for the treatment of KRAS wild type metastatic colorectal cancer (mCRC). Multiple phase III studies have demonstrated improvement in progression free survival (PFS) and overall survival (OS) with the use of anti-EGFR antibodies alone or in combination with chemotherapy among patients with wild type KRAS tumors4–9. Only a minority of patients in these studies were over the age of 70, and subgroup analyses of elderly patients demonstrated mixed efficacy results6,10. These drugs carry less of the typical adverse events associated with chemotherapy. However, they do carry significant toxicities including skin rash, diarrhea and electrolyte imbalance. Among older adults, side effects such as these can cause significant morbidity. While skin toxicity primarily causes cosmetic discomfort, diarrhea may predispose older patients to dehydration and risk for renal compromise.
European groups have studied the effects of these drugs on elderly patients in retrospective or small prospective studies. The largest cohort of older patients treated with anti-EGFR antibodies was reported in an observational study from Germany evaluating the efficacy and safety of these agents among 300 patients over the age of 65 compared to their younger counterparts. The study demonstrated similar toxicity and efficacy with the combination of cetuximab and irinotecan in older and younger patient cohorts11. The Spanish Group for Digestive Tumors Therapy (TTD) studied cetuximab as a single agent and in combination with irinotecan or capecitabine in the older population and demonstrated a similar toxicity profile to that seen among younger patients12–14. We sought to evaluate the use of anti-EGFR antibodies among older patients with mCRC treated at an academic center in the United States. In this report, we outline the pattern of care for use of anti-EGFR antibodies and the toxicity profile seen among elderly patients treated with these agents.
Materials and Methods
Patient characteristics
Patients over the age of 65 who had received cetuximab or panitumumab between February 2004 and March 2011 for the treatment of mCRC were identified through our pharmacy computer database. All patients had a histologically confirmed diagnosis of metastatic adenocarcinoma of the colon/rectum. We excluded patients with histologic type other than adenocarcinoma of the colon or rectum and patients with incomplete medical records.
Data collection
The following patient and disease characteristics were gathered through a retrospective review of the electronic medical record: age, gender, site of disease, stage at diagnosis, site of metastasis, number of metastatic sites, and initial performance status (PS). We further extracted data regarding the patient’s treatment pattern including: drugs, treatment duration, doses, line of therapy, treatment interruption and dose reductions. We defined a line of therapy as a change in therapy secondary to disease progression. To minimize the recall bias associated with a retrospective review, we recorded objective laboratory parameters as well as subjective parameters from the patient’s clinic visit provider notes. Hematologic toxicity was evaluated by review of the patient’s laboratory records during the treatment period. Non-hematologic toxicity was evaluated based on medical record documentation. In addition, we reviewed parameters that can serve as surrogates for non-hematologic toxicity such as decline in PS at the end of treatment, >10% weight loss, >10% decrease in albumin level, use of local or systemic therapy for rash, and hospitalization. Toxicity was graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) v.4.0. The study included patients who received anti-EGFR antibodies prior to as well as following the introduction of KRAS testing as a predictor of response. Therefore, a significant portion of patients on this study did not have their tumor tested for this mutation. Finally, we recorded the overall survival (OS) of patients in our cohort from date of diagnosis to death. The study protocol was approved by the Institutional Review Board at Fox Chase Cancer Center.
Statistical analysis
Descriptive statistics for demographic characteristics, disease presentation, and treatment related toxicity were summarized. Continuous variables were analyzed using the Mann-Whitney test or the Kruskal-Wallis test as appropriate, and categorical variables were analyzed using the Fisher’s exact test. Age was analyzed as a continuous variable in our univariate analysis, and as a categorical variable in the OS analysis. PS was analyzed as a categorical variable depicting two groups of PS 0–1 and 2–3. The two-sample, binomial test of proportions was used to compare proportions between different groups. All tests were two sided and utilized a 5% Type I Error. The R statistical language and environment (www.r-project.org) and the STATA package were used for computations15. Univariate log-rank tests and Cox proportional hazards models were used to correlate treatment, demographic, and clinical variables with OS. OS was calculated from date of diagnosis of colorectal cancer to date of death. Kaplan-Meier survival curves and hazard ratios (with corresponding 95% confidence intervals) were computed.
Results
Patient characteristics and patterns of care
Two hundred patients over the age of 65 who received cetuximab or panitumumab were identified from our pharmacy database. Of these, 117 patients were eligible for the final analysis. Patients were excluded based on histology other than adenocarcinoma and incomplete medical records. Ninety-nine were treated with cetuximab and 18 were treated with panitumumab (Table 1). The median age at treatment initiation was 73 years with male and Caucasian preponderance. Colon cancer was the predominant primary tumor type with metastatic disease present at diagnosis in half of patients. At the time of treatment initiation, most patients had a PS of 0 or 1. KRAS testing was not available for the majority of patients, and nearly all of those patients tested were wild-type KRAS. More patients treated with cetuximab had a PS of 0 at treatment initiation compared to patients in the panitumumab arm (27.3% versus 5.6%). Alternatively, more patients in the panitumumab arm had a PS of 1 at treatment initiation compared to patients in the cetuximab arm (77.7% versus 47.5%). These differences did not reach statistical significance, likely due to the low number of panitumumab-treated patients.
Table 1.
Patient characteristics and treatment pattern
| Total N=117 | Cetuximab N=99 | Panitumumab N=18 | |
|---|---|---|---|
|
| |||
| Median age at TX initiation (range) | 73(65–86) | 73 (65–86) | 72(66–82) |
|
| |||
| Gender N (%) | |||
| Male | 69(58.9%) | 61 (61.6%) | 8 (44.4%) |
| Female | 48 (41.1%) | 38 (38.4%) | 10 (56.6%) |
|
| |||
| Primary site N (%) | |||
| Colon | 96 (82%) | 81 (81.8%) | 15 (83.3%) |
| Rectum | 18 (18%) | 18 (18.2%) | 3 (16.6%) |
|
| |||
| Stage at diagnosis N(%) | |||
| 1 | 6 (5.1%) | 6 (6.1%) | 0 (0%) |
| 2 | 17 (14.5%) | 12 (12.1%) | 5 (27.8%) |
| 3 | 35 (29.9%) | 28 (28.3%) | 7 (38.9%) |
| 4 | 59 (50.5%) | 53 (53.5%) | 6 (33.3%) |
|
| |||
| Number of metastatic sites N(%) | |||
| 1 | 79 (67.5%) | 68 (68.7%) | 11 (61.1%) |
| 2 | 34 (29.1%) | 29 (29.3%) | 5 (27.8%) |
| ≥3 | 4 (3.4%) | 2 (2.0%) | 2 (11.1%) |
|
| |||
| Race N (%) | |||
| Caucasian | 91 (77.8%) | 78 (78.8%) | 13 (72.2%) |
| AA | 13 (11.1%) | 10 (10.1%) | 3 (16.6%) |
| Hispanic | 7 (5.9%) | 6 (6.1%) | 1(5.6%) |
| Other | 6 (5.2%) | 5 (5.0%) | 1 (5.6%) |
|
| |||
| PS at TX initiation N(%) | |||
| 0 | 28 (23.9%) | 27 (27.3%) | 1 (5.6%) |
| 1 | 61 (52.2%) | 47 (47.5%) | 14 (77.7%) |
| 2 | 22 (18.8%) | 20 (20.2%) | 2 (11.1%) |
| 3 | 3 (2.5%) | 2 (2.0%) | 1 (5.6%) |
| n/a | 3 (2.5%) | 3 (3.0%) | 0 (0%) |
|
| |||
| KRAS testing N (%) | |||
| N/A | 82 (70%) | 70(70.7%) | 12(66.7%) |
| Wild type | 30 (25.7%) | 24 (24.3%) | 6 (33.3%) |
| Mutant | 5 (4.3%) | 5 (5%) | 0 (0%) |
|
| |||
| Single agent N(%) | 44 (37.6%) | 34 (34.4%) | 10 (55.6%) |
|
| |||
| Combination with chemo N(%) | 73 (62.4%) | 65 (65.6%) | 8 (44.4%) |
|
| |||
| Type of chemo combination N(%) | |||
| Irinotecan/FOLFIRI | 52 (71.3%) | 45 (69.3%) | 7 (87.5%) |
| Oxaliplatin/FOLFOX/ XELOX | 8 (10.9%) | 8 (12.3%) | 0 (0%) |
| 5-fluorouracil / Capecitabine | 1 (1.3%) | 1 (1.5%) | 0 (0%) |
| Other | 12 (16.4%) | 11 (16.9%) | 1 (12.5%) |
|
| |||
| Line of therapy | |||
| 1st | 19 (16.2%) | 19 (19.2%) | 0 (0%) |
| 2nd | 41 (35.1%) | 34 (34.3%) | 7 (38.9%) |
| 3rd | 46 (39.3%) | 37 (37.4%) | 9 (50%) |
| 4th | 11 (9.4%) | 9 (9.1%) | 2 (11.1%) |
The pattern of use of anti-EGFR antibodies in our cohort is summarized in Table 1. Most patients received cetuximab in combination with chemotherapy, while panitumumab was more commonly used as a single agent. Irinotecan alone or with infusional 5-fluorouracil (FOLFIRI) was the most common chemotherapy agent used in combination with anti-EGFR antibodies. Cetuximab was utilized throughout all lines of therapy, and panitumumab was primarily used in the second line setting and beyond.
The median time on anti-EGFR therapy was 2.4 months (range: 0.3 to 38 months), 2.5 months for patients on cetuximab, 2.1 months for patients on panitumumab. The median OS was 2.6 years for the full cohort (range: 0.19–15.2 years), 2.5 years for patients treated with cetuximab and 3.7 years for patients treated with panitumumab.
Toxicity profile
The incidence of ≥ grade 3 hematologic and non-hematological toxicity was evaluated for the full cohort and the subgroups of patients treated with anti-EGFR antibodies alone or in combination with chemotherapy (Figure 1). The overall rate of non-hematologic and hematology ≥ grade 3 toxicity for the full cohort was 36.4% and 15.3% respectively. Twenty-two percent of patients in the full cohort required a treatment break and 13.6% of patients required dose reduction. No statistically significant difference was seen between the rates of overall non-hematologic and hematologic toxicities between patients treated with cetuximab and panitumumab. As would be expected due to the nature of these drugs, hypersensitivity reactions were seen only among patients treated with cetuximab. The small number of patients in the panitumumab cohort limits definitive conclusions regarding any differences in toxicity between patient groups treated with one antibody versus the other.
Figure 1.
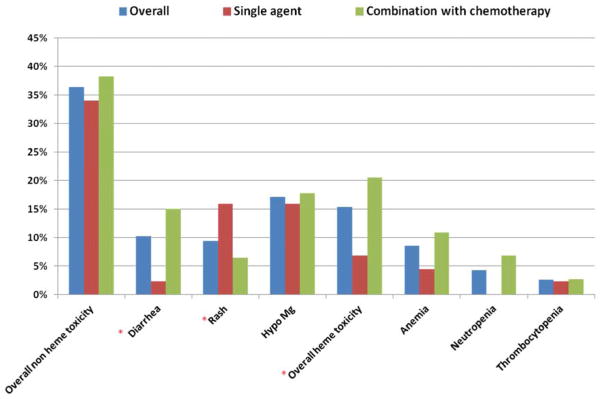
Incidence of grade 3 or higher hematologic and non-hematologic adverse events among older mCRC patients receiving anti EGFR agents alone or in combination with chemotherapy.
(*) Statistically significant difference between the incidence of adverse events seen among patients treated with monotherapy versus combination therapy.
Toxicity profile of single agent versus combination anti-EGFR use
No significant difference was noted in the incidence of ≥ grade 3 overall non-hematologic toxicity between patients treated with single agent anti-EGFR therapy versus combination treatment (34% and 38.3% respectively; p=0.64) (Figure 1). Patients receiving anti-EGFR antibodies in combination with chemotherapy were more likely to experience diarrhea compared to patients receiving single agent treatment (15.1% versus 2.3%, P=0.027). Conversely, rash was more commonly seen among patients treated with single agent as compared to combination therapy (15.9% and 5.5% respectively, P=0.047).
The rate of ≥ grade 3 overall hematologic toxicity was lower for patients treated with single agent compared to combination therapy (6.8% versus 20.5%; P=0.046) (Figure 1). Anemia was the most common hematologic adverse event seen among patients receiving an anti-EGFR antibodies alone or with chemotherapy, while grade 3 or higher neutropenia was seen only among patients treated with combination therapy.
Patients receiving anti-EGFR treatment in combination with chemotherapy were more likely to have breaks in therapy (31.5% versus 13.6% for single agent therapy; p=0.02). No difference in the rates of dose reductions during the treatment period was seen between the two groups. Decline in PS at treatment completion, hospitalization during therapy and use of topical/oral treatment for rash were seen in about a third of patients in both groups. Overall, there was no significant difference in the incidence of the evaluated objective surrogates of toxicity between patients treated with single agent anti-EGFR antibody versus those receiving combination treatments with chemotherapy. A trend towards increased incidence of weight loss and lower albumin levels were noted among patients receiving combination therapy.
Impact of age and performance status on clinical outcomes
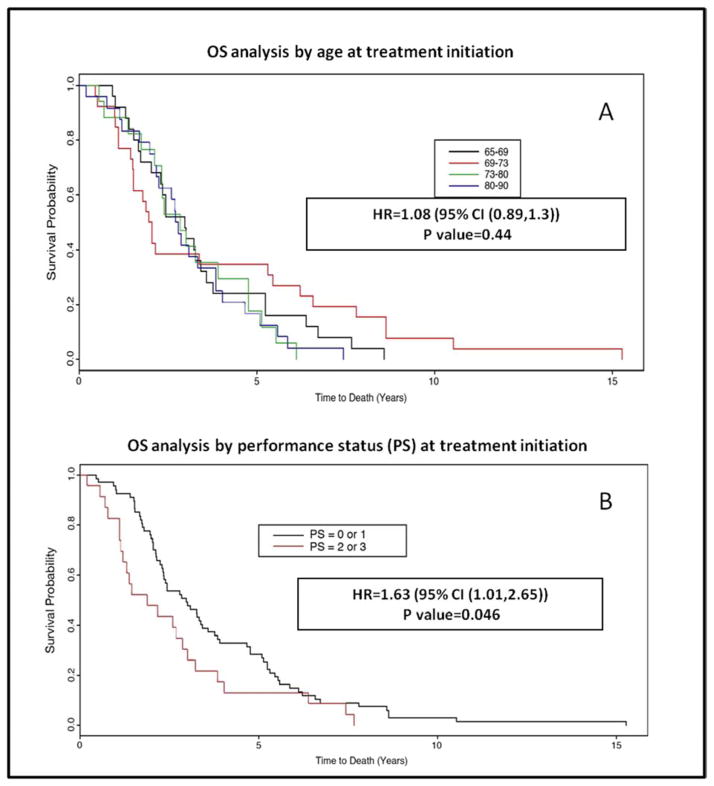
Univariate analysis of age at treatment initiation as a continuous variable demonstrated an association between increased age and use of anti-EGFR antibody alone as opposed to use in combination with chemotherapy (p=0.0009). No significant association was seen between increased age and OS (p=0.76), time on therapy (p=0.126), line of therapy in which anti-EGFR agents were used (0.8), or incidence of treatment breaks (0.07) (Figure 2). Lower PS at treatment initiation was associated with decreased time on treatment (p=0.011) and worse OS (p=0.013) (Figure 2). We found no association between PS and the use of anti-EGFR therapy as monotherapy or in combination with chemotherapy (p=0.1), evidence of treatment break (p=0.27), or line of therapy in which the drugs were used (p=0.8).
Figure 2.
Kaplan Meyer curves for overall survival by age (A) and performance status at treatment initiation (B)
We analyzed potential risk factors associated with the development of any ≥ grade 3 toxicity (hematologic or non-hematologic) during treatment with anti-EGFR antibodies (Table 2). Interestingly, age at treatment initiation was not associated with increased incidence of toxicity. Time on treatment was significantly higher among patients experiencing any grade 3 or higher toxicity (144.6 days versus 56.8 days respectively, P<0.0001). This trend was persistent among patients receiving anti-EGFR antibodies as a single agent (179.0 days versus 56.5 days, p=0.0004) and in combination with chemotherapy (141.2 days vs. 75.6 days, p=0.008). Patients with higher PS at treatment initiation were more likely to experience grade 3 or higher toxicities.
Table 2.
Clinical factors associated with the development of any grade 3 or higher toxicity (hematologic or non hematologic).
| Patients experiencing ≥ grade 3 hematologic/non-hematologic toxicity n=50 (43%) | Patients without ≥ grade 3 hematologic/non-hematologic toxicity n=67 (57%) | P Value | |
|---|---|---|---|
|
| |||
| Median Age (range) | 73 (65–85) | 73 (add 65–85) | 0.91 |
|
| |||
| PS at initiation* n(%): | |||
| 0–1 | 44 (88%) | 45 (67%) | 0.011* |
| ≥2 | 5(10%) | 20(29%) | |
|
| |||
| Time on therapy (full cohort) | 144.6 days | 56.8 days | <0.0001* |
|
| |||
| Time on therapy (single agent) | 179 days | 56.5 days | 0.0004* |
|
| |||
| Time on therapy (combination with chemotherapy) | 141.2 days | 75.6 days | 0.008* |
Statistically significant findings.
Discussion
There have been significant improvements in the treatment of metastatic colorectal cancer in recent years, with new agents added to the treatment arsenal including antibodies targeting the epidermal growth factor receptor. These agents have improved clinical outcomes when used as a single agent or in combination with chemotherapy4–9. Limited numbers of elderly patients were included in the randomized studies which preceded the approval of anti-EGFR antibodies for the treatment of mCRC. Several European studies have shown these agents to have acceptable tolerance and efficacy when used in older adults, both as a single agent and in combination with chemotherapy10,12,14. To our knowledge, this is the first study to evaluate the patterns of use and tolerance of anti-EGFR agents among older patients in an academic center in the United States.
Our analysis demonstrated increased use of anti-EGFR antibodies as monotherapy with increasing age. At the same time, advanced age at treatment initiation bore no correlation with OS, time on treatment, treatment breaks, or incidence of hematologic and non-hematologic toxicity. While single agent treatment carries a better toxicity profile, combination treatment with chemotherapy has generally been shown to be more efficacious and result in a higher response rate. Using age alone to determine treatment intensity may result in withholding optimal effective therapy from older adults who can tolerate treatment and benefit from more aggressive therapy.
Performance status has been accepted as a predictor for chemotherapy tolerance, and a screening tool for geriatric syndromes16. Our analysis demonstrated decreased OS (P=0.013) and treatment duration (P=0.011) among patients with a poor PS. Previous studies have demonstrated a correlation between poor PS and higher incidence of toxicity among in older patients17,18. In our study, better PS at treatment initiation was associated with higher incidence of ≥ grade 3 hematologic and non-hematologic toxicities. This is likely related to more aggressive therapy and longer treatment duration in this patient population. PS clearly influences the length of time a patient can remain on therapy and thus affects their likelihood of developing adverse events.
In terms of toxicity, our study demonstrates a toxicity profile that is similar to that documented among younger patients in the large phase III studies with these agents. This is also in concordance with the data published by European groups demonstrating safety of these drugs among older patients11–14. We found an incidence of any grade 3 or higher non-hematologic toxicity to be 36%, with an incidence of 10% for diarrhea and rash. The incidence of these toxicities in previously published large studies ranged between 8–15% for diarrhea and 11–30% for skin rash. Based on this comparison, it appears that the use of these agents among older mCRC patients carries a similar toxicity profile to that seen among younger adults. In reviewing factors that predisposed patients to increased toxicity, we could only demonstrate an association between longer time on treatment and higher rates of ≥ grade 3 hematologic/non-hematologic toxicity (P<0.0001), regardless of whether patients were treated with monotherapy (P=0.0004) or in combination with chemotherapy (P=0.008).
When comparing the use of cetuximab to panitumumab, we noted a higher use of panitumumab as a single agent, mostly in second line of therapy and beyond. This likely reflects the FDA approval of panitumumab during the timeframe in which many of our patients were treated9. Data regarding the use of this drug in combination with chemotherapy was forthcoming at the time6,7. The long median OS seen among the group of patients treated with panitumumab could be explained by a fit group of patients, with possibly more indolent disease who were able to make it beyond second or third line therapy. Survival data must be interpreted cautiously, however, as it was calculated from diagnosis of metastatic colorectal cancer, and may have been impacted by other factors during the patient’s treatment course. No significant difference in toxicity profiles were noted between the two agents (cetuximab and panitumumab) with the exception of hypersensitivity as expected9.
The strength of our study is in its evaluation of a real-world pattern of use of anti-EGFR antibodies, approved for use in 2004. Our analysis aimed to describe the use of these agents among older adults in the first seven years of commercial availability of these agents. Interestingly, we were able to identify only 117 patients over the age of 65 who received these agents during this period at our center. This may point to concerns among oncologists with regard to the use of anti-EGFR antibodies among older patients. Alternatively, this may reflect the slow adoption of this treatment, especially among the older patient population.
A limitation of our study is its retrospective nature. Retrospective analyses of toxicity are challenging due to concerns for reporting bias by the patient and the physician. In order to minimize this bias, we analyzed surrogate markers of toxicity. Our data demonstrated that approximately one third of patients treated with anti-EGFR antibodies experienced a decline in their PS, hospitalization during therapy, or >10% decrease in their albumin level. These parameters are rarely reported in clinical trials even though they can serve as surrogates for overall functional and/or physiologic decline. Other groups have utilized such surrogates markers for prognostication and prediction of treatment tolerance. A study by Cella and colleagues reported a strong correlation between declining PS and increasing rate of disease related symptoms19. Albumin level has been incorporated with C-reactive protein into the Glasgow Prognostic Score (GPS) which has been found to correlate with worsening PS, multiple laboratory abnormalities and worse OS in advanced cancer20,21. Given the significant percentages of patients in our cohort that experienced changes in these surrogate markers, it is clear that close observation is required when treating older adults with these agents. The lack of a control group of younger patients in which these markers are evaluated limits the ability to reach definitive conclusions regarding these data.
The lack of KRAS testing for the majority of cases in our cohort is another limitation of our study. Many of our patients were treated prior to the incorporation of routine KRAS testing for all mCRC patients. KRAS mutation status predicts for response to anti-EGFR agents, which may impact our survival analysis as some of the patients included in our analysis may have harbored a KRAS mutation and thus would not have benefited from anti-EGFR treatment. However, KRAS mutational status has not been shown to affect the toxicity pattern with these agents and thus the primary focus of our analysis should not be affected.
Overall, our retrospective study supports the safety of anti-EGFR antibodies in older patients with mCRC, with toxicity profiles similar to those reported in larger phase III studies of younger patients. The OS of our older patients was also similar to that shown in these trials with younger patients. Based on these data, elderly fit patients should be treated with anti-EGFR antibodies when indicated in treatment patterns similar to their younger counterparts. Future studies with these agents should include older adults to better understand the treatment approach to the typical patient commonly seen in clinic.
Clinical Practice Points.
With the aging of the population in the United States, the number of older metastatic colon cancer patients (mCRC) seen in oncology clinics is on the rise.
Data to guide oncologists in the management of older mCRC patients is lacking, with few older patients included in large randomized trials of anti-EGFR antibodies.
In a cohort of elderly (median age 73 years) mCRC treated at an academic center in the US, toxicity profile resembled that described in clinical trials of younger patients with no association between age and incidence of adverse events.
Poorer performance status at treatment initiation was associated with shorter treatment duration and shorter survival.
Our data supports the use of anti-EGFR agents as clinically indicated for treatment of older mCRC patients.
Acknowledgments
Research Support: Cancer Center Support Grant 3 P30 CA006927-47S4 and a Cancer Center Support Grant P30 CA43703
Footnotes
Previous presentations: Poster presentation at ASCO-Gastrointestinal Cancers symposium 2012 and International Society of Geriatric Oncology meeting 2012.
Conflict of Interest: Dr. Steven Cohen: Bristol Myers Squibb (BMS) – Advisory Board; Dwight Kloth: Amgen-advisory board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pallis AG, Papamichael D, Audisio R, et al. EORTC Elderly Task Force experts’ opinion for the treatment of colon cancer in older patients. Cancer Treat Rev. 36:83–90. doi: 10.1016/j.ctrv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program (SEER) Http://seer.cancer.gov/index.html.
- 3.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 5.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 7.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 10.Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67:255–62. doi: 10.1016/j.critrevonc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Jehn CF, Boning L, Kroning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer. 2012;106:274–8. doi: 10.1038/bjc.2011.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelwahab S, Azmy A, Abdel-Aziz H, et al. Anti-EGFR (cetuximab) combined with irinotecan for treatment of elderly patients with metastatic colorectal cancer (mCRC) J Cancer Res Clin Oncol. 2012;138:1487–92. doi: 10.1007/s00432-012-1229-8. [DOI] [PubMed] [Google Scholar]
- 13.Sastre J, Aranda E, Gravalos C, et al. First-line single-agent cetuximab in elderly patients with metastatic colorectal cancer. A phase II clinical and molecular study of the Spanish group for digestive tumor therapy (TTD) Crit Rev Oncol Hematol. 2011;77:78–84. doi: 10.1016/j.critrevonc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist. 2012;17:339–45. doi: 10.1634/theoncologist.2011-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team RDC. R: A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- 16.Owusu C, Koroukian SM, Schluchter M, et al. Screening older cancer patients for a Comprehensive Geriatric Assessment: A comparison of three instruments. J Geriatr Oncol. 2011;2:121–129. doi: 10.1016/j.jgo.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 18.Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:2529–36. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Eton D, Hensing TA, et al. Relationship between symptom change, objective tumor measurements, and performance status during chemotherapy for advanced lung cancer. Clin Lung Cancer. 2008;9:51–8. doi: 10.3816/CLC.2008.n.009. [DOI] [PubMed] [Google Scholar]
- 20.Brown DJ, Milroy R, Preston T, et al. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60:705–8. doi: 10.1136/jcp.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]