Abstract
Background:
Many HIV-infected children are diagnosed with tuberculosis (TB), but the effect of TB treatment on virologic and immunologic response to combination antiretroviral therapy (cART) is not well documented.
Methods:
Secondary analysis of a prospective cohort of cART-naïve HIV-infected South African children aged 0-8 years initiating cART to assess the effect of TB treatment at time of cART initiation on virologic suppression (HIV RNA <50 copies/mL), virologic rebound (HIV RNA >1000 copies/mL after suppression), and CD4 cell percentage (CD4%) increase during the first 24 months of cART.
Results:
Of 199 children (median age 2.1 years), 92 (46%) were receiving TB treatment at cART initiation. Children receiving and not receiving TB treatment at cART initiation had similar median baseline HIV RNA (5.4 vs. 5.6 copies/mL), median time to virologic suppression (6.2 months in each group, aHR 1.36, 95% CI 0.94-1.96) and rates of virologic rebound by 24 months (23% vs. 24%, aHR 1.53, 95% CI 0.71-3.30). Children on TB treatment had significantly lower median CD4% at baseline (15.3% vs. 18.8%, P<0.01) and during the first 12 months of cART, but experienced similar median increases in CD4% at 6 months (9.9% vs. 9.6%), 12 months (14.2% vs. 11.9%) and 24 months of cART (14.5% vs. 14.2%). Exploratory analyses suggest that children receiving lopinavir/ritonavir-based cART and TB treatment may have inferior virologic and immunologic response compared to children receiving efavirenz-based cART.
Conclusions:
Receiving TB treatment at time of cART initiation did not substantially affect virologic or immunologic responses to cART in young children.
Keywords: HIV, tuberculosis, antiretroviral therapy, children, therapy response
Introduction
In 2012, an estimated 530,000 tuberculosis (TB) cases and 74,000 TB deaths occurred in children <15 years.1 TB is common in HIV-infected children, with HIV prevalence among children with active TB ranging from 10% to 62%.2,3 In 2010, the World Health Organization recommended initiation of combination antiretroviral therapy (cART) as soon as TB therapy is tolerated in HIV/TB co-infected children.4 Consequently, many children initiate cART while receiving TB treatment. Co-treated individuals may experience a differential response to cART due to drug-drug interactions,2,5 increased risk of drug toxicity,2,5 immune reconstitution inflammatory syndrome (IRIS),6 and potentially lower adherence due to the large amount of medications.5 In particular, interactions between protease inhibitors (PIs) and rifampicin can result in subtherapeutic plasma concentrations of both types of drugs,7 causing the need for increased PI dosing in children to achieve the desired virologic and immunologic outcomes.
In adults, receiving TB treatment at time of cART initiation does not impair virologic or immunologic response to cART,8 though it may be associated with an increased risk of long-term mortality.9 Results of limited pediatric research to date suggest that starting cART during TB treatment improves survival,10-12 and that concomitant TB treatment and cART does not increase mortality.13-15 The few studies that assessed the effect of TB treatment on virologic or CD4 cell response to pediatric cART observed lower rates of virologic suppression in children receiving TB treatment, especially in those receiving PI-based cART other than super-boosted lopinavir/ritonavir (LPV/r),15-17 but similar CD4 reconstitution14,15,17,18 and proportion with severe immunodeficiency during cART.19
We aimed to evaluate the impact of receiving TB treatment at time of cART initiation on short- and long-term virologic and immunologic response to cART among a cohort of young children.
Methods
Study population
We performed a secondary analysis of data from the TB HIV IRIS and Nutrition in Kids (THINK) study. This study prospectively followed 1) children receiving TB treatment who became eligible for cART, and 2) children who were TB-free at the time of cART initiation. The primary objectives of the parent study were to determine the incidence, timing, and clinical manifestations of TB− and Bacillus Calmette–Guérin (BCG)-IRIS among children initiating cART, along with the association between malnutrition and the risk of developing TB− and BCG-IRIS.
cART-naive children aged 0 to 8 years presenting between September 2009 and March 2012 at the Harriet Shezi outpatient pediatric HIV clinic or the Pediatric Wards of Chris Hani Baragwanath Hospital in Soweto, South Africa, who were eligible for cART were offered study participation. Children were followed-up for 24 months or until August 2013. Children with at least one documented HIV RNA and/or CD4 cell percentage (CD4%) result following cART initiation were included in this secondary analysis.
Patient follow-up
The study included a pre-cART visit, cART initiation visit, and visits every three months thereafter (+/− one month) for 24 months. As part of routine care, caregivers of children who missed a scheduled visit were contacted to remind them of their missed appointment and encourage their return. Children who were transferred to a primary care facility were encouraged to return for the final 24-month visit. Patients who did not complete the 24-month visit were considered lost-to-follow-up and censored at the date of their last HIV RNA and/or CD4% measurement. Three patients were late for their 24-month visit and had laboratory measurements taken at 25.2, 26.0 and 26.6 months following cART initiation; these values were retained in analyses.
Study variables
CD4% (LSRII flow cytometer, BD Biosciences) and HIV RNA (Ultrasensitive Amplicor HIV Monitor assay, Roche Diagnostic Systems, Basel, Switzerland, lower limit of detection 50 copies/mL) were measured at or before cART initiation and during follow-up visits. Baseline values were those measured at cART initiation or the closest value within four months prior to initiation. Immunodeficiency and anemia were defined according to WHO age-specific classifications.20,21 Severe immunodeficiency was defined as CD4% <25% in children <11 months, <20% in children 12-35 months, <15% in children 36-59 months, and CD4 cell count <200 cells/μL or CD4% <15% in children >5 years.20 Weight-for-age z-scores were calculated using WHO Anthro (version 3.2.2) SAS macro22 for children <5 years and WHO AnthroPlus SAS macro23 for children 5-8 years.
Virologic suppression was defined as the first documented HIV RNA <50 copies/mL following cART initiation, whether or not confirmed by a subsequent measurement. Virologic rebound was defined as HIV RNA >1000 copies/mL following a prior measurement <50. The primary measure of immunologic response was median increase in CD4% from baseline at 3, 6, 12 and 24 months of cART.
TB and HIV clinical care
Children were diagnosed and treated for TB24,25 and HIV26,27 according to South African guidelines. TB diagnosis was made based on a combination of clinical signs, contact with an adult with active TB, positive tuberculin skin test, suggestive chest X-ray, or positive sputum smear microscopy or culture.24 The National Health Laboratory Service processed all sputum samples. Starting in July 2011, a sputum sample was also evaluated by Xpert MTB/RIF. All children diagnosed with active TB were treated and generally received isoniazid, rifampicin, pyrazinamide, and ethambutol for two months followed by isoniazid and rifampicin for four months.
Prior to April 2010 in South Africa, children were eligible for cART if they had recurrent or prolonged HIV-related hospitalizations, WHO stage II/III disease,20 CD4% <20% (if ≤18 months), or CD4% <15% (if >18 months).27 Children ≤3 years or ≤10 kilograms initiated LPV/r-based cART (stavudine/lamivudine/LPV/r), children >3 years and >10 kilograms initiated efavirenz-based cART (stavudine/lamivudine/efavirenz). Initiation of cART was delayed for at least two months in children receiving TB treatment, and children concomitantly on LPV/r-based cART and TB treatment received ritonavir at a 1:1 dosage with lopinavir (super-boosted LPV/r).
In April 2010, the South African guidelines changed. All children ≤1 year were eligible for cART upon HIV diagnosis.26 Children 1-5 years were eligible for cART if WHO stage III/IV, CD4% ≤25% or CD4 count <750 cells/μL. Children >5 years were eligible if WHO stage III/IV or CD4 count <350 cells/μL. Children ≥3 years and ≥10 kilograms initiated efavirenz-based cART (abacavir/lamivudine/efavirenz), and LPV/r-based cART (abacavir/lamivudine/LPV/r) was used for children <3 years or <10 kilograms. Initiation of cART was recommended to be delayed for 2-4 weeks after starting TB treatment, and children concurrently on TB treatment and LPV/r-based cART received added ritonavir, either super-boosted LVP/r for younger children or double-dose LPV/r for older children.26 The updated guidelines also recommended decentralization of cART initiation and follow-up to primary care clinics.
Statistical analysis
Wilcoxon rank-sum tests were used to compare continuous demographic and clinical variables between groups, Pearson’s X2 test was used for categorical variables, and exact P values were calculated when appropriate.
To examine virologic suppression, the median time to suppression was calculated. Distributions of event times in each exposure group were examined using the Kaplan-Meier function and compared by the log-rank test. Crude and multivariable Cox proportional hazards regression were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Time origin was the date of cART initiation; patients were censored at the earliest occurrence of death, loss to follow-up, or 24-month visit. Covariates included cART guidelines (pre-2010 vs. 2010 guidelines), age (<2.1 vs. <2.1 years), sex, weight-for-age z-score (<−3 vs. ≥−3), baseline HIV RNA (<5.5 vs. ≥5.5 log10 copies/mL), baseline CD4% (continuous), cART regimen (efavirenz-based vs. LPV/r-based), and baseline hemoglobin (continuous). Similar methods were used to assess virologic rebound, limiting the analysis to patients who ever experienced virologic suppression.
Median CD4% increases were calculated, both overall and stratified by TB treatment status and were compared using Wilcoxon rank sum tests. The proportion of children with severe age-specific immunodeficiency20 at each visit was also examined.
In addition to the main analysis, we performed exploratory sensitivity analyses stratified by (1) cART regimen and (2) timing of cART initiation relative to the 2010 change in guidelines. As new guidelines were implemented over several months, children who initiated cART from April to October 2010 were classified as initiating care according to the pre-2010 guidelines if their initial regimen included stavudine or as initiating according to the 2010 guidelines if their initial regimen contained abacavir. An additional sensitivity analysis examined an alternative virologic suppression definition of HIV RNA <400 copies/mL.
Study data were collected and managed using Research Electronic Data Capture (REDCap)28 hosted at The University of North Carolina-Chapel Hill. All analyses were performed using SAS (version 9.3, SAS Institute, Cary, North Carolina, USA).
Ethics approval
Children were enrolled following parental permission and child assent procedures (for children ≥7 years). Institutional Review Boards of University of North Carolina at Chapel Hill and University of Witwatersrand approved the study.
Results
Patient characteristics
Of the 246 children enrolled in the THINK study who initiated cART, 199 children had <1 follow-up HIV RNA and/or CD4%. Children included in analyses were representative of all 246 children who initiated cART (see Table, Supplemental Digital Content 1, for a comparison of children included vs. not included in analyses), except for lower baseline HIV RNA and CD4% and a greater proportion on LPV/r-based cART.
At cART initiation, 92 (46%) children were receiving TB treatment and 107 (54%) were not receiving TB treatment (Table 1). Median age was 2.1 years (interquartile range [IQR] 0.9-4.6); 52% were male, 12% presented with severe undernutrition (weight-for-age z-score <−3), and 52% had moderate or severe anemia. Median baseline CD4% was 17.1 (IQR 11.6-23.3); 108 (57%) had severe immunodeficiency at cART initiation. Median baseline HIV RNA was 5.6 log10 copies/mL (IQR 4.9-6.0). Two-thirds (66%) initiated cART according to 2010 guidelines. Sixty percent initiated an LPV/r-based first-line cART regimen; the remaining 40% received efavirenz-based cART. Children on LPV/r-based cART were younger and more likely to have severe undernutrition, lower baseline hemoglobin, higher baseline HIV RNA, higher baseline CD4%, and more severe age-specific immunodeficiency than children initiating efavirenz-based cART (see Table, Supplemental Digital Content 2, for a comparison of children initiating LVP/r-based vs. efavirenz-based cART).
Table 1.
Baseline characteristics of 199 children who initiated cART and had at least one follow-up HIV RNA and/or CD4 cell percentage, stratified by TB treatment status
| Characteristic | All patients | Children receiving TB treatment |
Children not receiving TB treatment |
P a | |||
|---|---|---|---|---|---|---|---|
| No. of patients (%) | 199 | (100) | 92 | (46.2) | 107 | (53.8) | |
| Median age, years (IQR) | 2.1 | (0.9 – 4.6) | 2.2 | (1.4 – 4.5) | 1.9 | (0.5 – 4.7) | 0.07 |
| <1 | 54 | (27.1) | 16 | (17.4) | 38 | (35.5) | 0.03 |
| 1-2 | 64 | (32.2) | 37 | (40.2) | 27 | (25.2) | |
| 3-4 | 36 | (18.1) | 19 | (20.7) | 17 | (15.9) | |
| 5-6 | 31 | (15.6) | 14 | (15.2) | 17 | (15.9) | |
| 7-8 | 14 | (7.0) | 6 | (6.5) | 8 | (7.5) | |
| Male sex, no. (%) | 103 | (51.8) | 55 | (59.8) | 48 | (44.9) | 0.04 |
| Weight-for-age z-score, median (IQR) | −1.52 | (−2.51 – −0.67) | −1.81 | (−2.63 – −0.97) | −1.29 | (−2.35 – −0.48) | 0.02 |
| <−2, underweight for age | 72 | (36.2) | 40 | (43.5) | 32 | (29.9) | 0.05 |
| <−3, very low weight for age | 24 | (12.1) | 13 | (14.1) | 11 | (10.3) | 0.41 |
| Median hemoglobin, g/dl (IQR)b | 10.0 | (9.0 – 11.0) | 9.6 | (8.9 – 10.6) | 10.3 | (9.4 – 11.1) | 0.01 |
| Mild anemia, no. (%)c | 45 | (24.9) | 17 | (19.8) | 28 | (29.5) | 0.24 |
| Moderate anemia, no. (%)d | 91 | (50.3) | 50 | (58.1) | 41 | (43.2) | |
| Severe anemia, no. (%)e | 4 | (2.2) | 2 | (2.3) | 2 | (2.1) | |
| Median HIV RNA, log10 copies/mL (IQR)f | 5.6 | (4.9 – 6.0) | 5.4 | (4.8 – 5.9) | 5.6 | (5.0 – 6.1) | 0.33 |
| Median CD4 count, cells/μL (IQR)g | 665 | (340 – 1069) | 591 | (300 – 914) | 769 | (407 – 1442) | <0.01 |
| Median CD4 cell percentage (IQR)g | 17.1 | (11.6 – 23.3) | 15.3 | (9.5 – 21.0) | 18.8 | (14.3 – 25.3) | <0.01 |
| WHO age-specific severity of immunodeficiency, no. (%)g |
|||||||
| Not significant | 28 | (14.7) | 10 | (11.1) | 18 | (17.8) | 0.14 |
| Mild | 22 | (11.5) | 7 | (7.8) | 15 | (14.9) | |
| Advanced | 33 | (17.3) | 15 | (16.7) | 18 | (17.8) | |
| Severe | 108 | (56.5) | 58 | (64.4) | 50 | (49.5) | |
| First-line cART regimen, no. (%) | |||||||
| Efavirenz-basedh | 79 | (39.7) | 39 | (42.4) | 40 | (37.4) | 0.47 |
| LPV/r-basedi | 120 | (60.3) | 53 | (57.6) | 67 | (62.6) | |
| Timing of cART initiation, no. (%) | |||||||
| According to pre-2010 guidelines | 67 | (33.7) | 34 | (37.0) | 33 | (30.8) | 0.36 |
| According to 2010 guidelines | 132 | (66.3) | 58 | (63.0) | 74 | (69.2) | |
| Median follow-up time, months (IQR) | 23.8 | (20.6 – 24.1) | 23.8 | (20.2 – 24.1) | 23.8 | (20.7 – 24.2) | 0.61 |
| Median duration of TB treatment at cART initiation, days (IQR) |
23 | (15 – 39) | |||||
Abbreviations: cART, combination antiretroviral therapy; IQR, interquartile range; LPV/r, lopinavir/ritonavir; TB, tuberculosis; WHO, World Health Organization.
Wilcoxon rank-sum testing was used to compare continuous variables; Pearson’s X2 test was used for categorical variables. Statistical significance defined as P <0.05 for all tests.
Baseline hemoglobin values were not available for 18 patients.
Mild anemia was defined as hemoglobin 10.0-10.9 g/dl in children <5 years or 11.0-11.4 g/dl in children ≥5 years.
Moderate anemia was defined as hemoglobin 7.0-9.9 g/dl in children <5 years or 8.0-10.9 g/dl in children ≥5 years.
Severe anemia was defined as hemoglobin <7.0 g/dl in children <5 years or <8.0 g/dl in children ≥5 years.
Baseline HIV RNA values were not available for 22 patients.
Baseline CD4 cell percentages and CD4 counts were not available for 8 patients.
Efavirenz-based cART was generally used for children ≥3 years and ≥10 kilograms.
Protease inhibitor-based cART was generally used for children <3 years or <10 kilograms.
Median duration of TB treatment at time of cART initiation was 23 days (IQR 15-39, range 0-180) (Table 1). Consequently, most children receiving co-treatment did so for ≥5 months. Children receiving TB treatment were more likely to be male (60% vs. 45%, P=0.04), were slightly older, and had lower baseline hemoglobin (9.6 vs. 10.3 g/dL, P=0.01), weight-for-age z-scores (−1.81 vs. −1.29, P=0.02), and median CD4% (15.3% vs. 18.8%, P<0.01) than children not on TB treatment. Baseline median HIV RNA was similar among those receiving and not receiving TB treatment (5.4 vs. 5.6 log10 copies/mL, P=0.3).
Duration of follow-up ranged from 2.2 to 26.6 months (median 23.8, IQR 20.6-24.1), and did not differ by TB treatment status (P=0.6, Table 1). Sixty (30%) children did not complete the 24-month follow-up visit and were censored at their date of last visit. Of these, 29 (48%) were receiving TB treatment at cART initiation and 22 (37%) did not have 24 months of follow-up before the study stopped in August 2013. During follow-up, 24 (12%) children switched cART: 7 from efavirenz-based to LPV/r-based cART, 1 from LPV/r-based to efavirenz-based cART, 12 within LPV/r-based cART, and 4 within efavirenz-based regimens. TB treatment was not associated with regimen switching (15% vs. 9%, P=0.2). Only 1 death was documented during follow-up; the child was not receiving co-treatment for TB and died at age 2.3 years, 23 months after cART initiation.
Virologic suppression
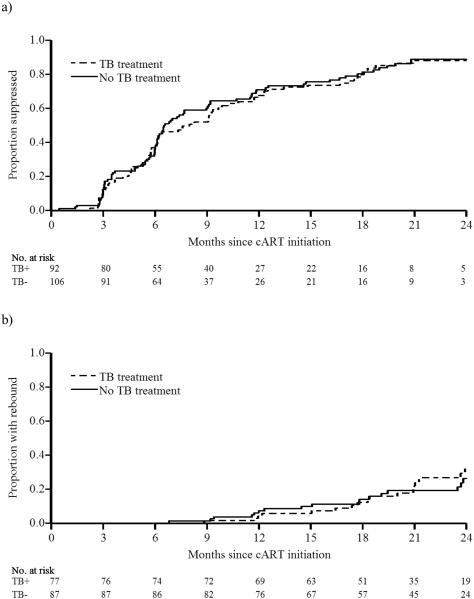
The number of children with available viral loads at each visit is described in Supplemental Digital Content 3. Time to virologic suppression ranged from 0.5 to 25.2 months, with a median of 6.2 months among both children receiving and not receiving TB treatment (Table 2), with consistently overlapping Kaplan-Meier curves in the two groups (logrank P=0.7, Figure 1a). TB treatment also did not significantly affect the hazard of virologic suppression (adjusted HR [aHR] 1.36, 95% CI 0.94-1.96, Table 2). By 12 months post cART initiation, 124 (62%) children experienced virologic suppression <50 copies/mL and had not yet rebounded >1000 copies/mL (63% vs. 62% in children receiving vs. not receiving TB treatment).
Table 2.
Virologic and immunologic response outcomes, stratified by TB treatment status
| Outcome | All patients | Children receiving TB treatment |
Children not receiving TB treatment |
P | |||
|---|---|---|---|---|---|---|---|
| Virologic suppression | |||||||
| Time to suppression, median months (IQR) | 6.2 | (3.9 – 11.5) | 6.2 | (4.6 – 11.7) | 6.2 | (3.5 – 10.7) | 0.50 |
| HR (95% CI) | |||||||
| 6 months (crude) | 1.09 | (0.67, 1.77) | 1.0 | 0.73 | |||
| 6 months (adjusted)a | 1.20 | (0.69, 2.08) | 1.0 | 0.53 | |||
| 12 months (crude) | 1.04 | (0.72, 1.49) | 1.0 | 0.85 | |||
| 12 months (adjusted)a | 1.23 | (0.82, 1.84) | 1.0 | 0.32 | |||
| 24 months (crude) | 0.94 | (0.69, 1.28) | 1.0 | 0.68 | |||
| 24 months (adjusted)a | 1.36 | (0.94, 1.96) | 1.0 | 0.10 | |||
| Virologic rebound b | |||||||
| HIV RNA >1000 copies/mL, no. (%) | 39/164 | (23.8) | 18/77 | (23.4) | 21/87 | (24.1) | 0.91 |
| Time to rebound, median months (IQR) | 18.3 | (12.2 – 23.5) | 18.3 | (15.0 – 21.0) | 17.8 | (12.0 – 23.7) | 0.89 |
| Crude HR (95% CI)c | 1.10 | (0.58, 2.08) | 1.0 | 0.77 | |||
| Adjusted HR (95% CI)a,c | 1.53 | (0.71, 3.30) | 1.0 | 0.27 | |||
| Immunologic response | |||||||
| CD4%, median (IQR) | |||||||
| cART initiation | 17.1 | (11.6 – 23.3) | 15.3 | (9.5 – 21.0) | 18.8 | (14.3 – 25.3) | <0.01 |
| 3 months | 25.5 | (19.1 – 32.3) | 22.6 | (16.7 – 29.1) | 26.5 | (20.0 – 33.7) | 0.03 |
| 6 months | 27.8 | (20.6 – 34.2) | 24.8 | (18.6 – 30.9) | 30.1 | (24.8 – 36.9) | <0.01 |
| 12 months | 29.5 | (23.6 – 34.7) | 27.3 | (21.4 – 33.2) | 30.9 | (25.0 – 36.2) | 0.04 |
| 24 months | 33.6 | (26.2 – 37.5) | 31.3 | (21.9 – 36.7) | 34.5 | (27.0 – 38.2) | 0.09 |
| Increase in CD4%, median (IQR) | |||||||
| 3 months | 7.2 | (3.3 – 11.4) | 7.2 | (3.6 – 11.0) | 6.5 | (3.3 – 11.9) | 0.90 |
| 6 months | 9.7 | (5.4 – 14.1) | 9.9 | (5.2 – 14.4) | 9.6 | (5.6 – 14.0) | 0.85 |
| 12 months | 13.3 | (7.9 – 17.5) | 14.2 | (9.2 – 18.7) | 11.9 | (7.0 – 16.3) | 0.06 |
| 24 months | 14.4 | (9.1 – 19.7) | 14.5 | (9.6 – 19.2) | 14.2 | (8.0 – 20.0) | 0.87 |
| Severe immunodeficiency, no. (%)d | |||||||
| 3 months | 57/115 | (49.6) | 31/54 | (57.4) | 26/61 | (42.6) | 0.11 |
| 6 months | 58/151 | (38.4) | 38/73 | (52.1) | 20/78 | (25.6) | <0.01 |
| 12 months | 27/116 | (23.3) | 15/48 | (31.3) | 12/68 | (17.6) | 0.09 |
| 24 months | 7/101 | (6.9) | 7/48 | (14.6) | 0/53 | (0.0) | <0.01 |
Abbreviations: cART, combination antiretroviral therapy; CD4%, CD4 cell percentage; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; TB, tuberculosis.
Multivariable models adjusted for timing of cART initiation relative to the 2010 change in guidelines, age at cART initiation, sex, cART regimen, and baseline HIV RNA, CD4 cell percentage, hemoglobin, and weight-for-age z-score. Adjusted models include individuals who had complete covariate data: n=164 for the suppression models and n=139 for the rebound model.
Virologic rebound was assessed among the 164 individuals who suppressed <50 copies/mL at any point following cART initiation.
Hazard ratios account for 24 months of follow-up after cART initiation.
Severe immunodeficiency was defined according to World Health Organization age-specific classifications 21: CD4% <25% in children <11 months, CD4% <20% in children 12-35 months, CD4% <15% in children 36-59 months, and CD4 cell count <200 cells/μL or CD4% <15% in children >5 years.
Figure 1. Kaplan-Meier graphs of virologic response, stratified by TB treatment status at cART initiation.
(a) Time to first virologic suppression. (b) Time to virologic rebound. Abbreviations: cART, combination antiretroviral therapy; TB, tuberculosis; TB+, receiving TB treatment at cART initiation; TB−, not receiving TB treatment at cART initiation.
Using an alternative suppression definition of <400 copies/mL resulted in decreased time to suppression (from a median of 6.2 to 5.7 months), increased proportion suppressed by 12 months (from 62% to 74%), and decreased time to rebound (from 18.3 to 17.5 months), but did not change the proportion that rebounded or any HRs of interest (see Tables, Supplemental Digital Content 4-6, for analyses using a suppression definition of <400 copies/mL).
Virologic rebound
Overall, 164 (82%) patients had experienced virologic suppression and were included in the virologic rebound analyses (Table 2). Similar proportions of patients receiving and not receiving TB treatment experienced virologic rebound (23% vs. 24%, P = 0.9), with substantial overlap between the Kaplan-Meier curves (logrank P=0.8, Figure 1b). Time to virologic rebound ranged from 6.8 to 26.0 months from cART initiation. TB treatment did not affect time to virologic rebound (18.3 months vs. 17.8 months, P=0.9) or hazard of virologic rebound over 24 months following cART initiation (aHR 1.53, 95% CI 0.71, 3.30).
CD4% response
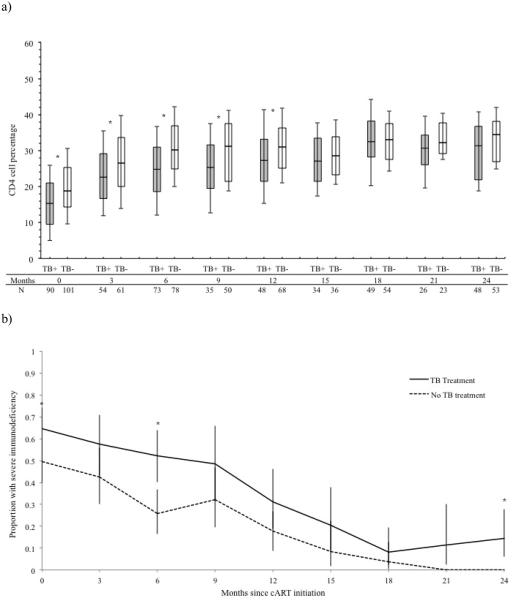
Children on TB treatment had lower CD4% in the first 12 months of cART, after which they caught up to children not on TB treatment (Table 2, Figure 2a). Children receiving and not receiving TB treatment at cART initiation had a similar median CD4% increase at 3 months (7.2% vs. 6.5%), 6 months (9.9% vs. 9.6%), 12 months (14.2% vs. 11.9%) and 24 months (14.5% vs. 14.2%) (all P≥0.06, Table 2), but a higher proportion of children on TB treatment had severe age-specific immunodeficiency throughout follow-up (Figure 2b).
Figure 2. Graphs of immunologic response, stratified by TB treatment status at cART initiation.
(a) Observed CD4 cell percentage evolutions among children receiving TB treatment and children not receiving TB treatment. Each box plot depicts the median and interquartile range, with the error bars marking the 10th and 90th percentiles. Time is indicated in months since cART initiation. The number of children in each group with a CD4 cell percentage measurement at each time point is indicated. (b) Proportion of children with severe age-specific immunodeficiency, with exact 95% confidence limits. * indicates a statistically significant difference between those receiving vs. not receiving TB treatment at that time point (P <0.05). Abbreviations: cART, combination antiretroviral therapy; TB, tuberculosis; TB+, receiving TB treatment at cART initiation; TB−, not receiving TB treatment at cART initiation.
Exploratory analysis by cART regimen
cART regimen had a greater effect on virologic suppression than TB treatment, with children on efavirenz-based regimens experiencing shorter median time to suppression (5.5 months vs. 7.7 months, P<0.01) as compared to children on LPV/r-based cART (Table 3, Supplemental Digital Content 7, for a cART regimen-stratified Kaplan-Meier suppression graph). cART regimen did not appear to influence virologic rebound (aHR 0.62, 95% CI 0.15, 2.52) or CD4% reconstitution.
Table 3.
The effect of TB treatment on virologic and immunologic response outcomes, stratified by cART regimen
| Outcome | Efavirenz-based cART (n=79) |
LPV/r-based cART (n=120) |
Efavirenz-based cART (n=79) | LPV/r-based cART (n=120) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Children receiving TB treatment (n=39) |
Children not receiving TB treatment (n=40) |
Children receiving TB treatment (n=53) |
Children not receiving TB treatment (n=67) |
|||||||||
| Virologic suppression | ||||||||||||
| Time to suppression, median | 5.5 | (3.1 – 7.6)a | 7.7 | (5.7 – 13.4) | 5.3 | (3.3 – 7.6) | 5.8 | (3.1 – 6.9) | 9.5 | (5.8 – 16.7) | 6.8 | (5.2 – 12.4) |
| months (IQR) | ||||||||||||
| Crude HR (95% CI)b | 1.78 | (1.30, 2.43)a | 1.0 | 1.06 | (0.66, 1.69) | 1.0 | 0.83 | (0.55, 1.26) | 1.0 | |||
| Adjusted HR (95% CI)b | 1.26 | (0.63, 2.51)c | 1.0 | 1.66 | (0.88, 3.16)d,e | 1.0 | 1.04 | (0.64, 1.69)d,f | 1.0 | |||
| Virologic rebound g | ||||||||||||
| HIV RNA >1000 copies/mL, no. (%) |
17/71 | (23.9) | 22/93 | (23.7) | 6/35 | (17.1) | 11/36 | (30.6) | 12/42 | (28.6) | 10/51 | (19.6) |
| Time to rebound, median months (IQR) |
18.4 | (15.1 – 23.7) | 17.8 | (12.0 – 21.3) | 19.2 | (17.4 – 21.0) | 18.4 | (14.5 – 23.8) | 18.0 | (13.6 – 21.1) | 15.1 | (11.7 – 23.5) |
| Crude HR (95% CI)b | 1.11 | (0.59, 2.11) | 1.0 | 0.65 | (0.24, 1.76) | 1.0 | 1.66 | (0.70, 4.00) | 1.0 | |||
| Adjusted HR (95% CI)b | 0.62 | (0.15, 2.52)c | 1.0 | 1.03 | (0.26, 4.12)d,e | 1.0 | 2.34 | (0.80, 6.83)d,f | 1.0 | |||
| Immunologic response | ||||||||||||
| CD4%, median (IQR) | ||||||||||||
| cART initiation | 15.8 | (9.6 – 20.7)a | 18.5 | (13.0 – 25.1) | 15.5 | (7.5 – 20.6) | 16.6 | (12.4 – 21.7) | 15.2 | (10.2 – 21.1)h | 22.0 | (16.0 – 27.4) |
| 3 months | 21.6 | (15.3 – 32.2) | 26.1 | (20.2 – 32.4) | 21.6 | (14.0 – 30.3) | 22.0 | (15.8 – 33.4) | 23.5 | (18.8 – 29.1)h | 29.1 | (22.2 – 35.6) |
| 6 months | 26.2 | (20.4 – 32.2) | 28.5 | (22.0 – 34.9) | 26.2 | (19.7 – 31.3) | 26.1 | (20.9 – 34.6) | 23.7 | (14.5 – 30.9)h | 32.7 | (26.7 – 36.9) |
| 12 months | 28.6 | (23.7 – 33.1) | 30.6 | (23.6 – 37.1) | 28.0 | (23.7 – 32.0) | 29.4 | (23.6 – 33.1) | 24.7 | (21.4 – 33.6)h | 31.7 | (28.0 – 39.5) |
| 24 months | 34.0 | (26.1 – 38.4) | 32.7 | (26.2 – 37.3) | 32.8 | (25.0 – 36.5) | 35.6 | (26.4 – 41.3) | 30.8 | (20.4 – 38.1) | 33.6 | (27.8 – 36.8) |
| Increase in CD4%, median (IQR) | ||||||||||||
| 3 months | 6.5 | (4.7 – 10.3) | 7.4 | (2.5 – 12.4) | 6.9 | (3.6 – 10.5) | 6.4 | (4.9 – 9.8) | 7.7 | (3.1 – 11.3) | 7.3 | (2.5 – 13.7) |
| 6 months | 10.9 | (6.9 – 14.8) | 9.7 | (5.1 – 14.0) | 11.6 | (6.7 – 15.3) | 9.3 | (7.2 – 13.5) | 9.7 | (4.7 – 12.2) | 10.0 | (5.3 – 14.2) |
| 12 months | 14.5 | (9.7 – 18.3) | 12.2 | (6.8 – 16.3) | 16.7 | (10.8 – 19.1) | 13.3 | (9.1 – 16.7) | 13.6 | (7.6 – 18.2) | 10.7 | (4.7 – 16.2) |
| 24 months | 15.6 | (12.2 – 20.7) | 13.2 | (5.2 – 19.1) | 15.5 | (12.3 – 19.3) | 15.6 | (8.6 – 20.8) | 12.9 | (7.0 – 19.1) | 13.2 | (3.2 – 18.7) |
| Severe immunodeficiency, no. (%)i | ||||||||||||
| 3 months | 26/43 | (60.5) | 31/72 | (43.1) | 13/21 | (61.9) | 13/22 | (59.1) | 18/33 | (54.5) | 13/39 | (33.3) |
| 6 months | 28/65 | (43.1) | 30/86 | (34.9) | 15/33 | (45.5) | 13/32 | (40.6) | 23/40 | (57.5)h | 7/46 | (15.2) |
| 12 months | 11/49 | (22.4) | 16/67 | (23.9) | 5/21 | (23.8) | 6/28 | (21.4) | 10/27 | (37.0)h | 6/40 | (15.0) |
| 24 months | 1/50 | (2.0) | 6/51 | (11.8) | 1/22 | (4.5) | 0/28 | (0.0) | 6/26 | (23.1)h | 0/25 | (0.0) |
Abbreviations: cART, combination antiretroviral therapy; CD4%, CD4 cell percentage; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LPV/r, lopinavir/ritonavir; TB, tuberculosis.
The efavirenz-based vs. LPV/r-based cART comparison was statistically significant (P <0.05).
Hazard ratios account for 24 months of follow-up after cART initiation.
Multivariable models included TB treatment at cART initiation, timing of cART initiation relative to the 2010 change in guidelines, age at cART initiation, sex, cART regimen, and baseline HIV RNA, CD4 cell percentage, hemoglobin, and weight-for-age z-score. Adjusted models include individuals who had complete covariate data: n=164 for the suppression model and n=139 for the rebound model.
Multivariable models included TB treatment at cART initiation, timing of cART initiation relative to the 2010 change in guidelines, age at cART initiation, sex, and baseline HIV RNA, CD4 cell percentage, hemoglobin, and weight-for-age z-score.
Adjusted models include individuals who had complete covariate data: n=61 for the suppression model and n=56 for the rebound model.
Adjusted models include individuals who had complete covariate data: n=103 for the suppression model and n=83 for the rebound model.
Virologic rebound was assessed among the 164 individuals who suppressed <50 copies/mL at any point following cART initiation.
The TB treatment vs. no TB treatment comparison was statistically significant (P <0.05).
Severe immunodeficiency was defined according to World Health Organization age-specific classifications 21: CD4% <25% in children <11 months, CD4% <20% in children 12-35 months, CD4% <15% in children 36-59 months, and CD4 cell count <200 cells/μL or CD4% <15% in children >5 years.
Among children on LPV/r-based regimens, children on TB treatment tended to have similar virologic suppression (aHR 1.04, 95% CI 0.64, 1.69), increased virologic rebound (29% vs. 20%, P=0.3), higher hazard of rebound (aHR 2.34, 95% CI 0.80, 6.83), and had significantly lower CD4% at 3, 6, and 12 months, and higher proportions with severe immunodeficiency at 6, 12, and 24 months compared to children on LPV/r not receiving TB treatment (Table 3). In contrast, among children on efavirenz-based cART, no significant effect of TB treatment was observed, with similar hazard of virologic suppression (aHR 1.66, 95% CI 0.88, 3.16), similar hazard of virologic rebound (aHR 1.03, 95% CI 0.26, 4.12), and no significant differences in immunologic response between children on efavirenz-based cART who did and did not receiving TB co-treatment.
Exploratory analysis by timing of cART initiation
Children initiating cART according to pre-2010 guidelines had a shorter median time to suppression (5.7 vs. 6.5 months, P=0.01) and greater hazard of suppression over time (aHR 1.94, 95% CI 1.35, 2.80) compared to those initiating cART according to 2010 guidelines (see Table, Supplemental Digital Content 8, for analyses stratified by timing of cART initiation). They also consistently experienced lower CD4% increases and higher proportions of severe immunodeficiency, though the differences were not statistically significant.
Discussions
Children receiving TB treatment at cART initiation did not have a substantially different response to cART, with similar rates of virologic suppression, virologic rebound, and CD4% increase. Children initiating cART while on TB treatment presented with more advanced disease at baseline and remained more vulnerable as they continued to have lower CD4% and a higher prevalence of severe immunodeficiency throughout the first 2 years of cART. Similar to observed responses in adults,29 efavirenz-based cART was superior to LPV/r-based cART, with more and quicker suppression compared to LPV/r-based regimens. In young children, our exploratory sensitivity analysis indicates that the combination of TB treatment and super-boosted LPV/r-based cART may increase the risk of rebound and diminish CD4% reconstitution, a finding in accordance with prior studies15,17 and with known drug-drug interactions between PIs and rifampicin, which can result in subtherapeutic plasma concentrations of both types of medications.7
We did not observe an effect of TB treatment on virologic suppression, a key measure of successful cART,2 among children on nonnucleoside reverse transcriptase inhibitor-based cART, a finding similar to that reported by Zanoni et al. Three studies have reported decreased virologic suppression rates in young children receiving concurrent PI-based cART and TB treatment. Zanoni et al. found that children <3 years on super-boosted or double-dosed PI-based cART and concomitant TB treatment experienced decreased suppression compared to children not receiving TB treatment.16 Frohoff et al. also observed that TB treatment decreased suppression among young children <2 years on double-dose LPV/r and ritonavir-based cART,15 and Reitz et al. found decreased suppression by 39 weeks among children <2 years on ritonavir or unboosted LPV/r-based cART being co-treated for TB.17 Among children on LPV/r-based cART, we did not observe a decrease in virologic suppression among children receiving concurrent TB treatment at cART initiation. However, our median observed time to virologic suppression was 6.2 months, which was longer than the 13 to 14 weeks reported by Reitz et al.17 and may be partially attributed to infrequent sampling for viral load in our cohort. The sparse viral load data, especially at the 3-month visit when there is overlapping administration of TB and cART medications, may have masked a true difference in virologic suppression rates. Even though we did not observe differences in suppression rates, we did observe an increased hazard of virologic rebound in children receiving LPV/r-based cART and TB treatment, supporting the potential for an inferior virologic response in children receiving LPV/r-based cART together with TB treatment in the first months of cART.
Virologic rebound may indicate an unsuccessful cART regimen, inadequate adherence, or the emergence of drug-resistant virus, and is an especially important indicator in children due to limited treatment options.2 We did not find TB treatment to affect virologic rebound overall, though there was a clinically meaningful increase in virologic rebound among children <3 years on LPV/r-based cART. Reitz et al. found a lower incidence of rebound among children on PI-based cART and TB treatment within 16 weeks after suppression (2.8% vs. 12%),17 while we found no significant difference at this time point (4.8% vs. 2.0%, P=0.6).
Immune reconstitution is crucial, as a longer time spent at low CD4% is associated with increased risk of morbidity and mortality.2 We found children receiving TB treatment to have lower CD4% in the short-term, but no impairment of median CD4% increase. This finding is in accordance with previous studies,14,15,17,18 and extends this observation to 24 months of cART.
This study possessed a number of important strengths. The THINK study was prospective, enabling us to firmly establish the timing between TB treatment and cART initiation and to obtain regular HIV RNA and CD4% measurements during follow-up. Children were followed-up for two years, allowing us to distinguish between short- and long-term effects of TB treatment. The occurrence of only one documented death allowed us to study response to cART without survival bias. Most children were very young (median age 2.1 years), an age group which tends to be underrepresented in cART programs and research studies but is becoming larger with increasing access to early infant diagnosis.19
This study was however not without limitations. The observational design nested within routine care resulted in loss-to-follow-up, missing data, and required adaptations to changing guidelines over time. Classification of virologic rebound using a single HIV RNA measurement >1000 copies/mL may have included blips. Adherence data for TB treatment and cART were not available and most TB diagnoses were presumptive, resulting in potential misclassification of active TB. However, given our primary exposure was administration of TB treatment at cART initiation, exposure misclassification is likely less of a concern, and results are applicable to all children on TB treatment at cART initiation. While TB treatment duration prior to cART initiation may affect response to cART, we could not assess this due to limited variability in time lag between start of TB treatment and cART initiation (median 23 days, IQR 15-39). Finally, as cART regimen was prescribed according to age and weight, it was difficult to separate the individual effects of these exposures.
In conclusion, despite a multitude of challenges posed by concomitant TB and HIV treatment in young children,30,31 our findings indicate that children ≥3 years on efavirenz-based cART and TB treatment do not experience an inferior cART response. This is similar to the situation in adults8 and reinforces WHO recommendations to initiate cART soon after TB treatment is tolerated.4 In young children, our exploratory sensitivity analyses suggest that the combination of TB treatment and super-boosted LPV/r-based cART may increase the risk of rebound and diminish CD4% reconstitution. As guidelines and treatment options evolve, further research is needed to determine optimal HIV/TB co-treatment regimens, especially for children aged <3 years.
Supplementary Material
Acknowledgements
The authors are grateful to Joseph J. Eron, Jr., Sonia Napravnik, and Alan Brookhart for their thoughtful comments and guidance, and to Andrew Edmonds for inspiring the side-by-side box plot. We acknowledge the contributions from study staff and wish to thank study participants and their caregivers for their contribution to this study.
Sources of Funding
This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health [grant number R01HD058972]. The use of REDCap was partially funded by the Clinical and Translational Science Award program of the Division of Research Resources, National Institutes of Health [grant number 1UL1TR001111-01]. The United States Agency for International Development’s United States President’s Emergency Plan for AIDS Relief Project at Wits Reproductive Health Institute provided partial funding for staff, equipment and technical support at the Harriet Shezi Children's Clinic.
Footnotes
Presented in part
44th World Conference on Lung Health, Paris, France, 30 October – 3 November 2013 (abstract OP-188-02).
Conflicts of Interest
No conflicts of interest were reported.
References
- 1.World Health Organization . Global Tuberculosis Report. WHO; Geneva: 2013. [Google Scholar]
- 2.Volberding P, Sande M, Lange J, Greene W. Global HIV/AIDS Medicine. Elsevier; Philadelphia: 2008. [Google Scholar]
- 3.Russell GK, Merle CS, Cooke GS, Casas EC, Silveira da Fonseca M, du Cros P. Towards the WHO target of zero childhood tuberculosis deaths: an analysis of mortality in 13 locations in Africa and Asia. Int J Tuberc Lung Dis. 2013 Dec;17(12):1518–1523. doi: 10.5588/ijtld.13.0238. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Antiretroviral therapy for HIV infection in infants and children: towards universal access - 2010 revision. Geneva: 2010. [PubMed] [Google Scholar]
- 5.Curran A, Falco V, Pahissa A, Ribera E. Management of Tuberculosis in HIV-Infected Patients. AIDS Rev. 2012 Oct;14(4):231–246. [PubMed] [Google Scholar]
- 6.Breton G, Bourgarit A, Pavy S, et al. Treatment for tuberculosis-associated immune reconstitution inflammatory syndrome in 34 HIV-infected patients. The International Journal of Tuberculosis and Lung Disease. 2012 Oct;16(10):1365–1370. doi: 10.5588/ijtld.11.0693. [DOI] [PubMed] [Google Scholar]
- 7.McIlleron H, Ren Y, Nuttall J, et al. Lopinavir exposure is insufficient in children given double doses of lopinavir/ritonavir during rifampicin-based treatment for tuberculosis. Antivir Ther. 2011;16(3):417–421. doi: 10.3851/IMP1757. [DOI] [PubMed] [Google Scholar]
- 8.Soeters HM, Napravnik S, Patel MR, Eron JJ, Jr., Van Rie A. The effect of tuberculosis treatment on virologic and CD4+ cell count response to combination antiretroviral therapy: a systematic review. AIDS. 2014;28(2):245–255. doi: 10.1097/01.aids.0000434936.57880.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soeters HM, Poole C, Patel MR, Van Rie A. The effect of tuberculosis treatment at combination antiretroviral therapy initiation on subsequent mortality: a systematic review and meta-analysis. PLoS One. 2013;8(10):e78073. doi: 10.1371/journal.pone.0078073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pensi T, Hemal A, Banerjee T. Simultaneous HAART improves survival in children coinfected with HIV and TB. Tropical medicine & international health : TM & IH. 2012 Jan;17(1):52–58. doi: 10.1111/j.1365-3156.2011.02884.x. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman CA, Schaaf HS, Cotton MF, et al. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2011 Jun;15(6):770–775. doi: 10.5588/ijtld.10.0501. [DOI] [PubMed] [Google Scholar]
- 12.Yotebieng M, Van Rie A, Moultrie H, et al. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010 Jun 1;24(9):1341–1349. doi: 10.1097/QAD.0b013e328339e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bong CN, Chen SC, Jong YJ, et al. Outcomes of HIV-infected children with tuberculosis who are started on antiretroviral therapy in Malawi. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2007 May;11(5):534–538. [PubMed] [Google Scholar]
- 14.van Dijk JH, Sutcliffe CG, Hamangaba F, Bositis C, Watson DC, Moss WJ. Effectiveness of efavirenz-based regimens in young HIV-infected children treated for tuberculosis: a treatment option for resource-limited settings. PLoS One. 2013;8(1):e55111. doi: 10.1371/journal.pone.0055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohoff C, Moodley M, Fairlie L, et al. Antiretroviral therapy outcomes in HIV-infected children after adjusting protease inhibitor dosing during tuberculosis treatment. PLoS One. 2011;6(2):e17273. doi: 10.1371/journal.pone.0017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Impact of tuberculosis cotreatment on viral suppression rates among HIV-positive children initiating HAART. AIDS. 2011 Jan 2;25(1):49–55. doi: 10.1097/QAD.0b013e32833f9e04. [DOI] [PubMed] [Google Scholar]
- 17.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. The Journal of infectious diseases. 2010 Apr 15;201(8):1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanoni BC, Phungula T, Zanoni HM, France H, Cook EF, Feeney ME. Predictors of poor CD4 and weight recovery in HIV-infected children initiating ART in South Africa. PLoS One. 2012;7(3):e33611. doi: 10.1371/journal.pone.0033611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatti G, Bock P, Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: an analysis in four provinces in South Africa, 2004-2009. J Acquir Immune Defic Syndr. 2011 Nov 1;58(3):e60–67. doi: 10.1097/QAI.0b013e3182303c7e. [DOI] [PubMed] [Google Scholar]
- 20.WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. World Health Organization; Geneva: 2007. [Google Scholar]
- 21.WHO Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. 2011 http://www.who.int/vmnis/indicators/haemoglobin Accessed August 15, 2013.
- 22.World Health Organization Child growth standards. 2011 http://www.who.int/childgrowth/software/en/ Accessed August 7, 2013.
- 23.World Health Organization Growth reference 5-19 years. 2013 http://www.who.int/growthref/tools/en/ Accessed August 7, 2013. [Google Scholar]
- 24.National Tuberculosis Management Guidelines. National Department of Health; Republic of South Africa: 2008. [Google Scholar]
- 25.Moore DP, Schaaf HS, Nuttall J, Marais BJ. Childhood tuberculosis guidelines of the Southern African Society for Paediatric Infectious Diseases. South Afr J Epidemiol Infect. 2009;24(3):57–68. [Google Scholar]
- 26.Guidelines for the management of HIV in children. 2nd National Department of Health; Republic of South Africa: 2010. [Google Scholar]
- 27.Antiretroviral treatment (ART) in children. National Department of Health; Republic of South Africa: 2004. [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008 May 15;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turkova A, Webb RH, Lyall H. When to start, what to start and other treatment controversies in pediatric HIV infection. Paediatric drugs. 2012 Dec 1;14(6):361–376. doi: 10.2165/11599640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Verhagen LM, Warris A, van Soolingen D, de Groot R, Hermans PW. Human immunodeficiency virus and tuberculosis coinfection in children: challenges in diagnosis and treatment. The Pediatric infectious disease journal. 2010 Oct;29(10):e63–70. doi: 10.1097/INF.0b013e3181ee23ae. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.