Abstract
Preeclampsia, a cardiovascular disorder of late pregnancy, is characterized as a low-renin hypertensive state relative to normotensive pregnancy. As other non-pregnant low-renin hypertensive disorders often exhibit and are occasionally dependent upon elevated arginine vasopressin (AVP) secretion, we hypothesized a possible use for plasma AVP measurements in the prediction of preeclampsia. Copeptin is an inert pro-segment of AVP that is secreted in a 1:1 molar ratio and exhibits a substantially longer biological half-life than AVP, rendering it a clinically useful biomarker of AVP secretion. Copeptin was measured throughout pregnancy in maternal plasma from preeclamptic and control women. Maternal plasma copeptin was significantly higher throughout preeclamptic pregnancies versus control pregnancies. While controlling for clinically significant confounders (age, BMI, chronic essential hypertension, twin gestation, diabetes, and history of preeclampsia) using multivariate regression, the association of higher copeptin concentration and the development of preeclampsia remained significant. Receiver operating characteristic analyses reveal that as early as the 6th week of gestation, elevated maternal plasma copeptin concentration is a highly significant predictor of preeclampsia throughout pregnancy. Finally, chronic infusion of AVP during pregnancy (24 ng/hr) is sufficient to phenocopy preeclampsia in C57BL/6J mice, causing pregnancy-specific hypertension, renal glomerular endotheliosis, proteinuria, and intrauterine growth restriction. These data (1) implicate AVP release as a novel predictive biomarker for preeclampsia very early in pregnancy, (2) identify chronic AVP infusion as a novel and clinically-relevant model of preeclampsia in mice, and are (3) consistent with a potential causative role for AVP in preeclampsia in humans.
Keywords: Preeclampsia, Vasopressin, Human Biologic Marker, Animal Model
INTRODUCTION
Preeclampsia affects 2–8% of all pregnancies, approximately 300,000 per year in the U.S. It causes 10–15% of all maternal mortality with close to 100,000 preeclampsia-related annual maternal deaths worldwide 1. Maternal death due to preeclampsia disproportionately affects developing countries with 99% of preeclampsia-related maternal deaths occurring in low and middle income countries. In addition, 25% of stillbirths and neonatal deaths in these developing countries are associated with preeclampsia/eclampsia 2. Preeclampsia is known to cause immediate and long term maternal-fetal morbidities such as fetal growth restriction, maternal-fetal death, and future adult neurological and cardiovascular diseases for mother and child 3–8. Because its pathogenesis is poorly understood, preventive, and therapeutic modalities for preeclampsia are elusive. This emphasizes the importance of identifying unifying pathways to predict and treat preeclampsia. One candidate is the vasopressin (AVP) pathway.
Selected non-pregnant populations including African Americans 9, the elderly 10, and patients with chronic heart 11 or renal failure 12 exhibit AVP-dependent hypertension 13. These populations are also characterized by low circulating renin-angiotensin system activity. Relative to non-preeclamptic pregnant women, preeclamptic pregnant women exhibit reduced circulating activity of the renin-angiotensin system 14. This body of literature led us to hypothesize a relationship between AVP hypersecretion and the development of preeclampsia.
AVP exhibits a short biological half-life (5–20 minutes in blood), which complicates assessment of AVP secretion by direct measurement of this hormone. AVP is translated in 1:1 stoichiometric ratio with a small, inactive pro-segment, copeptin. Copeptin is eliminated primarily by renal excretion and is very stable in plasma. Consequently, it is a very useful and reliable biomarker for AVP secretion 15. Zulfikaroglu et al. recently documented a late second/early third trimester elevation in circulating copeptin in preeclamptics16. Similarly, Foda et al. demonstrated increased maternal copeptin levels in preeclamptics at time of delivery 17. The first objective of the current study was to determine if there are differences in first trimester copeptin concentrations between pregnant women who did and did not develop preeclampsia. The second objective of this study was to determine whether chronic AVP infusion during pregnancy is sufficient to induce preeclampsia phenotypes in mice.
METHODS
The Methods section is available in the online-only Data Supplement.
RESULTS
A total of 54 control pregnant, non-preeclamptic women, 50 pregnant, preeclamptic women, and 33 non-pregnant women were analyzed in this study. A full complement of first, second, and third trimester pregnant plasma samples was not available for each pregnant participant. The numbers of analyzed samples for each trimester were as follows: first trimester: 26 controls and 20 cases; second trimester: 19 controls and 20 cases; and third trimester: 38 controls and 50 cases. Maternal age, body mass index, and percentage of those with chronic essential hypertension were similar between the non-pregnant, control, and preeclamptic groups (Table 1). The non-pregnant group had significantly fewer previous pregnancies (lower Gravida), lower percentage of subjects with a history of preeclampsia and history of preexisting diabetes in comparison to the control and preeclamptic pregnant groups. These data may suggest that the non-pregnant cohort represents a cohort at lower risk for preeclampsia. Yet when gravida, history of preeclampsia, and preexisting diabetes was compared between control and preeclamptic pregnant women, no statistically significant differences were noted (gravida: p=0.99, history of preeclampsia: p=0.97, preexisting diabetes: p=0.33). In addition, the racial distribution between these groups were also similar and reflective of the Iowa population with a predominantly Caucasian populace based on current Iowa census data 18. When evaluating the pregnancy characteristics between the control and preeclampsia groups (Table 1), typical differences were observed between groups. The preeclampsia group exhibited a significantly lower gestational age at delivery (36.1 ± 3.1 vs. 38.8 ± 3.1 weeks, p<0.001), lower birthweight (2749.6 ± 800.1 vs. 3386.5 ± 644.8 grams, p<0.001), and higher blood pressures particularly in the third trimester. These findings are consistent with the known clinical factors associated with preeclampsia: higher rate of preterm delivery, higher rate of twin gestation, and lower birthweight due to vascular causes and earlier delivery 19.
Table 1.
Group Characteristics
| Group Characteristics | Nonpregnant (n=33) |
Control (n=54) |
Preeclampsia (n=50) |
P Value for all groups compared |
|---|---|---|---|---|
| Maternal Characteristics | ||||
| Maternal Age (years) | 31.4 ± 7.2 | 29.9 ± 5.2 | 30.0 ± 5.6 | 0.47 |
| Gravida | 1.0 | 2.0 | 2.0 | <0.001 |
| Body Mass Index (kg/m2) | 29.6 ± 8.5 | 30.0 ± 8.7 | 31.9 ± 9.2 | 0.41 |
| Chronic Essential Hypertension | 9.1% | 25.9% | 20.0% | 0.16 (χ2=3.7) |
| Preexisting Diabetes | 3.0% | 20.4% | 22.0% | 0.05 (χ2=5.9) |
| History of Preeclampsia | 0.0% | 29.6% | 18.0% | 0.002 (χ2=12.1) |
| Race: Caucasian, not Hispanic | 90.9% | 92.6% | 90.0% | 0.56 (χ2=6.8) |
| Race: Hispanic | 0% | 3.7% | 4.0% | 0.56 (χ2=6.8) |
| Race: Asian | 6.1% | 1.8% | 0% | 0.56 (χ2=6.8) |
| Race: African-American | 3.0% | 1.9% | 4.0% | 0.56 (χ2=6.8) |
| Systolic Blood Pressure 1 (mmHg) | — | 120.4 ± 12.3 | 129.5 ± 12.3 | 0.041 |
| Diastolic Blood Pressure 1 (mmHg) | — | 69.1 ± 9.8 | 72.2 ± 5.6 | 0.26 |
| Systolic Blood Pressure 2 (mmHg) | — | 121.0 ± 13.6 | 124 ± 18.5 | 0.558 |
| Diastolic Blood Pressure 2 (mmHg) | — | 70.7 ± 9.0 | 70.7 ± 11.3 | 0.998 |
| Systolic Blood Pressure 3 (mmHg) | — | 121.9 ± 17.9 | 129.2 ± 16.7 | 0.049 |
| Diastolic Blood Pressure 3 (mmHg) | — | 70.7 ± 9.9 | 76.3 ± 11.6 | 0.028 |
| Pregnancy Characteristics | ||||
| Gestational Age at Delivery (wk) | — | 38.8 ± 1.7 | 36.1 ± 3.1 | < 0.001 |
| Mode of Delivery: Vaginal | — | 58.5% | 32.7% | < 0.001 (χ2=16.6) |
| Mode of Delivery: C-Section | — | 11.3% | 67.3% | < 0.001 (χ2=16.6) |
| Mode of Delivery: Operative Vaginal Delivery | — | 30.2% | 0% | < 0.001 (χ2=16.6) |
| Twin Gestation | — | 13.0% | 22.0% | 0.34 (χ2=0.92) |
| Birthweight (grams) | — | 3386.5 ± 644.8 | 2749.6 ± 800.1 | < 0.001 |
| 1 minute APGAR | — | 8.0 | 8.0 | 0.64 |
| 5 minute APGAR | — | 9.0 | 9.0 | 0.76 |
Continuous variables are expressed as Mean ± SD or Median
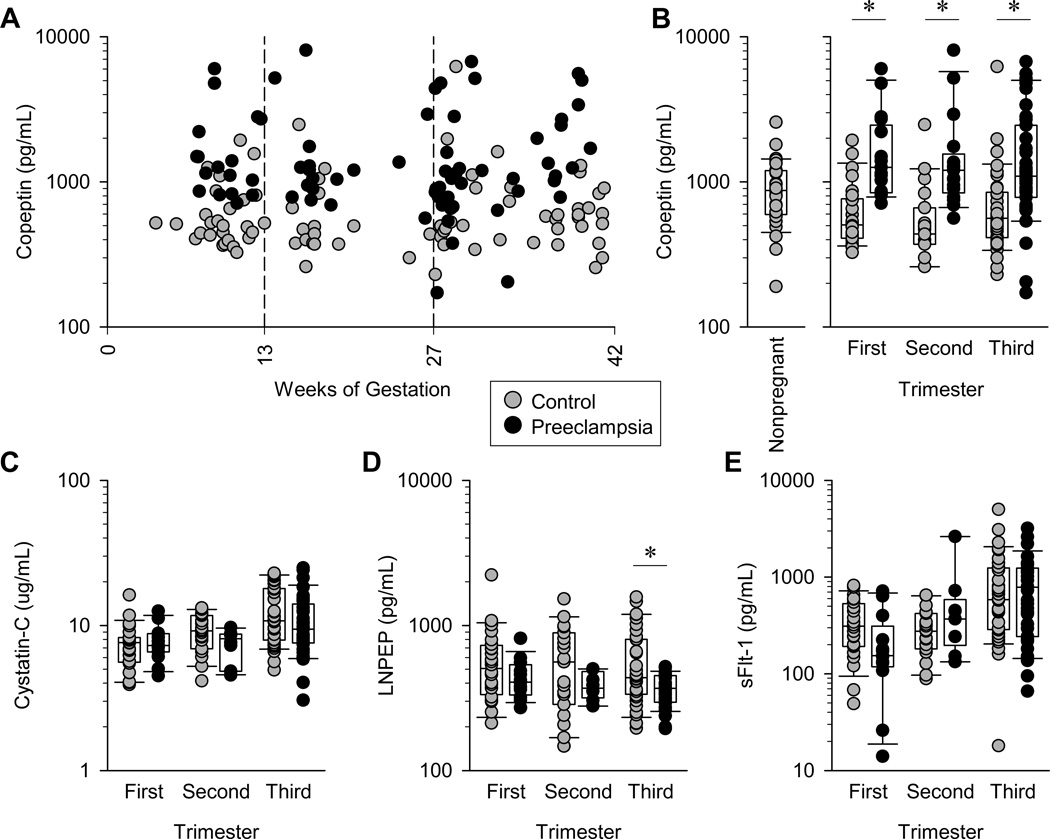
As seen in Figure 1a and 1b, maternal plasma copeptin concentration is significantly higher in pregnant women who developed preeclampsia in comparison with control, non-preeclamptic women in the first trimester, second trimester, and third trimester. In addition, the trimester-specific copeptin concentrations in preeclamptic women are higher than the plasma copeptin concentration of non-pregnant women presenting to our institution for gynecologic care. The non-pregnant cohort data suggests that the rise in copeptin concentration is both pregnancy- and preeclampsia-specific. These group differences in plasma copeptin are likely not associated with changes in renal function and AVP degradation as measured by plasma Cystatin-C and vasopressinase (LNPEP), respectively, as these levels were similar between groups in each trimester (Figure 1c and 1d). Furthermore, significant differences in soluble fms-like tyrosine kinase-1 (sFLT-1) were not detected between the two groups but with a trend of increased sFLT-1 in preeclamptics in the second and third trimesters (Figure 1e).
Figure 1. Maternal plasma copeptin is significantly elevated throughout pregnancies that eventually develop preeclampsia.
(A) Maternal plasma copeptin concentrations throughout gestation from women with normal pregnancies and pregnancies that eventually developed preeclampsia. (B) Comparison of plasma copeptin concentrations within each trimester of pregnancy among control pregnancies, pregnancies that eventually developed preeclampsia, and in non-pregnant women (n=33). (C) Maternal plasma cystatin C within each trimester from pregnancies with and without preeclampsia. (D) Plasma LNPEP within each trimester from pregnancies with and without preeclampsia. (E) Plasma sFLT-1 within each trimester from pregnancies with and without preeclampsia. Data points all represent individual maternal blood samples from control pregnancies n=26, 19, and 38, and preeclamptic pregnancies n=20, 20, and 50 for first, second and third trimesters, respectively. Boxes illustrate median, 25th and 75th percentiles, and whiskers illustrate 10th and 90th percentiles. *p<0.05.
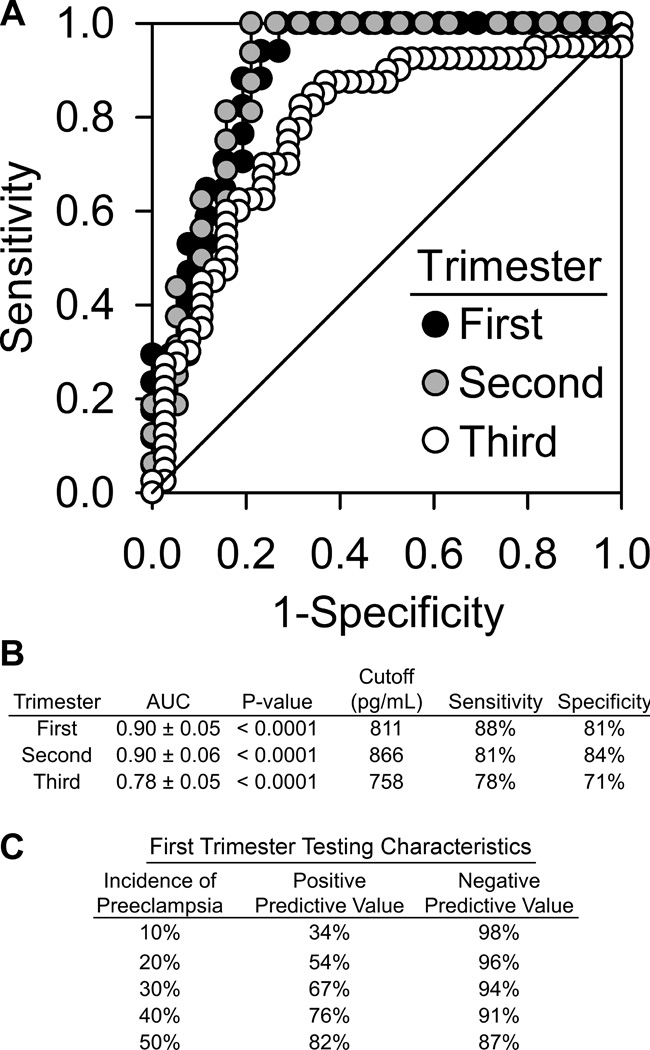
Receiver operating characteristic (ROC) curves for each trimester were constructed to interrogate if this elevated plasma copeptin concentration was predictive of the development of preeclampsia. Furthermore, optimal copeptin concentration cutoffs were determined from these curves. As seen in Figure 2a and 2b, the ROC curves demonstrated significant areas under the curve in the first trimester (AUC=0.90±0.05, p<0.0001), second trimester (AUC=0.90±0.06, p<0.0001), and third trimester (AUC=0.78±0.05, p<0.0001). These data indicate that maternal plasma copeptin concentration is very strongly predictive of the development of preeclampsia in all three trimesters with clinically significant sensitivities and specificities. Copeptin prediction of preeclampsia demonstrates significantly high negative predictive values at all ranges of clinically significant preeclampsia risk and high positive predictive values in those who would be at higher risk of developing preeclampsia (Figure 2c).
Figure 2. Maternal plasma copeptin concentration is significantly predictive of the development of preeclampsia in all three trimesters of pregnancy.
Receiver operating characteristic (ROC) curves illustrate performance of maternal plasma copeptin in each trimester to identify pregnancies that will eventually develop preeclampsia (area under the curve, AUC). Trimester-specific cutoffs were calculated to maximize the true positive rate (sensitivity), while minimizing the false positive rate (1-specificity). AUC values from all three trimesters are statistically significant at p<0.0001 (A and B). Furthermore, first trimester maternal plasma copeptin concentrations exhibit clinically significant positive and negative predictive values (C).
Further, we determined if clinically significant and regression identified covariates would alter the association of preeclampsia and copeptin concentration at particular trimesters. Logistic regression models were constructed with the diagnosis of preeclampsia as the dependent variable. Participants were dichotomized according to being above or below the ROC curve determined cutoff for a particular trimester (Figure 2b). Models were generated using the status of being above or below the cutoff as an independent variable while controlling for significant covariates such as maternal age, body mass index, diabetes, chronic essential hypertension, history of preeclampsia, and twin gestation. Copeptin concentration remained the only variable in all the models that continued to be significantly associated with preeclampsia (Table 2). These clinical data in total suggest that the robust elevation in copeptin concentration occurs early in the first trimester and remains elevated throughout pregnancy despite potential confounding effects of clinically significant obstetrical and vascular covariates.
Table 2.
Using trimester specific cutoffs, maternal plasma copeptin remains significantly associated to the development of preeclampsia despite adjustment of significant clinical covariates
| First Trimester Model [Copepin] Cutoff = 811 pg/mL | β [Copeptin] | Adjusted Odds Ratio | P Value |
|---|---|---|---|
| 1st Trimester [Copeptin] | 3.5 | 33.1 | < 0.001 |
| 1st Trimester [Copeptin] + Maternal Age | 3.8 | 44.7 | < 0.001 |
| 1st Trimester [Copeptin] + Body Mass Index | 3.5 | 33.1 | < 0.001 |
| 1st Trimester [Copeptin] + Diabetes | 3.8 | 44.7 | < 0.001 |
| 1st Trimester [Copeptin] + Chronic Essential Hypertension | 3.7 | 40.4 | < 0.001 |
| 1st Trimester [Copeptin] + History of Preeclampsia | 4.5 | 90.0 | < 0.001 |
| 1st Trimester [Copeptin] + Twin Gestation | 3.4 | 30.0 | < 0.001 |
| 1st Trimester [Copeptin] + All Clinical Covariates | 6.1 | 446.0 | = 0.001 |
| Second Trimester Model [Copeptin] Cutoff = 866 pg/mL | β [Copeptin] | Adjusted Odds Ratio | P Value |
| 2nd Trimester [Copeptin] | 3.1 | 22.2 | < 0.001 |
| 2nd Trimester [Copeptin] + Maternal Age | 3.4 | 30.0 | < 0.001 |
| 2nd Trimester [Copeptin] + Body Mass Index | 3.2 | 24.5 | < 0.001 |
| 2nd Trimester [Copeptin] + Diabetes | 3.1 | 22.2 | < 0.001 |
| 2nd Trimester [Copeptin] + Chronic Essential Hypertension | 3.8 | 44.7 | < 0.001 |
| 2nd Trimester [Copeptin] + History of Preeclampsia | 3.8 | 44.7 | < 0.001 |
| 2nd Trimester [Copeptin] + Twin Gestation | 3.4 | 30.0 | < 0.001 |
| 2nd Trimester [Copeptin] + All Clinical Covariates | 7.1 | 1212.0 | = 0.015 |
| Third Trimester Model [Copeptin] Cutoff = 758 pg/mL | β [Copeptin] | Adjusted Odds Ratio | P Value |
| 3rd Trimester [Copeptin] | 2.1 | 8.2 | < 0.001 |
| 3rd Trimester [Copeptin] + Maternal Age | 2.2 | 9.0 | < 0.001 |
| 3rd Trimester [Copeptin] + Body Mass Index | 2.1 | 8.2 | < 0.001 |
| 3rd Trimester [Copeptin] + Diabetes | 2.5 | 12.2 | < 0.001 |
| 3rd Trimester [Copeptin] + Chronic Essential Hypertension | 2.3 | 10.0 | < 0.001 |
| 3rd Trimester [Copeptin] + History of Preeclampsia | 2.3 | 10.0 | < 0.001 |
| 3rd Trimester [Copeptin] + Twin Gestation | 2.5 | 12.2 | < 0.001 |
| 3rd Trimester [Copeptin] + All Clinical Covariates | 2.9 | 18.2 | < 0.001 |
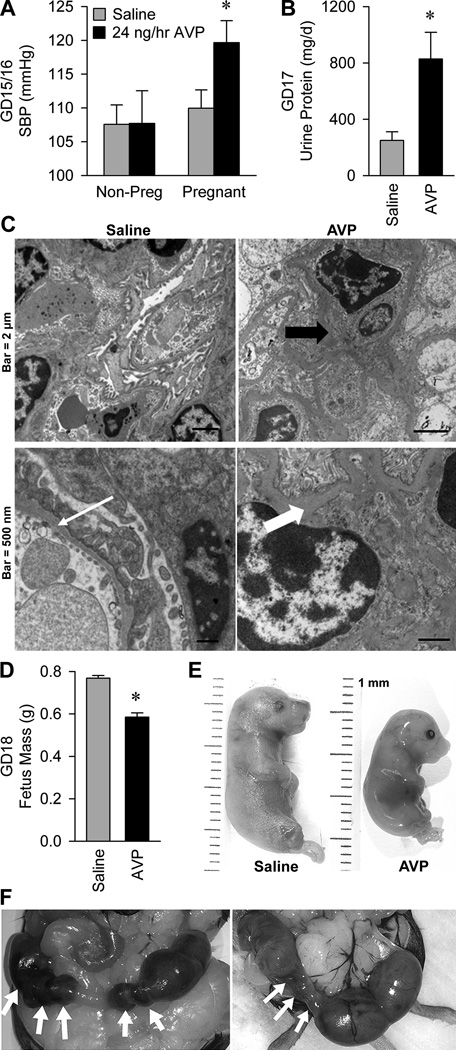
Finally, to examine a causal role for AVP in the pathogenesis of preeclampsia, we tested the sufficiency of chronic AVP infusion throughout gestation to induce the major preeclampsia phenotypes in wildtype C57BL/6J mice. AVP infusion (24 ng/hr) had no effect on systolic blood pressure (SBP) in non-pregnant female mice, but caused a robust elevation in SBP in pregnant mice (Figure 3a and Figure S1). Heart rate (HR) was increased during pregnancy, but vasopressin had no significant modulatory effect (non-pregnant+saline 494±13, non-pregnant+AVP 491±21, pregnant+saline 546±12, pregnant+AVP 516±14 beats/min; pregnancy P=0.01, infusion P=0.29, infusion x pregnancy interaction P=0.38). Pregnant mice infused with AVP exhibited a gross elevation in protein loss to urine (Figure 3b). This proteinuria was associated with robust renal glomerular endotheliosis (Figure 3c), a pathognomonic finding in preeclampsia.
Figure 3. Infusion of vasopressin during pregnancy is sufficient to cause the cardinal phenotypes of preeclampsia in mice.
(A) Systolic blood pressure on gestational days 15 and 16. Non-pregnant mice infused with saline (n=15) or AVP at 24 ng/hr (n=5) for 15–16 days, and pregnant mice infused with saline (n=16) or AVP (n=11). * P<0.05 versus all other groups. (B) Total protein lost to urine per day, on gestational day 17. Pregnant mice infused with saline (n=5) or AVP at 24 ng/hr (n=9). * P<0.05 versus saline. (C) Electron micrographs of renal cortex, illustrating glomerular endotheliosis. Left panels are from a saline infused animal which had a glomerular basement membrane thickness within normal limits (thin white arrow). Right panels are from animals that received vasopressin infusion (24 ng/hr). Redundant endothelial cell membrane is present (thick black arrow), and basement membranes are moderately to markedly thickened with electron dense material (thick white arrow). (D) Average GD18 fetus mass from mice infused with saline (117 fetuses from 16 pregnancies) or AVP at 24 ng/hr (115 fetuses from 16 pregnancies). * P<0.05 versus saline. (E) Photographs of representative gestational day 18 fetuses from pregnant dams infused with saline and 24 ng/hr AVP, illustrating intrauterine growth restriction. (F) Photographs of feto-placental units in utero on gestational day 18 during 24 ng/hr AVP infusion. White arrows indicate resorbed feto-placental units.
Chronic AVP infusion during pregnancy also significantly influenced fetal growth and development. Fetus masses on gestational day 18 were suppressed approximately 24% by AVP infusion (Figure 3d and 3e). One of seventeen AVP-infused dams underwent preterm labor on GD17. AVP infusion may also increase the rate of spontaneous feto-placental unit resorption (Figure 3f). In the AVP-infused (n=16) and saline-infused (n=16) pregnancies, similar numbers of fetuses were carried to GD18 (AVP: 115 vs. Saline: 117). Yet, there was a trend for increased feto-placental resorption in the AVP infused pregnancies (12% vs. 6%; P=0.17).
DISCUSSION
Significant information regarding the relationship between AVP/copeptin and preeclampsia are clear from these data. First, AVP hypersecretion, assessed via maternal plasma copeptin, is grossly elevated in the first few weeks of human pregnancies that eventually develop preeclampsia. Second, maternal plasma copeptin is significantly predictive of the development of preeclampsia, regardless of clinical covariates, at least as early as the sixth week of pregnancy. Third, chronic infusion of AVP during pregnancy in wildtype C57BL/6J mice is sufficient to induce all of the major maternal and fetal phenotypes associated with human preeclampsia including pregnancy-specific hypertension. Together, these findings support the use of AVP/copeptin measurements as a novel very early-pregnancy predictive biomarker for the late-pregnancy development of preeclampsia. Further, these findings are consistent with a causal role for AVP in the pathogenesis of preeclampsia.
The determination that AVP hypersecretion predicts preeclampsia as early as the sixth week of pregnancy represents a major advance in the prediction of this disorder. Currently, anti-angiogenic factors like soluble fms-like tyrosine kinase-1 (sFLT-1) and endoglin are elevated only as early as 12 weeks before the diagnosis of preeclampsia20. Follow up analyses of sFLT-1, endoglin, and other anti-angiogenic factors suggest that testing characteristics of these factors are poor in application to clinical practice21–23. Additional studies have examined first-trimester circulating interleukin-1b 24, high sensitivity C-reactive protein 25, and pregnancy-associated plasma protein-A (PAPP-A) 25, but these factors have been shown to be poor to moderately predictive of preeclampsia. Given the promise of antiangiogenic markers such as sFLT-1, endoglin, and placental growth factor (PlGF) in the pathogenesis of preeclampsia, they have also been investigated in the first trimester as possible predictors of preeclampsia. In conjunction with uterine artery Doppler (UAD) analyses, these anti-angiogenic factors have been shown to be predictive (AUC=0.74) of preeclampsia but the requirements of performing both an assay and a UAD analysis is not practical for clinical screening, particularly in developing countries 22. An elevated uterine artery Doppler pulsatile index (UAD-PI) alone in the first trimester is strongly correlated with the development of preeclampsia. Poon et al. demonstrated that UAD-PI coupled with maternal history and aneuploidy markers in the first trimester can be very predictive of preeclampsia with AUC=0.96. In and of itself, UAD-PI has an AUC of 0.9126,27. Although UAD-PI may be a powerful, predictive tool, reliable measurement requires substantial training for sonographers through verified programs such as the Fetal Medicine Foundation 28 to decrease significant interassay variability. Such training may not be as available in all hospital settings, again with the most susceptible populations in developing countries having the least access. In comparison, we report that first trimester plasma copeptin measurement, which could be easily simplified for point of care testing, is independently, highly predictive of preeclampsia much earlier in pregnancy, with clinically significant sensitivity, specificity, negative predictive value, and positive predictive value. Clearly, there is utility in finding a simple, high-fidelity predictor of preeclampsia as early in pregnancy as possible, and copeptin represents exactly this type of simple, powerful and individually predictive biomarker.
Modeling preeclampsia in animals is difficult, both because of a lack of spontaneous preeclampsia in most species, and a general lack of understanding of the early-pregnancy events that initiate this disorder. One common, powerful model of preeclampsia is the reduced uterine perfusion pressure (RUPP) model, which involves physical constriction of the uterine arteries 29. This model is effective to emulate the late-pregnancy vascular dysfunction that is typical of preeclampsia by modeling uterine artery vasoconstriction. Similarly, viral delivery of sFLT-1 during pregnancy in mice results in many of the phenotypes associated with preeclampsia30. While both methods effectively model the late-pregnancy pathogenesis of preeclampsia, neither intervention can recapitulate the early-pregnancy initiating stimuli that cause sFLT-1 elevation and subsequent vascular dysfunctions. One genetic mouse model of preeclampsia (the BPH/5 model) has been documented, which may help inform the search for the early-pregnancy pathogenic processes 31,32. The primary criticism of the BPH/5 model is the presence of a baseline hypertension which models chronic hypertension as opposed to pregnancy induced elevations in blood pressure. The AVP infusion of preeclampsia model demonstrates a pregnancy-specific hypertension, a phenotype that is not associated with any of these models. This finding represents a major breakthrough in the modeling of preeclampsia. Our study strongly supports the clinical and translational relevance of this new model as the model was based on our observation that AVP secretion is chronically and robustly elevated throughout preeclamptic human pregnancies. Further studies into the mechanisms that cause elevated AVP secretion may lead to the discovery of even earlier-pregnancy biomarkers.
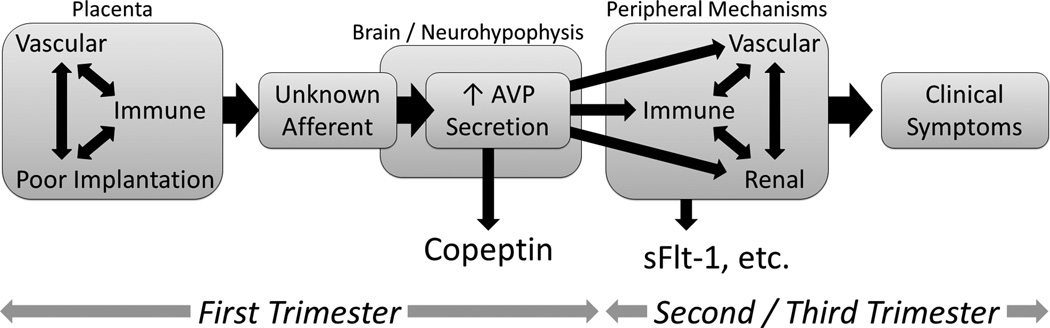
Our human prediction and murine model data leads us to hypothesize that AVP production, secretion, steady-state, and/or action at various receptors may represent novel and rational therapeutic targets for the prevention and treatment of preeclampsia. Pregnancy mechanisms that mediate preeclampsia such as immune dysfunction, endothelial and vascular dysfunction, oxidative stress, and angiogenesis 33, must be initiated by an earlier event or signal. AVP is known to interact with essentially all of these processes 34,35. Therefore, we contend that AVP hypersecretion represents an initiating event / signal (Figure 4).
Figure 4. Working model.
Conceptualized time-course for the development of maximal plasma concentrations of copeptin (and thus vasopressin release), markers and mediators of immune function, vascular function, angiogenesis and clinical symptoms of preeclampsia. We posit that the hypersecretion of AVP in the first trimester is an initiator of other previously-identified mediators of preeclampsia, which are known to become more active later in pregnancy.
AVP induces expression of the vascular endothelial growth factor (VEGF) at nanomolar concentrations in human vascular smooth muscle cells 36 and human mesangial cells 37. As AVP and VEGF act to increase vascular resistance and may therefore contribute to reduced uterine blood flow in preeclampsia, one may hypothesize a reflexive and protective induction of sFLT-1, as sFLT-1 functions to reduce VEGF signaling 38. Excessive subsequent induction of sFLT-1 then contributes to the pathogenesis of preeclampsia through its anti-angiogenic effects.
Furthermore, AVP is known to be affected and is an effector of multiple immune cells. The Redman and Sargent model of the pathogenesis of preeclampsia holds that poor immunoregulation leads to immune rejection of the placenta. This immune rejection leads to downstream poor placentation and placental vascular dysfunction leading to the phenotype of preeclampsia 39. Natural (NK) cells are in direct contact with the placenta and are likely to play a critical role in the tolerance of the placenta as a significant source of IFNγ and TNFα 40,41. IFNγ and TNFα are elevated in preeclamptic women with a concurrent decrease in IL-10 and IL-4 production. This creates a milieu rich in cytokines that drive a T helper 1 T cell (TH1) response promoting a placental cytotoxic response leading to poor placentation. Production of AVP is stimulated by proinflammatory cytokines including: IL-6, IL-1β, IFNg, and TNF-a, which are affected by preeclampsia 35,42. Furthermore, Johnson et al. demonstrated AVP replaces the IL-2 help requirement for production of IFNg and that it acts upon an AVP receptor on lymphocytes 43,44. These processes are very important in the development of the immune phenotype of preeclampsia 39. Future studies to understand the complex interactions among AVP, immunologic dysregulation, angiogenesis, and vascular dysfunction are clearly needed to understand the early-pregnancy mechanisms that initiate preeclampsia. These mechanisms can identify additional molecular targets to treat – and more importantly, prevent – preeclampsia.
The current study has benefitted from high quality clinical data and biosamples from the University of Iowa Department of OB/Gyn Maternal Fetal Tissue Bank and Women’s Health Tissue Repository. Further, the study was appropriately powered to evaluate our desired outcomes. One weakness of our study is the predominantly Caucasian population of our sample. As aforementioned, the racial distribution in this cohort is consistent with the current population of the state of Iowa. Even though the relationship of copeptin and preeclampsia is robust after clinical covariate adjustment, we are not appropriately powered to analyze potential variance due to race. Numerous additional human and animal translational studies are also required to identify the sites and causes of AVP hypersecretion, to identify the tissue sites of action and receptors involved, and to isolate the critical timeframes for AVP hypersecretion to elicit various preeclampsia phenotypes. Such knowledge will identify the specific targets and timing at which interference with AVP signaling could prevent or treat preeclampsia. Future, large-scale clinical studies confirming the prediction of preeclampsia by copeptin and investigating the safety and effectiveness of interference with AVP signaling during pregnancy are required, as are studies to investigate further improvements in predictive power of copeptin in combination with other predictor of preeclampsia.
PERSPECTIVES
We posit that a very early-pregnancy role for AVP in preeclampsia supports the novel concept that the central nervous system, and more specifically the neurohypophysis, is critically involved in the pathogenesis of preeclampsia. This challenges and expands the current understanding of preeclampsia as a second-trimester disease of the vasculature and immune system. Measurement of AVP release in the first few weeks of pregnancy holds great promise as a novel diagnostic tool to predict the development of preeclampsia, and inhibition of AVP release or action may represent a novel and rational therapeutic approach to preventing and treating preeclampsia. Finally, these data identify AVP infusion as a new, simple, and clinically-relevant means of modeling preeclampsia in rodents.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
-
What Is New?
Maternal plasma copeptin predicts late-pregnancy onset of preeclampsia at least as early as the sixth week of pregnancy, regardless of clinical covariates.
Infusion of vasopressin during pregnancy is sufficient to induce the cardinal phenotypes of preeclampsia in mice.
-
What Is Relevant?
Current diagnostic biomarkers for preeclampsia are largely limited to the second trimester, shortly before the onset of clinical symptoms.
Early-pregnancy events that activate known late-pregnancy mediators of preeclampsia (e.g. - vascular, immune, renal) are essentially unknown.
-
Summary
Copeptin represents a novel diagnostic biomarker for the prediction of preeclampsia, and is predictive far earlier in pregnancy than all other known biomarkers.
Chronic vasopressin infusion represents a new, rational, clinically-relevant method to model preeclampsia in mice.
These findings are consistent with a role for the neurohypophysis in the very-early pregnancy pathogenesis of preeclampsia.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the technical support of the staff of the University of Iowa Maternal-Fetal Tissue Bank and Women’s Health Tissue Repository, the Santillan laboratory, the Grobe laboratory, and Amy Trent in the University Of Iowa Department Of Pathology for her expertise in electron microscopy. The authors also thank James N. Martin, MD, University of Mississippi, and Allyn L. Mark, MD, and Curt D. Sigmund, PhD, University of Iowa, for their critical reviews of the manuscript.
SOURCES OF FUNDING
This work was supported through grants from the NIH to MKS (HD000849, RR024980) and JLG (HL098276, HL084207), American Heart Association to JLG and MKS (14IRG18710013), American Diabetes Association to JLG (1-14-BS-079), Roy J. Carver Charitable Trust to MKS and JLG, University of Iowa Office of the Vice President for Research and Economic Development to JLG, Preeclampsia Foundation to MKS, Immunology Postdoctoral Training Grant to SMS (5T32AI007260-27), University of Iowa Medical Student Research Program to JYM, and the University of Iowa Department of Obstetrics and Gynecology.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Authors MKS and JLG currently hold a provisional patent addressing the use of copeptin and AVP measurements for the early pregnancy prediction of preeclampsia, and a second provisional patent addressing the inhibition of the AVP system for the treatment of preeclampsia. No direct financial or other conflicts of interest exist.
REFERENCES
- 1.Villar J, Say L, Gulmezoglu AM, Merialdi M, Lindheimer MD, Betran AP, Piaggio G. Eclampsia and preeclampsia: a worldwide health problem for 2000 years. In: H Critchley, A MacLean, L Poston, J Walker., editors. Preeclampsia. London (UK): RCOG Press; 2003. [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Garovic VD, Hayman SR. Hypertension in pregnancy: An emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 4.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 5.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114:961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 6.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The helsinki birth cohort study. Stroke. 2009;40:1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 7.Wu CS, Sun Y, Vestergaard M, Christensen J, Ness RB, Haggerty CL, Olsen J. Preeclampsia and risk for epilepsy in offspring. Pediatrics. 2008;122:1072–1078. doi: 10.1542/peds.2007-3666. [DOI] [PubMed] [Google Scholar]
- 8.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am J Obstet Gynecol. 2009;201:269 e261–269 e210. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 9.Bakris G, Bursztyn M, Gavras I, Bresnahan M, Gavras H. Role of vasopressin in essential hypertension: Racial differences. J Hypertens. 1997;15:545–550. doi: 10.1097/00004872-199715050-00011. [DOI] [PubMed] [Google Scholar]
- 10.de Paula RB, Plavnik FL, Rodrigues CI, Neves Fde A, Kohlmann O, Jr., Ribeiro AB, Gavras I, Gavras H. Contribution of vasopressin to orthostatic blood pressure maintenance in essential hypertension. Am J Hypertens. 1993;6:794–798. doi: 10.1093/ajh/6.9.794. [DOI] [PubMed] [Google Scholar]
- 11.Gavras H. Pressor systems in hypertension and congestive heart failure. Role of vasopressin. Hypertension. 1990;16:587–593. doi: 10.1161/01.hyp.16.5.587. [DOI] [PubMed] [Google Scholar]
- 12.Argent NB, Burrell LM, Goodship TH, Wilkinson R, Baylis PH. Osmoregulation of thirst and vasopressin release in severe chronic renal failure. Kidney international. 1991;39:295–300. doi: 10.1038/ki.1991.36. [DOI] [PubMed] [Google Scholar]
- 13.Burrell LM, Risvanis J, Johnston CI, Naitoh M, Balding LC. Vasopressin receptor antagonism--a therapeutic option in heart failure and hypertension. Experimental physiology. 2000;85(Spec No):259S–265S. doi: 10.1111/j.1469-445x.2000.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 14.Shah DM. The role of ras in the pathogenesis of preeclampsia. Current hypertension reports. 2006;8:144–152. doi: 10.1007/s11906-006-0011-1. [DOI] [PubMed] [Google Scholar]
- 15.Argent NB, Burrell LM, Goodship TH, Wilkinson R, Baylis PH. Osmoregulation of thirst and vasopressin release in severe chronic renal failure. Kidney international. 1991;39:295–300. doi: 10.1038/ki.1991.36. [DOI] [PubMed] [Google Scholar]
- 16.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. The journal of obstetrics and gynaecology research. 2011;37:1198–1202. doi: 10.1111/j.1447-0756.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 17.Foda AA, Abdel Aal IA. Maternal and neonatal copeptin levels at cesarean section and vaginal delivery. European journal of obstetrics, gynecology, and reproductive biology. 2012;165:215–218. doi: 10.1016/j.ejogrb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Bureau USC. Iowa state and county quickfacts. 2013 [Google Scholar]
- 19.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 20.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 21.Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von Dadelszen P, Mol BW, Pajkrt E, Collaboration EC. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: A systematic review and meta-analysis. BJOG. 2012;119:778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 22.Odibo AO, Rada CC, Cahill AG, Goetzinger KR, Tuuli MG, Odibo L, Macones GA, England SK. First-trimester serum soluble fms-like tyrosine kinase-1, free vascular endothelial growth factor, placental growth factor and uterine artery doppler in preeclampsia. J Perinatol. 2013;33:670–674. doi: 10.1038/jp.2013.33. [DOI] [PubMed] [Google Scholar]
- 23.McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407. doi: 10.1016/j.ajog.2012.08.010. e401–407. [DOI] [PubMed] [Google Scholar]
- 24.Siljee JE, Wortelboer EJ, Koster MP, Imholz S, Rodenburg W, Visser GH, de Vries A, Schielen PC, Pennings JL. Identification of interleukin-1 beta, but no other inflammatory proteins, as an early onset pre-eclampsia biomarker in first trimester serum by bead-based multiplexed immunoassays. Prenat Diagn. 2013:1–6. doi: 10.1002/pd.4219. [DOI] [PubMed] [Google Scholar]
- 25.D’Antonio F, Rijo C, Thilaganathan B, Akolekar R, Khalil A, Papageourgiou A, Bhide A. Association between first-trimester maternal serum pregnancy-associated plasma protein-a and obstetric complications. Prenat Diagn. 2013;33:839–847. doi: 10.1002/pd.4141. [DOI] [PubMed] [Google Scholar]
- 26.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: A multivariate approach. J Hum Hypertens. 2009 doi: 10.1038/jhh.2009.45. [DOI] [PubMed] [Google Scholar]
- 27.Poon LC, Karagiannis G, Leal A, Romero XC, Nicolaides KH. Hypertensive disorders in pregnancy: Screening by uterine artery doppler imaging and blood pressure at 11–13 weeks. Ultrasound Obstet Gynecol. 2009;34:497–502. doi: 10.1002/uog.7439. [DOI] [PubMed] [Google Scholar]
- 28.Papageorghiou AT, To MS, Yu CK, Nicolaides KH. Repeatability of measurement of uterine artery pulsatility index using transvaginal color doppler. Ultrasound Obstet Gynecol. 2001;18:456–459. doi: 10.1046/j.0960-7692.2001.00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Hodari AA. Chronic uterine ischemia and reversible experimental “toxemia of pregnancy”. American journal of obstetrics and gynecology. 1967;97:597–607. doi: 10.1016/0002-9378(67)90448-6. [DOI] [PubMed] [Google Scholar]
- 30.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sflt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. American journal of obstetrics and gynecology. 2007;196:396. doi: 10.1016/j.ajog.2006.12.024. e391–397; discussion 396.e397. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in bph/5 mouse model of preeclampsia. Hypertension. 2008;51:1058–1065. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- 32.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–342. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 33.Santillan MK, Santillan DA, Sigmund CD, Hunter SK. From molecules to medicine: A future cure for preeclampsia? Drug News Perspect. 2009;22:531–541. doi: 10.1358/dnp.2009.22.9.1435464. [DOI] [PubMed] [Google Scholar]
- 34.Russell JA, Walley KR. Vasopressin and its immune effects in septic shock. Journal of innate immunity. 2010;2:446–460. doi: 10.1159/000318531. [DOI] [PubMed] [Google Scholar]
- 35.Chikanza IC, Petrou P, Chrousos G. Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Annals of the New York Academy of Sciences. 2000;917:825–834. doi: 10.1111/j.1749-6632.2000.tb05448.x. [DOI] [PubMed] [Google Scholar]
- 36.Tahara A, Saito M, Tsukada J, Ishii N, Tomura Y, Wada K, Kusayama T, Yatsu T, Uchida W, Tanaka A. Vasopressin increases vascular endothelial growth factor secretion from human vascular smooth muscle cells. European journal of pharmacology. 1999;368:89–94. doi: 10.1016/s0014-2999(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 37.Tahara A, Tsukada J, Tomura Y, Yatsu T, Shibasaki M. Vasopressin induces human mesangial cell growth via induction of vascular endothelial growth factor secretion. Neuropeptides. 2011;45:105–111. doi: 10.1016/j.npep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 40.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 41.Wilczynski JR. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia - the same basic mechanism? Human immunology. 2006;67:492–511. doi: 10.1016/j.humimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Campos LA, Couto AS, Iliescu R, Santos JA, Santos RA, Ganten D, Campagnole-Santos MJ, Bader M, Baltatu O. Differential regulation of central vasopressin receptors in transgenic rats with low brain angiotensinogen. Regul Pept. 2004;119:177–182. doi: 10.1016/j.regpep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Johnson HM, Farrar WL, Torres BA. Vasopressin replacement of interleukin 2 requirement in gamma interferon production: Lymphokine activity of a neuroendocrine hormone. The Journal of Immunology. 1982;129:983–986. [PubMed] [Google Scholar]
- 44.Johnson HM, Torres BA. Regulation of lymphokine production by arginine vasopressin and oxytocin: Modulation of lymphocyte function by neurohypophyseal hormones. Journal of immunology. 1985;135:773s–775s. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.