Abstract
Most patients with essential hypertension do not exhibit substantial renal damage. Renal autoregulation, by preventing glomerular transmission of systemic pressures has been postulated to mediate this resistance. Conversely, malignant nephrosclerosis (MN) has been postulated to develop when severe hypertension exceeds a critical ceiling. If the concept is valid, even modest BP reductions to below this threshold regardless of antihypertensive class (i) should prevent MN and (ii) lead to the healing of the already developed MN lesions. Both predicates were tested using BP radiotelemetry in the stroke prone spontaneously hypertensive rats (SHRsp) receiving 1% NaCl as drinking fluid for 4 weeks. Severe hypertension (final 2 weeks average systolic BP, >200 mmHg) and MN (histologic damage score 36±5; n=27), developed in the untreated SHRsp but were prevented by all antihypertensive classes [enalapril (n=15), amlodipine (n=13) or a hydralazine/hydrochlorothiazide combination (n=15)], if the final 2 week systolic BP remained <190mmHg. More impressively, modest systolic BP reductions to 160–180mmHg (Hydralazine/Hydrochlorothiazide regimen) initiated at ∼4 wks in additional untreated rats after MN had already developed (injury score 35±4 in the right kidney removed before therapy) led to a striking resolution of the vascular and glomerular MN injury over 2–3 weeks (post therapy left kidney injury score 9±2, p<0.0001; n=27). Proteinuria also declined rapidly from 122±9.5 mg/24 hr before therapy to 20.5±3.6mg 1 week later. These data clearly demonstrate the barotrauma mediated pathogenesis of MN and the striking capacity for spontaneous and rapid repair of hypertensive kidney damage if new injury is prevented.
Keywords: hypertension, autoregulation, radiotelemetry, calcium channel blockers, renin angiotensin system
INTRODUCTION
Hypertension induced renal damage (HIRD) is second only to diabetic nephropathy as a primary cause of end-stage renal disease (ESRD).1 However, except for some genetically susceptible subgroups such as African-Americans2,3, the vast majority of patients with essential hypertension exhibit a very low individual risk of developing ESRD as is apparent when the relatively small prevalence of ESRD (<0.5%) is contrasted with the huge prevalence of hypertension in the general population.1,4–6 We and others have suggested that intact renal autoregulatory mechanisms in individuals with essential hypertension protect the glomerular capillaries from BP elevations within the autoregulatory range so that only the slowly progressive vascular pathology of benign nephrosclerosis with late and modest ischemic nephron loss is observed4–7. Severe renal damage in such individuals is usually only seen when they develop the syndrome of accelerated/malignant hypertension with severe systolic BP elevations (>200mmHg) presumably exceeding a critical threshold and resulting in a syndrome of malignant nephrosclerosis (MN) with acute disruptive vascular and glomerular injury, proteinuria, hematuria, and renal failure.4–12
If such a formulation as to the pathogenesis of the MN pathology is valid, even modest BP reductions to below such a threshold should be able to prevent its development. The fact that salt-supplemented SHRsp exhibit preserved renal autoregulation prior to the development of MN13, renders the SHRsp model particularly suitable for an examination of these concepts14–16. Additionally, such BP reductions to below the critical threshold for injury, even after MN lesions have already developed, should nevertheless, result in the repair/regression of such MN lesions despite continued hypertension. However, this has not been directly demonstrated. Moreover, only limited data exists as to the fate of the already developed MN lesions7,8 when BP is subsequently reduced but without complete normalization, as is often the case clinically8–12. The present studies were performed to address these aspects of MN in the SHRsp model using BP radiotelemetry.
METHODS
Detailed methods are provided in the On-Line Data Supplement
Two sets of studies were performed
Protocol A (Prevention of MN by modest BP reductions)
Saline drinking SHRsp rats were randomly allocated to one of the 4 groups; they were left untreated or received one of the following 3 antihypertensive regimens for the following ∼4 weeks: enalapril 50mg/L, amlodipine 50mg/L or a combination of hydralazine (100–200mg/L) and hydrochlorothiazide (25–50mg/L) in the drinking fluid (H&H).
Protocol B (Repair of MN lesions after modest BP reductions)
Similar to the untreated rats in Protocol A, male SHRsp were placed on a Japanese style diet and 1% NaCl for ∼3–4 weeks till they were noted to have a systolic BP >200mmHg and a significant increase in proteinuria. The rats were then anesthetized and the right kidney removed to quantitate the severity of existing renal damage. Following uninephrectomy (UNX), the rats were continued on the same diet but additionally received the combination H&H regimen in the drinking fluid so as to maintain a systolic BP of < ∼190mmHg. The rats were followed for 2 (n=11) or 3 (n=13) weeks after which they were sacrificed and the remaining kidney was harvested to assess the extent of histologic repair/regression. Five additional rats, which also underwent UNX at 3–4 wks but did not exhibit significant pretreatment histologic injury, were not included in the analysis.
RESULTS
Protocol A studies (prevention of MN by modest BP reductions)
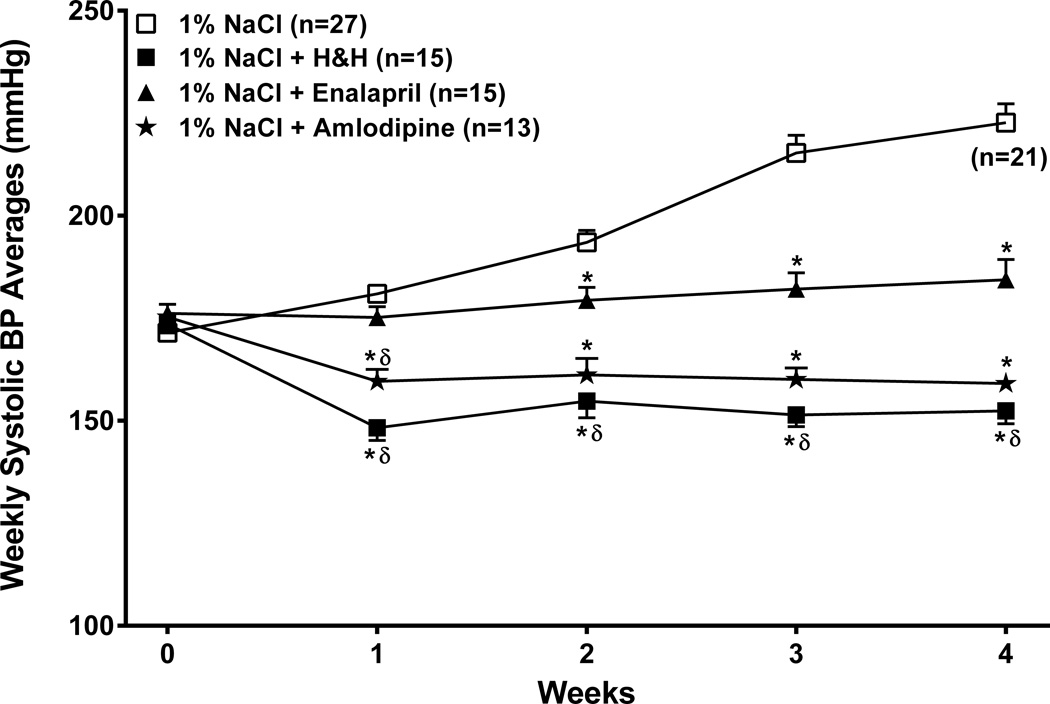
At baseline, there were no significant differences in body weight, 24 hour urinary protein excretion rates (<10mg/24hours in all groups) or average systolic BP between the groups (Table 1). However, the institution of the Japanese style diet and 1% NaCl as drinking fluid, rapidly led to progressive increases in systolic BP (Fig. 1). Co-administration of enalapril with the 1% NaCl as drinking fluid essentially abrogated such BP increases in all but 2/15 rats. By contrast, both the H&H combination and amlodipine were more effective and produced significant BP reductions from baseline that were maintained despite the continued salt-supplementation (Fig. 1). The final body weights and protein excretion rates prior to euthanasia after 4 weeks are also presented in Table 1. Body weight was significantly lower in the more severely hypertensive untreated SHRsp, although the amlodipine treated rats also tended to gain less weight for unclear reasons.
TABLE 1.
Baseline body weights, systolic BP, proteinuria and final body weights and proteinuria
| Baseline | Final at 4 weeks | ||||
|---|---|---|---|---|---|
| Group (n) | Body weight (g) |
Systolic BP mmHg |
Proteinuria mg/24h |
Body weight (g) |
Proteinuria mg/24h |
| 1% NaCl (27) | 244 ± 4.7 | 171.5 ± 1.8 | 7.3 ± 0.8 | 261 ± 6.7 | 115.5 ± 12.8 |
| 1% NaCl (15) + enalapril | 262 ± 5.4 | 176.2 ± 2.2 | 5.6 ± 0.4 | 301* ± 2.7 | 24.3* ± 3.1 |
| 1% NaCl (15) + H&H | 249 ± 4.6 | 173.6 ± 2.1 | 6.8 ± 0.7 | 295* ± 5.0 | 14.1* ± 0.9 |
| 1% NaCl (13) + amlodipine | 247 ± 6.1 | 175.3 ± 2.1 | 6.2 ± 0.6 | 282 ± 6.2 | 22.2* ± 2.4 |
Footnote: H&H – a combined antihypertensive regimen of hydralazine and hydrochlorothiazide
p < 0.05 vs. the untreated 1% NaCl only group. The final data at 4 weeks for the untreated 1% NaCl groups includes data obtained from 6 rats before the 4 weeks who were euthanized for humane reasons between the 3rd and 4th week.
Figure 1. Course of radiotelemetrically measured systolic BP.
Weekly averages of radiotelemetrically recorded systolic BP (mean ± SEM) at baseline (week 0) and over the subsequent 4 weeks in the 4 groups of SHRsp which received as drinking fluid (i) 1% NaCl (ii) 1% NaCl + enalapril (iii) 1% NaCl + a combination of hydralazine and hydrochlorothiazide (H/H) and (iv) 1% NaCl + amlodipine. The baseline BP was the average systolic BP during the last 3 days before the initiation of a Japanese style diet and salt-supplementation. Six of 27 untreated rats were euthanized for humane reasons between the 3rd and 4th week (see Methods for details). * p<0.05 maximum vs. the untreated 1% NaCl only group, δ p<0.05 maximum vs 1% NaCl + enalapril group.
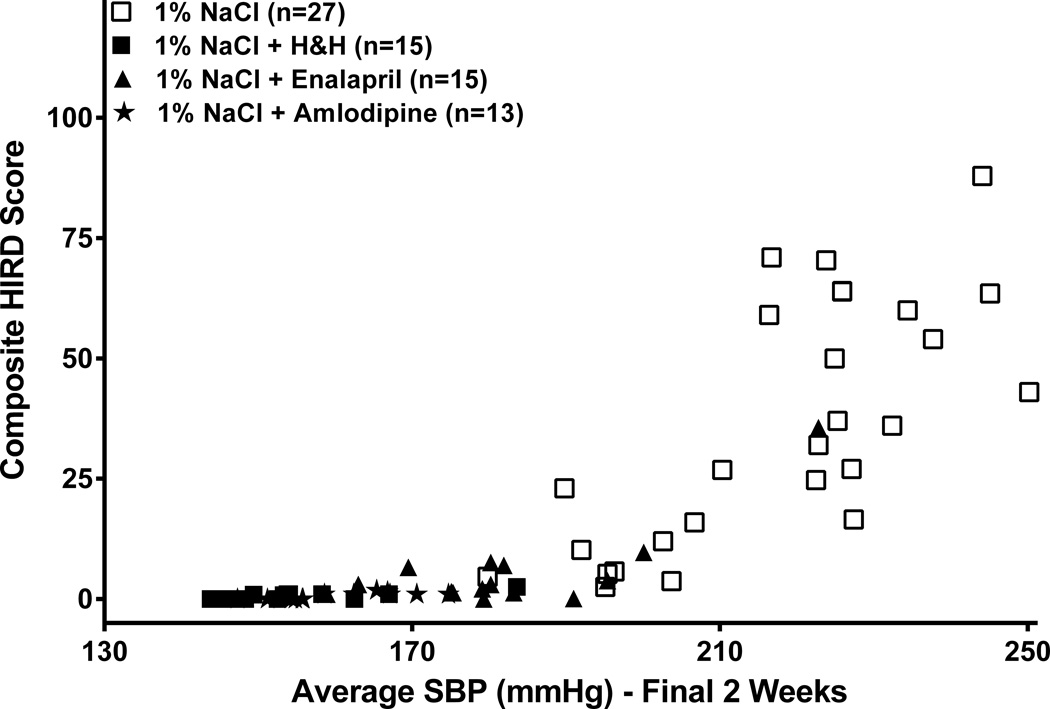
The increases in proteinuria in the 4 groups of rats after salt-supplementation followed the same pattern as the BP response to salt-supplementation, with severe increases only being seen in the severely hypertensive untreated SHRsp and the 2/15 enalapril-treated rats. Similarly, histologic renal damage was also essentially confined to these same more severely hypertensive untreated and 2/15 enalapril-treated rats (Table 2). Substantial vascular and glomerular injury was observed, with glomerular ischemia being more prominent than glomerular fibrinoid necrosis and/or thrombosis. Segmental glomerulosclerosis (GS) was observed infrequently and was sometimes difficult to separate from acute necrotic injury. Therefore, for purposes of this quantitation (Table 2), these two patterns of glomerular injury have been combined. Linear regression analysis was used to examine the quantitative relationship between BP and composite renal damage score in individual rats from all 4 groups. While strong correlations were observed between BP parameters and the HIRD scores, the threshold relationship between BP and renal damage was most clearly revealed when the composite HIRD score in individual rats was correlated with their average systolic BP during the final 2 weeks of the course (Fig. 2). As can be noted, the slope of the relationship (increase in HIRD score/mmHg increase in systolic BP during the final 2 weeks) is essentially flat for systolic BP <190 mmHg and increases sharply and linearly at systolic BP >190 mmHg.
TABLE 2.
Quantitation and distribution of histologic hypertensive injury
| Group (n) | Vascular Injury | % Glomerular Injury | Total HIRD Score | |
|---|---|---|---|---|
| Score | sclerosis/necrosis | Ischemia | ||
| 1% NaCl (27) | 15.5±2.2 | 4.4±0.8 | 11.9±2.3 | 35.9±4.9 |
| 1% NaCl (15) + Enalapril | 2.0±0.7* | 0.3±0.2* | 3.3±1.4* | 5.6±2.3* |
| 1% NaCl (15) + H&H | 0.1±0.1* | 0.1±0.1* | 0.1±0.1* | 0.6* |
| 1% NaCl (13) + Amlodipine | 0* | 0* | 0.2±0.2* | 0.6±0.2* |
Histologic renal damage was quantitated in a blinded fashion in perfusion fixed kidneys of untreated salt supplemented SHRsp and those which additionally received enalapril, a combination of hydralazine and hydrochlorothiazide (H&H) or amlodipine (see Methods for details).
p<0.05 maximum vs. the untreated 1% NaCl only group.
Figure 2. Correlation between systolic BP and renal damage.
Linear regression analysis of the correlation of histologic composite renal damage score in individual SHRsp with their respective average systolic BP during the final 2 weeks before study termination (n=70). For individual rats with average final 2 week systolic BP <190mmHg (n=41), slope = 0.17±0.04, r2 = 0.33, p<0.0001; for rats with average final 2 week systolic BP >190mmHg (n=29), slope = 1.2±0.2, r2 = 0.6, p<0.0001.
Protocol B studies (Repair of MN lesions after modest BP reductions)
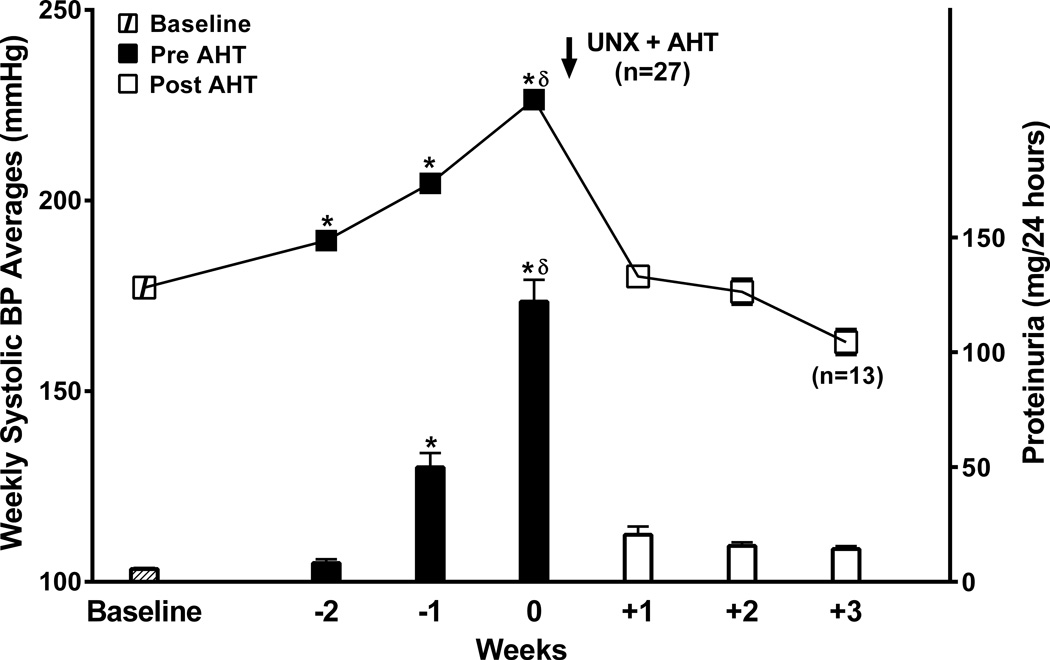
Fig. 3 presents the results of these studies. Due to the individual differences in the time to UNX (3–4.5 weeks), the group data for the weekly systolic BP averages and proteinuria are presented as at-baseline, for the 2–3 weeks before UNX, and for 2–3 weeks following the initiation antihypertensive therapy (AHT) with the H&H regimen. As can be noted, sharp increases in BP and proteinuria were observed during the final week before UNX. Acute reductions in systolic BP with AHT of ∼20–30 mmHg to below the BP threshold noted in Protocol A studies resulted in a rapid and dramatic decrease in proteinuria which was sustained throughout the follow-up period.
Figure 3.
Group BP data for all the SHRsp rats (n=27) that underwent Protocol B studies. Systolic BP (mmHg) and proteinuria (mg/24 hrs) are shown for baseline, the final 2 weeks before right uninephrectomy and initiation of antihypertensive therapy (AHT) and for 2 (n=14) or 3 weeks (n=13) after initiation of AHT. *p<0.001 vs. baseline; δ p<0.01 maximum vs. all other groups.
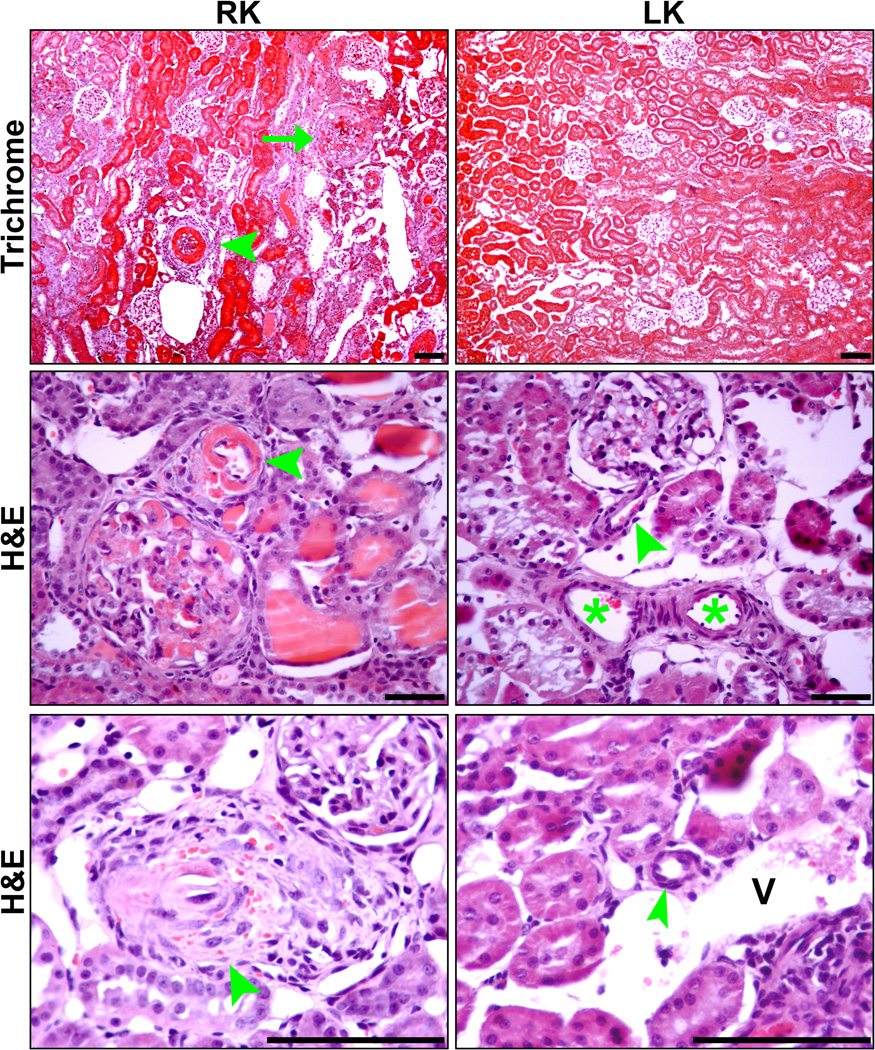
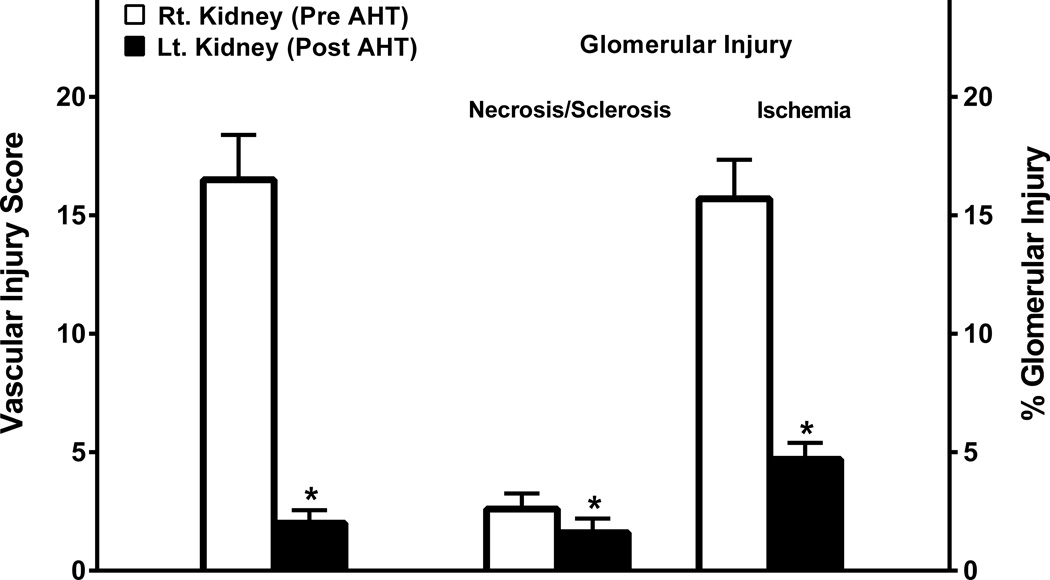
Figure 4 (a–d) provides a histologic illustration of the acute hypertensive vascular and glomerular injury in the right kidneys of salt-supplemented SHRsp rats which were removed before the initiation of the AHT and contrasts it with the much improved histology observed in the remaining left kidney at the termination of the studies after 2 to 3 weeks of H&H therapy. No significant differences were observed between the left kidneys of rats sacrificed after either 2 or 3 weeks of AHT. Accordingly, the results have been combined for the presentation of the quantitative data (Fig. 5). A striking resolution of vascular injury and glomerular ischemia was observed. A modest but significant decrease in the percentage of glomeruli exhibiting glomerular injury was also observed and the lesions at this stage appeared to be predominantly sclerotic rather than necrotic.
Figure 4.
Representative photomicrographs of right kidneys removed from rats on Japanese style diet and 1% salt drinking water prior to antihypertensive treatment (RK) and left kidneys remaining in rats on the same regimen following Hydralazine and Hydrochlorothiazide treatment (LK). Top panels (Masson Trichrome) show arteriolosclerosis (arrowhead), glomerular necrosis (arrow), interstitial infiltrates and fibrosis in RK but not in LK. Middle and bottom panels (hematoxylin and eosin) show arteriolar necrosis and glomerular necrosis in RK (middle panel), proliferative occlusive arteriolosclerosis with RBC in arteriolar wall in RK (bottom panel), and arterioles with nearly normal morphology in LK (middle and bottom panels). Arrowheads – arterioles. Asterisks – interlobular artery. V—Vein. Micron Bar: 100µm
Figure 5.
Quantitation and distribution of hypertensive vascular and glomerular injury lesions for the right kidney before antihypertensive therapy and the perfused-fixed left kidney harvested at termination of studies after 2–3 weeks of antihypertensive therapy with the H&H regimen.
* p<0.02 vs. pre AHT; maximum
DISCUSSION
The precise pathogenesis of the progressive renal damage in individuals with essential hypertension continues to be investigated and debated. While individuals with diabetic and non-diabetic chronic kidney disease (CKD) are generally acknowledged to exhibit an enhanced susceptibility to the adverse effects of even moderate hypertension4–7, there is considerable controversy as to whether the benign nephrosclerosis pathology of essential hypertension causes ESRD in the absence of an enhanced genetic predisposition such as has been identified in African-Americans2,3. By contrast, there is a general consensus that in the absence of adequate antihypertensive management, MN usually does progress to ESRD7–12. However, controversy persists as to whether in addition to the severity of hypertension (systolic BP exceeding ∼200mmHg), activation of the renin-angiotensin-aldosterone system (RAAS) and/or other downstream injury pathways is needed for the development of the MN pathology in both human and experimental hypertension7–12,14–25. The present studies do not directly examine the role of RAAS in the pathogenesis of MN. Rather, they are addressed to the relative importance of BP per se in the development of MN. In this context, several previous studies have reported that RAAS blockade is able to provide protection against MN with little or no BP lowering in the SHRsp model of MN7,9–12,21–23. However, these studies had almost exclusively relied on tail-cuff BP measurements that have been demonstrated to be inadequate for such interpretations26,27. When BP radiotelemetry was used in the SHRsp model, the protection by RAS blockade or aldosterone antagonists was found to strictly parallel the associated BP reductions, but comparisons with other antihypertensive agents were not performed17. The present studies extend these previous observations by demonstrating that protection against MN may depend on preventing the BP from reaching a critical threshold independent of the antihypertensive class, as three different antihypertensive regimens were equally effective in protecting against MN, by maintaining BP below the critical threshold and within the described autoregulatory range for SHRsp rats (mean arterial pressure between 100 and ∼175 mmHg)13.
Such interpretations and the concept of a critical BP threshold for MN injury are further buttressed by the striking demonstration that BP reductions of 20–30 mmHg to below such a threshold result in a dramatic resolution of the already developed MN lesions. Thus, the maintenance of a BP below the critical threshold may be both necessary and sufficient to not only prevent MN but also for the regression of the already developed MN lesions, although the precise mechanisms/pathways mediating such repair remain to be defined. In this context, it is of note that these data are in sharp contrast to previous studies that have found BP reductions with hydralazine based regimens per se to be ineffective in achieving repair/regression of already developed renal pathology in the NG-nitro-L-arginine methylester (L-NAME) model of MN as well as in the 5/6 renal ablation model28–30. In general, these studies have concluded that producing regression of such renal lesions requires supramaximal doses of RAS blockers, aldosterone antagonists, and/or endothelin receptor blockade and depends less on BP reductions but more on the modulation of specific cellular/molecular pathways that include plasminogen activation inhibitor (PAI-1), matrix metalloproteinases (MMPs), growth factor signaling, etc 28–33. By contrast, the present data illustrate the considerable capacity for spontaneous repair of hypertensive renal injury if new hypertensive injury is prevented. While some of these differences in results, may represent differences in the mechanisms and/or sites of renal damage and/or repair in these models (vascular and arteriolar vs. glomerular), they may also partly reflect the limitations of the tail-cuff BP measurements used in these previous studies for investigating the BP-dependence of repair/regression. It is also worth emphasizing that the acuity and magnitude of the reduction in proteinuria within a week of the initiation of modest BP reductions suggests a functional rather than a structural repair basis for at least the initial response. Given the previously demonstrated relationships between acute changes in glomerular pressure (PGC) and proteinuria34–35, it is possible that the initial reduction in proteinuria results from the restoration of the normal autoregulatory responses (and PGC) when BP is reduced into the autoregulatory range. In any event, the present data are consistent with past clinical data reporting recovery from dialysis requiring renal failure in patients with MN using non-specific antihypertensives before RAS blockade was clinically available8,36,37.
The success of modest BP reductions to 160–180 mmHg in preventing MN is in sharp contrast to the apparent need for BP reductions into the normotensive range (systolic BP < 140mmHg) to prevent progressive GS in CKD (reduced renal mass) models. The substantially different BP thresholds above which significant renal damage starts to develop in MN vs. CKD models likely reflect differences in autoregulatory capacity and which probably also account for the differences in the histologic pattern/phenotype of renal damage that is observed between MN and CKD models5,38–41. In MN, a breach of the normal autoregulatory ceiling by the severe hypertension (systolic BP exceeding ∼200 mmHg) results in an acute exposure of the downstream resistance vessels and microvasculature to very high intravascular pressures and barotrauma. Accordingly, MN is characterized by evidence of acute disruptive injury to the intrarenal vasculature with distal glomerular ischemia and less frequently, active capillary injury as observed in the present study5,7–9,15–17. Lesions of segmental GS are uncommon in MN. By contrast, segmental GS is the predominant lesion in CKD models and acute vascular injury is usually not observed5–7,38,39. It is likely that the more moderate hypertension in CKD states is insufficient to cause acute disruptive vascular injury, but nevertheless exposes the glomerular capillaries to chronically increased local pressures due to the preglomerular vasodilation and impaired autoregulation, resulting in GS. These data also suggest that the threshold for hypertensive injury may differ between vascular segments (arteries and arterioles vs. glomerular capillaries). Such intrinsic differences in the ability to withstand barotrauma may also be relevant to the issue of repair/regression of the hypertensive lesions after the initiation of antihypertensive therapy. Modest BP reductions of 20–30 mmHg to below the critical threshold are expected to both promote vascular healing and also allow restoration of the autoregulatory mechanisms to protect glomerular capillaries from further barotrauma, even though it is likely that the capacity for complete repair/regression may be more limited in glomerular capillaries.
These differences in the pathogenesis and anatomic distribution of the hypertensive injury in malignant nephrosclerosis and CKD states may also be relevant to the differential effects of CCBs in these states/models. As in the present study, CCBs have also been noted to be protective in other MN models such as the SHR given L-NAME and the DOCA + salt model.42,43 Such data support the interpretation that vascular injury is primarily dependent on the increased vascular pressures and therefore is prevented and/or ameliorated by CCB mediated BP reductions below the threshold for vascular injury. By contrast, CCBs may be less effective in protecting the glomerular capillaries from hypertensive injury. For instance, while amlodipine was successful in ameliorating the vascular injury in the DOCA + salt models, GS was not prevented.43 Similarly, despite their antihypertensive effectiveness in CKD models, CCBs do not consistently reduce proteinuria and/or GS in CKD models.4,5,44 We have postulated that this failure is due to the concurrent deleterious effects of CCBs on renal autoregulation.4,5,44,45 Even though the systemic BP is reduced, a greater fraction of the BP is transmitted distally to the glomerular capillaries and glomeruloprotection proportionate to the achieved BP reductions is not obtained. Consistent with such a postulate, using BP radiotelemetry, we have shown that the slope of the relationship between BP and GS is made steeper by CCB therapy in the 5/6 renal ablation model such that greater GS is observed at any given BP elevation in CCB treated as compared to untreated rats with remnant kidneys.4,5,44 These interpretations may also help explain the parallel clinical data showing the effectiveness and general equivalence of CCBs and other antihypertensive agents including RAAS blockade, in preventing vascular events such as stroke and/or myocardial infarction.46 By contrast, CCBs, particularly dihydropyridine (DHP) CCBs, have been noted to be less effective than RAAS blockade in slowing progression in proteinuric CKD states where the glomerular capillaries are the primary site of hypertensive injury.5,47
PERSPECTIVES
The results of the present studies provide a potential explanation for the substantially greater success that has been achieved clinically with antihypertensive therapy in preventing MN as compared to slowing the progression of CKD states. They additionally demonstrate the very considerable capacity for vascular repair/healing after acute MN injury, even with relatively moderate systolic BP reductions to 160–180 mmHg. These data thus suggest that the magnitude of protection provided by antihypertensive agents may depend not only on the magnitude of the BP reduction, but also on the prevailing threshold and slope relationships between BP and renal damage in a given model, disease state or individual. Conversely, for the same reasons, the contribution of any given BP increase to the observed renal damage may also differ between individuals. In this context, it also needs to be emphasized that while malignant nephrosclerosis can be prevented such BP reductions of 20–30 mmHg to below the critical threshold, the long-term risk for benign nephrosclerosis and more importantly, for other target organ damage continues with such suboptimally controlled hypertension. And it is of note that the risk of adverse cardiovascular events significantly exceeds that for progression to ESRD even in patients with pre-existent renal disease48 emphasizing the importance of adequate BP control and the lack of clinically meaningful differences between antihypertensive agents for the prevention of such macrovascular events in most patients with essential hypertension.46
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
These data demonstrate that a striking and rapid regression of acute hypertensive vascular and glomerular injury may be achievable with only modest BP reductions.
These salutary effects, independent of antihypertensive class, indicate a barotrauma mediated pathogenesis of such acute hypertensive renal injury.
What is Relevant?
The variable effectiveness of antihypertensive agents in mitigating hypertensive renal damage may depend on the threshold and slope relationships between BP and renal damage that may exist in individual hypertensive states.
Summary.
Modest BP reductions independent of antihypertensive class to below a critical threshold are not only sufficient to prevent the development of malignant nephrosclerosis, but even to result in a striking and rapid resolution of already developed acute hypertensive injury despite substantial continued hypertension.
ACKNOWLEDGEMENTS
The authors wish to generally acknowledge Theresa Herbst and Rizalita Redovan for technical support and Martha Prado for secretarial support.
SOURCE OF FUNDING
This work was supported by National Institutes of Health grants DK-40426 (Bidani), DK-61653 (Griffin), a Career Development Award 1K2BX001285 (Polichnowski) and a Merit Review Award (Griffin) from the Office of Research and Development of the Department of Veterans Affairs.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
None
REFERENCES
- 1.US Renal Data System. USRDS 2013 Annual Data Report. National Kidney Foundation, Inc: Published by Elsevier Inc; 2013. [Google Scholar]
- 2.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African-Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants race and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension. 2004;44:1–7. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 5.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- 7.Olson JL. Renal disease caused by hypertension. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s pathology of the kidney. 6th Ed. Vol II Philadelphia: Lippincott Williams & Wilkins; 937–990. [Google Scholar]
- 8.McCormack LJ, Beland JE, Schneckloth RE, Corcoran AC. Effects of antihypertensive treatment on the evolution of the renal lesions in malignant nephrosclerosis. Am J Pathol. 1958;34:1011–1021. [PMC free article] [PubMed] [Google Scholar]
- 9.Kincaid-Smith P. Malignant hypertension. J Hypertens. 1991;9:893–899. [PubMed] [Google Scholar]
- 10.Lip GYH, Beevers M, Beevers DG. Complications and survival of 315 patients with malignant-phase hypertension. J Hypertens. 1995;13:915–924. doi: 10.1097/00004872-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Van den Born BJ, Honnebier UP, Koopmans RP, van Montfrans GA. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension. 2005;45:246–251. doi: 10.1161/01.HYP.0000151620.17905.ee. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez R, Morales E, Segura J, Ruilope LM, Praga M. Long-term renal survival in malignant hypertension. Nephrol Dial Transplant. 2010;25:3266–3272. doi: 10.1093/ndt/gfq143. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol. 2005;289:F305–F313. doi: 10.1152/ajprenal.00349.2004. [DOI] [PubMed] [Google Scholar]
- 14.Smeda JS. Hemorrhagic stroke development in spontaneously hypertensive rats fed a North-American, Japanese-style diet. Stroke. 1989;20:1212–1218. doi: 10.1161/01.str.20.9.1212. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Schmidlin O, Olsen IL, Yi SL, Morris RC. Chloride-sensitive renal microangiopathy in the stroke-prone spontaneously hypertensive rat. Kidney Int. 2001;59:1066–1076. doi: 10.1046/j.1523-1755.2001.0590031066.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens. 2001;14:311–320. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- 17.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure dependent. Hypertension. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 18.Giese J. The Pathogenesis of Hypertensive Vascular Disease. Copenhagen, Denmark: Munksgaard; 1966. [Google Scholar]
- 19.Byrom FB. The Hypertensive Vascular Crisis: An Experimental Study. Heinemann Monograph. London, England: Pitman Press; 1969. [Google Scholar]
- 20.Belin LJ, Goldby FS, Mohring J. High arterial pressure versus humoral factors in the pathogenesis of the vascular lesions of malignant hypertension The case of pressure alone. Clin Sci Mol Med. 1977;52:111–117. doi: 10.1042/cs0520111. [DOI] [PubMed] [Google Scholar]
- 21.Stier CT, Jr, Benter IF, Ahmad S, Zuo HL, Selig N, Roethel S, Levine S, Itskovitz HD. Enalapril prevents stroke and kidney dysfunction in salt-loaded stroke-prone spontaneously hypertensive rats. Hypertension. 1989;13:115–121. doi: 10.1161/01.hyp.13.2.115. [DOI] [PubMed] [Google Scholar]
- 22.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT., Jr Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension. 1998;31:451–458. doi: 10.1161/01.hyp.31.1.451. [DOI] [PubMed] [Google Scholar]
- 23.Fleming S. Malignant hypertension – the role of the paracrine renin-angiotensin system. J Pathol. 2000;192:135–139. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH674>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Zatz R, Baylis C. Chronic nitric oxide inhibition model six years ago. Hypertension. 1998;32:958–964. doi: 10.1161/01.hyp.32.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, Bidani A. Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol Renal Physiol. 2012;302:F173–F182. doi: 10.1152/ajprenal.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz T. False claims of blood pressure-independent protection by blockade of the renin angiotensin aldosterone system? Hypertension. 2003;41:193–196. doi: 10.1161/01.hyp.0000049882.23078.eb. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. AHA Scientific Statement. Recommendation for blood pressure measurements in humans and animals Part 2: Blood pressure measurements in experimental animals. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- 28.Boffa J-J, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–1144. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- 29.Piecha G, Koleganova N, Gross ML, Geldyyev A, Adamczak R, Ritz E. Regression of glomerulosclerosis in subtotally nephrectomized rats: effects of monotherapy with losartan, spironolactone, and their combination. Am J Physiol. 2008;295:F137–F144. doi: 10.1152/ajprenal.00065.2008. [DOI] [PubMed] [Google Scholar]
- 30.Kavvadas P, Weis L, Abed AB, Feldman DL, Dussaule JC, Chatziantoniou C. Renin inhibition reverses renal disease in transgenic mice by shifting the balance between profibrotic and antifibrotic agents. Hypertenions. 2013;61:901–907. doi: 10.1161/HYPERTENSIONAHA.111.00639. [DOI] [PubMed] [Google Scholar]
- 31.Boffa J-J, Tharaux P-L, Dussaule J-C, Chatziantoniou C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension. 2001;37([part 2]):490–496. doi: 10.1161/01.hyp.37.2.490. [DOI] [PubMed] [Google Scholar]
- 32.Adamczak M, Gross M-L, Krtil J, Koch A, tyralla K, Amann K, Ritz E. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol. 2003;14:2833–2842. doi: 10.1097/01.asn.0000095248.91994.d3. [DOI] [PubMed] [Google Scholar]
- 33.Ma LJ, Nakamura S, Aldigier JC, Rossini M, Yang H, Liang X, Nakamura I, Marcantoni C, Fogo AB. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol. 2005;16:966–976. doi: 10.1681/ASN.2004060492. [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka T, Rennke HG, Salant DJ, Deen WM, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res. 1987;61:531–538. doi: 10.1161/01.res.61.4.531. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka T, Shiraga H, Yoshida Y, Fogo A, Glick AD, Deen WM, Hoyer JR, Ichikawa I. “Intact Nephrons” as the primary origin of proteinuria in chronic renal disease Study in the rat model of subtotal nephrectomy. J Clin Invest. 1988;82:1614–1623. doi: 10.1172/JCI113773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods JW, Blythe WB, Huffines WD. Management of malignant hypertension complicated by renal insufficiency. N Engl J Med. 1974;291:10–14. doi: 10.1056/NEJM197407042910103. [DOI] [PubMed] [Google Scholar]
- 37.Mamdani BH, Lim VS, Mahurkar SD, Kats AI, Dunea G. Recovery from prolonged renal failure in patients with accelerated hypertension. N Engl J Med. 1974;291:1343–1344. doi: 10.1056/NEJM197412192912509. [DOI] [PubMed] [Google Scholar]
- 38.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric BP monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 39.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int. 1994;46:1010–1018. doi: 10.1038/ki.1994.361. [DOI] [PubMed] [Google Scholar]
- 40.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:1003–1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 41.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. ‘Step’ vs ‘Dynamic’ autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Ono H, Frohlich ED. Differential effects of T- and L-type calcium antagonists on glomerular dynamics in spontaneously hypertensive rats. Hypertens. 1999;34:273–278. doi: 10.1161/01.hyp.34.2.273. [DOI] [PubMed] [Google Scholar]
- 43.Karam H, Clozel JP, Brumeval P, Gonzalez MF, Menard J. Contrasting effects of selective T- and L-type calcium channel blockade on glomerular damage in DOCA hypertensive rats. Hypertension. 1999;34:673–678. doi: 10.1161/01.hyp.34.4.673. [DOI] [PubMed] [Google Scholar]
- 44.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol. 2004;286:F1136–F1143. doi: 10.1152/ajprenal.00401.2003. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomized trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 47.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure and antihypertensive drug class on progression of hypertensive kidney disease. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 48.Foley RN, Murray AM, Li Sh, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medical population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.