Abstract
Endothelial-progenitor-cells participate in renal repair, but their number and function may be impaired by exposure to cardiovascular risk factors. The number of circulating endothelial-progenitor-cells is decreased in essential and renovascular hypertensive patients, but the effects of hypertension on endothelial-progenitor-cell function are incompletely understood. We hypothesized that endothelial-progenitor-cell function was preserved under well-controlled conditions in treated hypertensive patients. Patients with atherosclerotic-renal-artery-stenosis (n=22) or essential-hypertension (n=24) were studied during controlled sodium intake and anti-hypertensive regimen. Late-outgrowth-endothelial-progenitor-cells were isolated from the inferior vena cava and renal vein blood of atherosclerotic-renal-artery-stenosis and essential-hypertension patients, and a peripheral vein of matched normotensive controls (n=18). The angiogenic function of endothelial-progenitor-cells was assessed in vitro and multi-detector computer tomography used to measure single-kidney hemodynamics and function in atherosclerotic-renal-artery-stenosis and essential-hypertension patients. Inflammatory biomarkers and endothelial-progenitor-cell homing signals levels and renal release were calculated. Inferior vena cava and renal vein-obtained endothelial-progenitor-cell function were similar in atherosclerotic-renal-artery-stenosis and essential-hypertension patients, and comparable to that in normal controls (tube length 171.86±16.846, 191.09±14.222, 174.925±19.774μm, respectively). Function of renal vein-obtained endothelial-progenitor-cells directly correlated with stenotic-kidney glomerular filtration rate, endothelial-progenitor-cell homing factors and anti-inflammatory mediator levels in atherosclerotic-renal-artery-stenosis patients. Therefore, endothelial-progenitor-cell function was relatively preserved in atherosclerotic-renal-artery-stenosis patients, although it directly correlated with renal function. Adequate endothelial-progenitor-cell function supports the feasibility of using autologous endothelial-progenitor-cells as a therapeutic option in essential and renovascular hypertensive patients. Homing signals and inflammatory mediators may potentially regulate endothelial-progenitor-cell angiogenic function.
Keywords: Renovascular hypertension, essential hypertension, endothelial progenitor cells, renal hemodynamics, inflammatory markers
Introduction
Atherosclerotic renal artery stenosis (ARAS) is the major cause for secondary hypertension, which is characterized by reduced renal perfusion. Population studies revealed that ARAS constitutes an important risk factor for cardiovascular morbidity and mortality compared with essential hypertension (EH)1. We have also previously shown that ARAS exacerbates renal inflammation, oxidative stress, and renal release of cytokines in both experimental models2 and patients3.
A large body of evidence has shown that circulating bone-marrow derived endothelial progenitor cells (EPCs)-contribute to endothelial repair in ischemic tissues4. These cells have been described to include two subpopulations, based on the stage at which they appear in culture. Early-outgrowth-endothelial-cells (EOEC) appear after 4–7 days, and show monocytic features, and late-outgrowth-endothelial-cells (LOEC) appear after 14–21 days in culture, possess salient angiogenic capabilities, and exhibit many features resembling bone marrow-derived circulating EPC5. Furthermore, a combination of autologous EOEC and LOEC expanded in vitro has been used to replenish injured kidneys and improve their function in chronic experimental ARAS 6–8.
However, EPCs function may be impaired by exposure to cardiovascular risk factors like hypertension or diabetes9, 10, partly due to decreased nitric oxide availability or activation of the renal-angiotensin-aldosterone system (RAAS). Activation of angiotensin-II (Ang-II) in ARAS is involved in atherogenesis, inflammation, oxidative stress and endothelial damage11, and is particularly prominent within the kidney. Furthermore, we have previously shown that the number of systemic circulating CD34+/KDR+ EPC is decreased in both ARAS and EH patients compared to healthy volunteers (HV), yet is further decreased in the renal rein (RV) effluent of the post-stenotic ARAS kidneys (STK) compared to EH kidneys. Pertinently, we have demonstrated that inflammatory cytokines released from the human STK recruited and sequestered circulating EPCs to participate in the reparative process3. Nevertheless, EPC crossing the circulation of the STK might be functionally damaged because of the noxious microenvironment. Importantly, impaired LOEC function would imply impaired endogenous renal regeneration capacity in ARAS patients, and argue against use of LOEC to replenish the diminished population of EPCs available for renal repair.
However, it remains unknown whether LOEC function in ARAS patients is adequate and affords reparative activity. The current study was designed to test the hypothesis that angiogenic function of LOEC expanded from the systemic circulation and STK of ARAS patients is preserved compared to that in EH and HV.
Methods
This study was approved by the Institutional Review Board of the Mayo Clinic. ARAS (n=22) or EH (n=24) patients were prospectively enrolled, and HV (n=18) without cardiovascular risk factors prospectively recruited through the Mayo Clinic Biobank.
In all EH or ARAS patients, the antihypertensive regimen included RAAS blockade. During a 3-day inpatient protocol, STK renal blood flow (RBF) and glomerular filtration rate (GFR) were measured using multi-detector CT (MDCT). Blood samples were collected from the inferior vena cava (IVC) and renal vein (RV) of ARAS and EH patients and from peripheral vein of HV for measurements of inflammatory markers levels, EPC isolation, and subsequent evaluation of their proliferation, migration, and tube formation function in-vitro. For detailed methodologies please see the online Data Supplement.
Results
Patients Characteristics
The characteristics of the study subjects are summarized in Table 1. Systolic blood pressure was higher in EH and ARAS patients compared with HV. Antihypertensive medications were similar between EH and ARAS.
Table 1.
Characteristics of healthy volunteers, essential hypertensive and atherosclerotic renal artery stenosis patients
| Characteristics | HV(n=18) | EH(n=24) | ARAS(n=22) | P value |
|---|---|---|---|---|
| Age (years) | 69.1±1.8 | 69.8±1.5 | 70.2±1.5 | 0.88 |
| Gender (male/female) | 11/7 | 15/9 | 12/10 | 0.85† |
| BMI (kg/m2) | 26.3±1.0 | 27.0±0.9 | 27.8±0.9 | 0.54 |
| Degree of stenosis (%) | 0.0±0.0 | 0.0±0.0 | 74.2±0.7 | <0.0001 |
| Systolic pressure (mmHg) | 120.4±2.8 | 132.5±2.4* | 137.8±2.6* | <0.0001 |
| Diastolic pressure (mmHg) | 70.8±1.9 | 69.3±2.3 | 69.9±2.8 | 0.57 |
| MAP (mmHg) | 87.7±1.7 | 90.3±1.9 | 90.9±2.1 | 0.50 |
| Total cholesterol (mg/dl) | 177.7±6.7 | 174.8±5.8 | 170.8±6.0 | 0.76 |
| Triglycerides (mg/dl) | 117.1±17.5 | 123.8±11.4 | 133.7±13.3 | 0.26 |
| HDL (mg/dl) | 57.0±3.6 | 53.1±2.7 | 55.1±4.4 | 0.42 |
| LDL (mg/dl) | 90.3±4.6 | 96.8±4.4 | 88.9±4.7 | 0.43 |
| Serum creatinine (mg/dl) | 0.93±0.04 | 1.03±0.06 | 1.24±0.07* | 0.005 |
| Proteinuria (mg/24h) | 86.9±7.4 | 90.5±13.2 | 76.6±11.0 | 0.24 |
| Microalbuminuria (mg/24h) | 0/0 | 33.0±5.9 | 29.1±8.1 | 0.68 |
| Systemic PRA (ng/ml/h) | 0.51±0.1 | 6.4±1.4* | 8.9±1.8* | 0.0002 |
| Calcium-channel blocker | 0/0 | 9/37.5 | 8/36.4 | 0.84† |
| ACE-I | 0/0 | 16/66.7 | 12/54.5 | 0.40† |
| Angiotensin receptor blockers | 0/0 | 12/50.0 | 15/68.2 | 0.21† |
| Alpha-blocker | 0/0 | 2/8.3 | 1/4.5 | 0.60† |
| Statins | 0/0 | 13/54.2 | 14/63.6 | 0.51† |
Data are mean±SEM or (n, %). HV: healthy volunteers; EH: essential hypertension; ARAS: atherosclerotic renal artery stenosis patients; BMI: body-mass index; MAP: mean arterial pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein, PRA: plasma renin activity; ACE-I: angiotensin-converting enzyme inhibitor.
p<0.05 vs HV.
fisher’s exact test, between EH and ARAS.
Kidney function
Serum creatinine levels were elevated in ARAS compared to HV (Table 1), but urine protein excretion was unaltered. Systemic PRA levels were elevated in both ARAS and EH compared with HV.
Single-kidney function in the three groups is presented in Table S1. Of the ARAS patients, 10/22 were found to have bilateral disease, in which case both kidneys were considered as STK. Single-kidney cortical perfusion was decreased in STK compared with both EH and the non-stenotic, contralateral kidneys (CLK), and in the CLK compared with EH. Medullary perfusion was decreased in the STK compared to EH, while STK-RBF and GFR were both reduced compared to EH and CLK. RV PRA was elevated in the STK compared with EH.
LOEC function in vitro
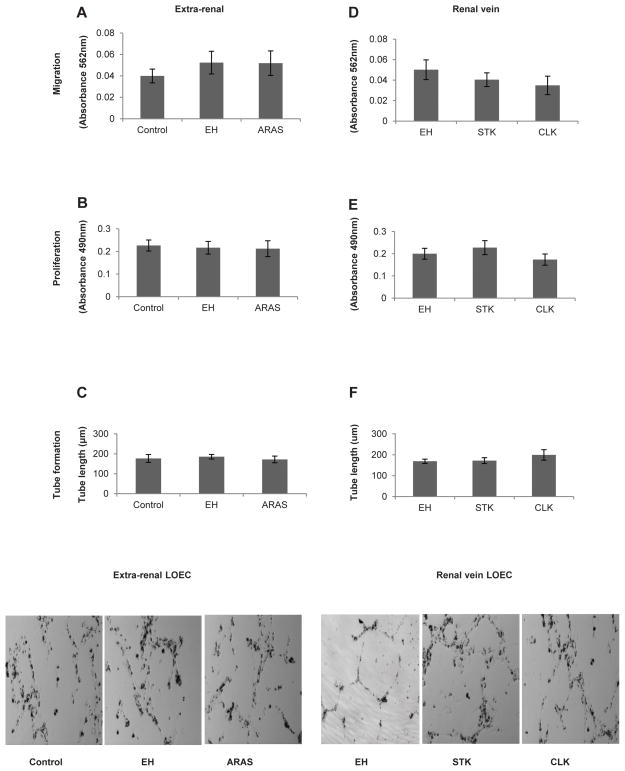
Cultured LOEC had a cobblestone appearance (indicative of endothelial-like phenotype) and expressed CD34 and KDR (Figure S1 A–B). Migration activity, proliferation, and tube formation of LOEC obtained from either collection site was all similar among the three groups (Figure 1A–F).
Figure 1.
Extra-renal (peripheral or inferior vena cava) and renal vein levels of late outgrowth endothelial cell (LOEC) migration, proliferation and tube formation capacity in healthy controls, essential hypertensive (EH) patients, or the stenotic kidneys (STK) and contralateral kidneys (CLK) of patients with atherosclerotic renal artery stenosis (ARAS). LOEC function was similar among the groups regardless of whether they were expanded from the extra-renal (A–C) or renal (D–F) veins. Bottom panel: Representative tube formation images.
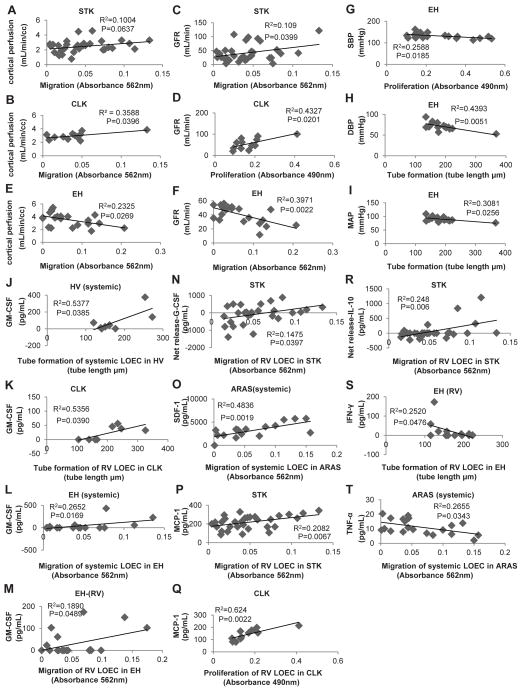
The STK and CLK-RV-LOEC migration and proliferation, respectively, directly correlated with the corresponding single-kidney GFR in ARAS, and their migration with CLK cortical perfusion. The STK-RV-LOEC migration tended to correlate with STK cortical perfusion (Figure 2A–D). The presence of bilateral disease did not significantly affect these relationships (Figure S2).
Figure 2.
Correlation of late-outgrowth-endothelial-cell (LOEC) function with renal hemodynamics, glomerular filtration rete (GFR), inflammatory markers or homing signals in healthy volunteers (HV), atherosclerotic-renal-artery-stenosis (ARAS) and essential hypertensive (EH) patients. (A–D) In ARAS, renal vein (RV)-LOEC migration and proliferation directly correlated with single-kidney GFR in stenotic (STK) and contralateral (CLK) kidneys, and migration with CLK cortical perfusion. The STK-RV- LOEC migration tended to correlate with STK cortical perfusion. (E–F) Conversely, EH-RV-LOEC migration inversely correlated with single-kidney cortical perfusion and GFR. (G–I) The EH-RV-LOEC proliferation correlated inversely with systolic blood pressure (SBP), and tube formation with diastolic blood pressure (DBP) and mean arterial pressure (MAP). (J–K) Tube formation of CLK-RV and HV systemic LOEC directly correlated with the level of granulocyte-macrophage colony-stimulating factor (GM-CSF). (L–M) Similarly, both systemic and RV-LOEC migration respective directly correlated with the level of GM-CSF in EH patients. (N–R) In ARAS, the STK-RV-LOEC migration directly correlated with the renal release of granulocyte CSF (G-CSF) and interleukin (IL)-10. Also, the systemic-LOEC migration directly correlated with the of stromal-derived factor (SDF)-1 level, and RV-LOEC migration with monocyte chemoattractant protein (MCP)-1. (R–S) IVC-LOEC migration inversely correlated tumor necrosis factor (TNF)-α level in ARAS and tube formation of EH RV-LOEC with interferon (IFN)-γ.
Conversely, the EH-RV-LOEC migration correlated inversely with the corresponding cortical perfusion and GFR (Figure 2E–F). In addition, EH-RV-LOEC proliferation inversely correlated with systolic blood pressure, and tube formation with diastolic and mean arterial pressure (Figure 2G–I).
We have also observed significant correlations between LOEC functions and homing and inflammatory mediator levels in the corresponding sites. The HV and CLK-RV-LOEC tube formation directly correlated with granulocyte-macrophage colony-stimulating factor (GM-CSF) (Figure 2J–K). Similarly, both systemic and RV-LOEC migration directly correlated with IVC and RV level of GM-CSF in EH, respectively (Figure 2L–M), and the STK-RV-LOEC migration directly correlated with the renal release of granulocyte-CSF (G-CSF) and interlukin (IL)-10 (Figure 2N, R). The ARAS-IVC-LOEC migration also directly correlated with stromal-derived factor (SDF)-1, and that of STK and CLK-RV with monocyte chemoattractant protein (MCP)-1 (Figure 2O–Q). Moreover, the ARAS-systemic-LOEC migration inversely correlated with tumor necrosis-factor (TNF)-α and of EH-RV-LOEC tube formation with interferon (IFN)-γ (Figure 2S–T). Indeed, both the SDF-1 (CXCR4) and MCP-1 (CCR2) receptors were expressed on our patients’ LOEC (Figure S1 C–D).
Stratification based on statin treatment did not reveal significant differences between LOEC obtained from treated and untreated patients (Figure S3).
No other statistically significant correlations were detected.
Discussion
In the present study, we demonstrate that LOEC angiogenic activity is preserved in treated renovascular and essential hypertensive patients compared to normotensive controls. Moreover, LOEC expanded from either the renal vein or the systemic circulation showed comparable migratory, proliferative and tube-forming capacities to those HV-systemic-LOEC. These findings therefore support the use of ARAS and EH-LOEC for reparative purposes during renal injury.
We have previously found that the numbers of EPC were diminished in the systemic circulation of patients with EH or renovascular hypertension, and further reduced in the STK compared to the EH RV3. However, after 12 weeks of experimental renovascular hypertension in swine, LOECs showed unaltered proliferation and tube formation compared to normotensive pigs12. Similarly, the present study shows that in patients with prolonged but medically-treated hypertension under controlled conditions, LOECs expanded from RV and systemic blood showed angiogenic capacity comparable to HV.
Previous studies have shown that hypertension attenuates the numbers of circulating EPC and colony forming units via increased tissue Ang-II13. Ang-II induces reactive oxygen species (ROS) production, which reduces EPC levels, impairs their function10, 14 and accelerates EPC senescence13. Inhibitors of the RAAS, like ACE-I or ARB, may have beneficial effects on the number and function of EPC in cardiovascular disease, including hypertension and atherosclerosis15, 16. For example, Losartan decreased the expression of ROS-forming enzymes in the rat aorta, heart and kidney, resulting in increased EPC number and function15. Similarly, ARB improved the impaired EPC angiogenic capacity in EH patients17. In the present study, the angiogenic capacity of LOEC obtained from the RV or systemic blood in our hypertensive patients was not impaired compared with healthy controls, possibly because they were all treated with RAAS inhibitors. Additionally, the inverse correlation between LOEC function and blood pressure in EH patients suggested reduction of blood pressure as a potential mechanism by antihypertensive drugs enhance LOEC angiogenic capacity.
LOEC migration, proliferation and tube formation capacity in ARAS patients were also maintained. ARAS is characterized by renal hypoperfusion, activation of Ang-II, increased ROS generation and microvascular loss, as well as vascular wall and microvascular remodeling18. Coexistence of atherosclerosis and persistent hypoperfusion in ARAS kidneys magnifies oxidative stress and inflammation. As observed in other chronic kidney diseases, RAAS inhibitors attenuate oxidative stress and inflammation19, slow the progression of microvascular remodeling, and restore EPC levels and function15, 20. Collectively, these effects may account for the preserved LOEC function in ARAS patients. Contrarily, this strategy was less effective in sustaining circulating EPC numbers that decline in such patients3, suggesting a different regulatory mechanism of EPC number and function.
Notably, most of our hypertensive patients were treated with statins and calcium-channel-blockers, which might also attenuate EPC apoptosis and increase EPCs mobilization and differentiation21, 22. Thus, their pleiotropic effects may have also improved LOEC function. However, this similar relationship between LOEC and renal function in patients stratified by statin treatment argues against its effect on this relationship. Effects of other drugs will need to be tested in future studies designed for this purpose.
The type of EPC may also determine their functionality. Giannotti et al have shown that EOEC obtained from patients with untreated prehypertension and hypertension, and expanded for 7 days, have impaired function9. We studied function of LOEC obtained from patients treated with antihypertensive drugs under dietary and therapeutically-controlled conditions, and with only mildly elevated systolic pressures. Hence, collectively, these results suggest that antihypertensive treatment in hypertensive patients may improve angiogenic function of circulating progenitor cells.
Nevertheless, despite medical treatment, we observed a significant relationship between LOEC function and single-kidney hemodynamics assessed using MDCT. The migration capacity of only RV-LOEC correlated with STK-GFR, and tended to correlate with cortical perfusion. Furthermore, we found direct correlations between the migratory activity of RV-LOEC and CLK cortical perfusion, and between their proliferation and CLK-GFR. These observations suggest that in the ARAS STK and CLK, hemodynamics and function are determinants of the angiogenic potency of reparative cells that transit within their circulation, possibly by virtue of the intra-renal inflammatory and pro-oxidant microenvironment. Interestingly, we conversely observed an inverse correlation between IVC or RV-LOEC migration and both cortical perfusion and GFR in EH patients. Possibly in the EH kidney, hyperfiltration produces a noxious microenvironment that impairs EPC function akin to that caused by ischemia in the STK. Yet these effects on EPC function are likely subtle, given that overall EPC function in ARAS and EH patients were not different than those in HV.
In response to renal injury in ARAS, the STK releases inflammatory cytokines and specific injury homing signals to recruit and retain circulating EPC to stimulate its reparative process3, 7. We previously observed an inverse correlation between the number of EPC and RV level of TNF-α and IFN-γ in EH and ARAS patients3, 23. The current study extends our previous observations and demonstrates a significant inverse relationship between LOEC function and TNF-α or IFN-γ in ARAS and EH patients. Conversely, the anti-inflammatory cytokine IL-10 showed a direct correlation with LOEC migration. Therefore, inflammatory cytokines may play a role in regulating LOEC angiogenic function. Furthermore, levels of GM-CSF, G-CSF and SDF-1 correlated with LOEC function in all groups. These homing factors are all involved in the recruitment and mobilization of circulating progenitor cells from the bone marrow24, 25, partly by bolstering their function. These molecules and their cognate receptor expressed on progenitor cells mediate the homing and engraftment process. Indeed, the SDF-1 receptor CXCR4 was expressed on our patients’ EPC. Thus, homing signals and inflammatory cytokines may regulate LOEC angiogenic function. Notably, RV level of MCP-1 correlated directly with STK and CLK RV-LOEC function. Indeed, MCP-1 has been shown to induce stem-cell migration and recruitment26, and the expression of its CCR2 receptor on EPC in this study poses this chemokine as a homing and retention factor in human ARAS.
Our study is limited by a relatively small sample size and cross-sectional nature. We also excluded patients with diabetes or serum creatinine levels >1.7 mg/dl, because of application of iodinated contrast for MDCT scanning. Therefore, changes of EPCs function in these patients should be determined in future studies. The cause and effect relationships between renal hemodynamics, GFR, inflammatory markers and progenitor cell function, and the effects of anti-hypertensive regimen on the relationship between LOEC and renal function also merit further investigation. In addition, the mechanism by which inflammatory mediators modulate LOEC function, and changes in EOEC function in our patient groups, all await further studies.
Supplementary Material
Perspectives.
Our results demonstrate preserved function of systemic and RV LOEC in medically-treated renovascular and essential hypertensive patients. The correlation between LOEC function and renal function may imply that the ambient microenvironment exerts small but potentially meaningful effects on the function of EPC that transit across the renal circulation. Moreover, the correlation between LOEC function and inflammatory markers and homing signals in both ARAS and EH patients suggest that inflammation may regulate EPC function. Identification of these effects may allow refining novel therapeutic options for patients with ARAS. Therefore, in medically-treated patients with mildly elevated SBP under well-controlled conditions, LOEC function is relatively maintained and may permit their utilization for autologous delivery for potential reparative applications in case of imminent renal injury.
Novelty and Significance.
What Is New?
Our study demonstrates preserved EPC angiogenic function in patients with atherosclerotic renal artery stenosis compared with essential hypertensive patients and healthy volunteers. Yet, EPC function correlated with renal vein levels of anti-inflammatory mediator and endothelial progenitor cell homing factors.
What Is Relevant?
The adequate angiogenic function underscores EPC as a viable therapeutic option in essential and renovascular hypertensive patients.
Summary
The relatively preserved EPC function supports the feasibility of using autologous EPCs as a therapeutic tool in essential and renovascular hypertensive patients. Furthermore, homing signals and inflammatory mediators may modulate EPC angiogenic function.
Acknowledgments
Sources of funding
This study was partly supported by NIH grants: DK73608, HL77131, HL12156, DK100081, C06-RR018898, and the Mayo Clinic Center for Regenerative Medicine.
Footnotes
Disclosures
None.
References
- 1.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hypertens. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 2.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 3.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540–548a. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versari D, Lerman LO, Lerman A. The importance of reendothelialization after arterial injury. Curr Pharm Des. 2007;13:1811–1824. doi: 10.2174/138161207780831239. [DOI] [PubMed] [Google Scholar]
- 5.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol. 2013;33:1006–1013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 10.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 11.Wassmann S, Nickenig G. Pathophysiological regulation of the at1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24:S15–21. doi: 10.1097/01.hjh.0000220402.53869.72. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XY, Urbieta Caceres VH, Favreau FD, Krier JD, Lerman A, Lerman LO. Enhanced endothelial progenitor cell angiogenic potency, present in early experimental renovascular hypertension, deteriorates with disease duration. J Hypertens. 2011;29:1972–1979. doi: 10.1097/HJH.0b013e32834ae611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endtmann C, Ebrahimian T, Czech T, Arfa O, Laufs U, Fritz M, Wassmann K, Werner N, Petoumenos V, Nickenig G, Wassmann S. Angiotensin ii impairs endothelial progenitor cell number and function in vitro and in vivo: Implications for vascular regeneration. Hypertension. 2011;58:394–403. doi: 10.1161/HYPERTENSIONAHA.110.169193. [DOI] [PubMed] [Google Scholar]
- 14.Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 15.Yao EH, Fukuda N, Matsumoto T, Kobayashi N, Katakawa M, Yamamoto C, Tsunemi A, Suzuki R, Ueno T, Matsumoto K. Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect. Hypertens Res. 2007;30:1119–1128. doi: 10.1291/hypres.30.1119. [DOI] [PubMed] [Google Scholar]
- 16.Chen DD, Dong YG, Yuan H, Chen AF. Endothelin 1 activation of endothelin a receptor/nadph oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension. 2012;59:1037–1043. doi: 10.1161/HYPERTENSIONAHA.111.183368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki R, Fukuda N, Katakawa M, Tsunemi A, Tahira Y, Matsumoto T, Ueno T, Soma M. Effects of an angiotensin ii receptor blocker on the impaired function of endothelial progenitor cells in patients with essential hypertension. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt208. [DOI] [PubMed] [Google Scholar]
- 18.Eirin A, Lerman LO. Darkness at the end of the tunnel: Poststenotic kidney injury. Physiology (Bethesda) 2013;28:245–253. doi: 10.1152/physiol.00010.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: Effects of acei and arb. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Eirin A, Li ZL, Crane JA, Krier JD, Ebrahimi B, Pawar AS, Zhu XY, Tang H, Jordan KL, Lerman A, Textor SC, Lerman LO. Angiotensin receptor blockade has protective effects on the poststenotic porcine kidney. Kidney Int. 2013;84:767–775. doi: 10.1038/ki.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, Kimura G, Ueda R, Rabelink TJ, Murohara T. Nifedipine improves endothelial function: Role of endothelial progenitor cells. Hypertension. 2008;52:491–498. doi: 10.1161/HYPERTENSIONAHA.108.111914. [DOI] [PubMed] [Google Scholar]
- 23.Eirin A, Zhu XY, Woollard JR, Herrmann SM, Gloviczki ML, Saad A, Juncos LA, Calhoun DA, Rule AD, Lerman A, Textor SC, Lerman LO. Increased circulating inflammatory endothelial cells in blacks with essential hypertension. Hypertension. 2013;62:585–591. doi: 10.1161/HYPERTENSIONAHA.113.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, Che Y, Ou L, Liu L, Kong D. Sdf-1/cxcr4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2:845–854. doi: 10.1007/s13238-011-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grudzinska MK, Kurzejamska E, Bojakowski K, Soin J, Lehmann MH, Reinecke H, Murry CE, Soderberg-Naucler C, Religa P. Monocyte chemoattractant protein 1-mediated migration of mesenchymal stem cells is a source of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33:1271–1279. doi: 10.1161/ATVBAHA.112.300773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.