Abstract
The tubular hypothesis of glomerular filtration and nephropathy in diabetes is a pathophysiological concept that assigns a critical role to the tubular system, including proximal tubular hyperreabsorption and growth, which is relevant for early glomerular hyperfiltration and later chronic kidney disease. Here we focus on how harnessing the bioactivity of hormones released from the gut may ameliorate the early effects of diabetes on the kidney in part by attenuating proximal tubular hyperreabsorption and growth. The endogenous tone of the glucagon-like peptide 1 (GLP-1)/GLP-1 receptor (GLP-1R) system and its pharmacologic activation are nephroprotective in diabetes independent of changes in blood glucose. This is associated with suppression of increases in kidney weight and glomerular hyperfiltration, which may reflect at least in part its inhibitory effects on tubular hyperreabsorption and growth. Inhibition of dipeptidyl peptidase 4 (DPP-4) is also nephroprotective independent of changes in blood glucose and involves GLP-1/GLP-1R dependent and independent mechanisms. The GLP-1R agonist exendin-4 induces natriuresis via activation of the GLP-1R. In contrast, DPP4 inhibition, which albeit increases circulating GLP-1, drives a GLP-1R independent natriuretic response implying a role for other DPP-4 substrates. The extent to which the intrarenal DPP-4/GLP-1 receptor system contributes to all these changes remains to be established as does the direct impact of the system on renal inflammation.
Keywords: diabetic kidney disease, tubular hypothesis, gut hormones, kidney growth, dipeptidyl peptidase 4, glucagon-like peptide 1, incretin, hyperfiltration, uroguanylin, gut-kidney-communication
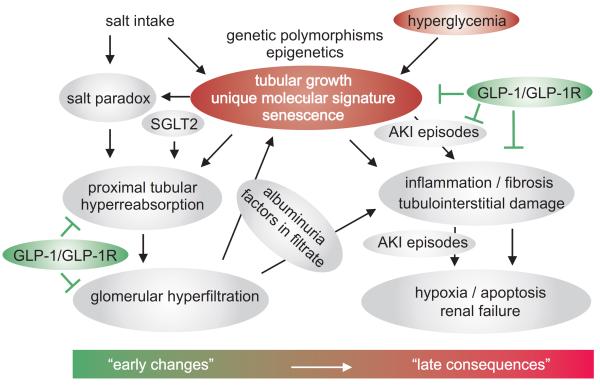
Diabetes is the single largest contributor to the growing prevalence of chronic kidney disease (CKD) (Collins et al. 2009). In a subset of diabetic patients the kidneys grow large and GFR becomes supranormal early during the disease, which both are risk factors for the later development of CKD. The tubular hypothesis of glomerular filtration and nephropathy is a pathophysiological concept to explain these changes (Figure 1).
Figure 1. The tubular hypothesis of glomerular filtration and nephropathy in diabetes mellitus - an emerging role for the GLP-1/GLP-1R system?
The tubular growth response to hyperglycemia differs from patient to patient, in part due to genetic and environmental influences. Diabetic tubular growth and its unique molecular signature determine early functional changes in the diabetic kidney like proximal tubular hyperreabsorption, the salt paradox and glomerular hyperfiltration. The latter promotes inflammation, fibrosis and tubulointerstitial damage through enhanced glomerular filtration of factors and proteins (including albumin) that interact with the tubular system. The molecular signature of diabetic tubular growth includes activation of TGF-β and senescence, which release pro-inflammatory factors, induce tubulointerstitial damage and fibrosis, and thereby facilitate episodes of acute kidney injury (AKI) and promote the development of late consequences including hypoxia, apoptosis and renal failure. Recent studies provide evidence that the endogenous tone and therapeutic activation of the GLP-1/GLP-1R system may inhibit proximal reabsorption and attenuate tubular growth and glomerular hyperfiltration, and induce anti-inflammatory effects in the diabetic kidney. SGLT2, sodium glucose cotransporter 2.
The Tubular Hypothesis of Glomerular Hyperfiltration and Nephropathy in Diabetes
The early distal tubule of every nephron makes contact with the vascular pole of its own glomerulus. At this site, the macula densa cells sense the luminal Na-Cl-K concentration and adjust single nephron GFR to stabilize delivery to the further distal nephron, where fine regulation of salt and fluid homeostasis is established. Diabetes causes a primary hyperreabsorption in the tubular segments upstream of the macula densa, especially in the proximal tubule (Vallon 2011; Vallon and Thomson 2012). Through the physiology of the described tubuloglomerular feedback, SNGFR is increased in diabetes in order to normalize salt delivery to the distal nephron. The hyperreabsorption in the diabetic kidney is due to increased filtered glucose load, which enhances glucose and sodium uptake through sodium-glucose cotransport (SGLT) (Vallon 2011; Vallon and Thomson 2012). As a consequence, genetic and pharmacologic inhibition of SGLT2 blunts diabetic hyperfiltration independent of changes in blood glucose (Vallon et al. 2013; Vallon et al. 2014). The second mechanism that enhances tubular reabsorption upstream of the macula densa in diabetes is tubular growth (Vallon 2011; Vallon and Thomson 2012).
The diabetic milieu triggers early tubular cell proliferation but the induction of TGF-β and cyclin-dependent kinase inhibitors causes a G1 cell cycle arrest and switch to tubular hypertrophy and a senescence-like phenotype (Vallon 2011; Vallon and Thomson 2012). While this growth phenotype explains unusual responses like the salt paradox of the early diabetic kidney (an inverse relationship between NaCl intake and GFR)(Vallon et al. 2002), the activated molecular pathways may set the stage for tubulointerstitial injury and diabetic nephropathy (Vallon 2011; Vallon and Thomson 2012). In other words, we hypothesize that the tubular growth response to hyperglycemia differs from patient to patient and explains early changes in the diabetic kidney, like glomerular hyperfiltration or the salt paradox, but also sets the stage for later diabetic nephropathy and CKD (Vallon 2011; Vallon and Thomson 2012). Complex interactions of glucose and growth factors act on the tubular cells to promote inflammation, fibrosis, oxidative stress and hypoxia.
Evidence exists to suggest that the processes described above may be amenable to inhibition via the tonic and post-prandial actions of hormonal signals derived from the gut which link sensing of nutrient intake in the gastrointestinal lumen to modulation of renal tubular function.
The Gut-Kidney Natriuretic Axis
Seminal studies in humans and in experimental animal models have indicated that oral ingestion of sodium chloride (NaCl) has a greater natriuretic effect than intravenous administration and that these effects appeared to be independent of changes in circulating aldosterone or atrial natriuretic peptide (ANP) as reviewed in (Michell et al. 2008).
Many gut-derived hormones are natriuretic including glucagon, GLP-1, amylin, secretin, VIP, gastrin/CCK, PYY and uroguanylin (Michell et al. 2008; Forte, Jr. 2004). Whereas some of these hormones activate adenylyl cyclase, others inhibit this enzyme (or the local isoform). Since inhibition of reabsorption can be induced by activation of adenylyl cyclase in the proximal tubule but also by inhibition of this enzyme in the distal nephron and collecting duct, we hypothesize that gut derived natriuretic hormones act on different sites in the kidney. To speculate further, dietary composition (incl. Na, Cl, K, Ca, phosphate, glucose, amino acids and protons) may also determine the qualitative make up of gut hormone “cocktails” in order for adjustments in renal excretion to closely track the specific composition of dietary intake. Very little is known about this latter issue, and in the following we focus the discussion on only one of the natriuretic gut-derived peptides, namely GLP-1.
Natriuretic Responses to GLP-1 Receptor Agonists and Dipetidyl Peptidase-4 (DPP-4) Inhibitors
GLP-1 is an incretin hormone secreted from enteroendocrine L cells in the intestine during the post-prandial phase that stimulates glucose-dependent insulin release (Drucker and Nauck 2006). After secretion, active GLP-1 is rapidly cleaved by membrane bound DPP-4 in capillaries, thereby limiting systemic levels of bioactive GLP-1 and reducing its half-life to less than 3 minutes. Therefore, therapeutic manipulation of the endocrine actions of the GLP-1 system rely on strategies that inhibit the degradation of GLP-1 by DPP-4 (i.e. DPP-4 inhibitors) or the use of degradation-resistant GLP-1 receptor agonists and analogues with a longer half-life, such as exendin-4 (EX4) or liraglutide.
The GLP-1 receptor (GLP-1R) is expressed in the brush-border of proximal tubules (Schlatter et al. 2007; Crajoinas et al. 2011). GLP-1/EX4 inhibited proximal tubular reabsorption, and induced diuresis and natriuresis and increased GFR in rats (Moreno et al. 2002; Schlatter et al. 2007; Thomson et al. 2013) and mice (Rieg et al. 2012). GLP-1 induced natriuresis associated with no change in GFR in healthy subjects and a small reduction in GFR in obese insulin-resistant subjects (Gutzwiller et al. 2004; Gutzwiller et al. 2006). GLP-1/EX4 decreases proximal tubular Na+-H+ exchanger 3 (NHE3)-mediated bicarbonate reabsorption, associated with phosphorylation of NHE3 at Serine 552 and Serine 605 in rat and porcine tubular cells (Schlatter et al. 2007; Crajoinas et al. 2011). A recent study in mice proposes that activation of the GLP-1R in the heart releases ANP, which then acts on the kidney to induce natriuresis (Kim et al. 2013). More recent data in human, however, do not support such a GLP-1/ANP axis (Skov et al. 2014).
DPP-4 is also expressed in proximal tubular brush border and assembles with NHE3 (Kenny et al. 1976; Girardi et al. 2001). DPP-4 inhibition also induces natriuresis (Girardi et al. 2001; Rieg et al. 2012; Crajoinas et al. 2011; Girardi et al. 2008; Girardi et al. 2004). It has been proposed that proximal tubular DPP-4 may affect the breakdown of endogenous factors derived from glomerular filtration that might regulate sodium reabsorption via effects on NHE3. GLP-1 is one attractive candidate in this regard.
To further define the role of the GLP-1/GLP-1R system, we performed studies in mice lacking the GLP-1R (Glp1r−/−). Glp1r−/− mice have an increased GFR (Rieg et al. 2012); one explanation could be that GLP-1R knockout removed its tonic inhibitory influence on proximal tubular reabsorption, which lowers the NaCl concentration at the macula densa and increases GFR via tubuloglomerular feedback. The presence of the GLP-1R was critical for EX4-induced natriuresis and phosphorylation of NHE3. Natriuretic responses to acute oral NaCl loading and the natriuretic effects of the DPP-4 inhibitor, alogliptin, were however surprisingly both independent of the GLP-1R. Moreover, the alogliptin-induced natriuresis was not associated with changes in NHE3 phosphorylation, arguing that the latter may not be a critical step in the induction of natriuresis (Rieg et al. 2012).
In the Glp1r−/− mice as well as in response to the application of GLP-1 or other GLP-1R agonists like EX-4, the integrated GFR response will be determined by the magnitude of the effects on proximal reabsorption and tubuloglomerular feedback versus a potential direct renal vasodilator effect of GLP-1R activation (Crajoinas et al. 2011; Green et al. 2008). Similar to studies in obese humans (Gutzwiller et al. 2004), we found that EX-4 lowered GFR in obese db/db mice (Rieg et al. 2012). The observed decrease in GFR in diabetes in response to GLP-1/EX-4 may relate to a stronger influence of proximal transport inhibition on GFR via tubuloglomerular feedback. The tubular hypothesis outlined above proposes that a primary proximal tubular hyperreabsorption contributes to glomerular hyperfiltration in the diabetic kidney. The GFR-lowering effect of GLP-1/EX-4 in diabetes may indicate that this tubular hyperreabsorption is particularly sensitive to inhibition by GLP-1R activation; as a consequence, with regard to the net GFR response the tubular effect of GLP-1R activation may dominate any potential direct renal vascular effect in diabetes. Alternatively or in addition, the renal vasodilator response to GLP-1R activation could be blunted in diabetes. We also noted a blunted natriuretic response to alogliptin in db/db mice, indicating that diabetes may affect the availability of the natriuretic factor regulated by DPP-4 inhibition (Rieg et al. 2012).
Direct Effects of GLP-1 on Kidney Injury?
Kodera and colleagues demonstrated a glucose-independent anti-inflammatory and protective role of GLP-1R signaling in type 1 diabetic rats (Kodera et al. 2011; Kodera et al. 2014); beneficial effects of EX4 in diabetic rats included a reduction in glomerular hypertrophy, mesangial matrix expansion, albuminuria, and macrophage infiltration, and markers of fibrosis and oxidative stress. The authors reported GLP-1 receptor expression on monocytes/macrophages and glomerular endothelial cells and the capacity for GLP-1 to oppose pro-inflammatory activation of both cell types in vitro. While EX-4 did not affect kidney weight or tubular hypertrophy, it prevented the increase in creatinine clearance in diabetic rats. The latter effect is consistent with the above described GFR-lowering response to GLP-1/EX4 observed in obese humans (Gutzwiller et al. 2004) and in obese db/db mice (Rieg et al. 2012).
Fujita and colleagues reported that the GLP-1R agonist liraglutide attenuated diabetes-induced renal changes in wild-type Akita mice (a genetic model of type 1 diabetes), including increases in kidney weight and GFR without significantly impacting upon glycaemic control (Fujita et al. 2014). Vice versa, GLP-1R knockout Akita mice showed increased albuminuria, mesangial expansion, markers of glomerular and renal oxidative stress, and further increases in kidney weight and GFR. DPP-4 inhibition also induced glucose-independent nephroprotective effects in type 1 diabetes that involved anti-inflammatory actions (Kodera et al. 2014) and suppression of the advanced glycation end products-receptor axis (Nakashima et al. 2014).
This provides evidence to support the contention that the endogenous GLP-1/GLP-1R system and its therapeutic activation suppress diabetic kidney growth and injury and glomerular hyperfiltration, which may be due to its inhibition of tubular growth and hyperreabsorption and affording protection against the cytotoxic effects of hyperglycaemia and through enhancement of anti-inflammatory signaling (Figure 1).
The argument for a more direct nephroprotective effect for DPP-4 inhibition and GLP-1 action is supported by findings in euglycaemic renal injury models with recent studies showing that DPP-4 inhibition protects the kidney against nephrotoxins through GLP-1 independent (indoxyl sulfate)(Wang et al. 2014) and GLP-1R-dependent mechanisms (cisplatin)(Katagiri et al. 2013).
Summary and Perspectives
Gut hormones can regulate urinary sodium and fluid excretion, but the physiological relevance and their specific roles remain unclear. The anti-diabetic drug, EX-4, induces natriuresis via activation of the GLP-1R. In contrast, the natriuretic effect of the DPP4 inhibitor, alogliptin, is independent of the GLP-1R. The GLP-1R is not critical to the ability to excrete an oral NaCl load, but the observed increase in GFR in Glp1r−/− mice may reflect a loss of tonic inhibition of proximal reabsorption by the endogenous GLP1/GLP1R system, which normally suppresses GFR through the physiology of tubuloglomerular feedback. Inhibition of DPP-4 can be nephroprotective independent of changes in blood glucose through GLP-1/GLP-1R dependent and independent mechanisms. Pre-clinical evidence points to a role for an endogenous tone within the GLP-1/GLP-1R system acting to limit inflammation and attenuate diabetic kidney growth and glomerular hyperfiltration. Therapeutic activation of the GLP-1R can further enhance these effects. Further studies are needed to better define the effects of GLP-1 on the vasculature and tubular system in the diabetic kidney, including the role of the intrarenal DPP-4/GLP-1R system.
These data support a future focus on the efficacy of GLP-1 and DPP-4 based anti-diabetic regimens in relation to renal end-points in large cohort studies. As GLP-1 levels are also augmented following Roux-en-Y gastric bypass, sleeve gastrectomy and endoluminal sleeve delivery, the establishment of a potential blood pressure lowering and nephroprotective gut-kidney axis after these bariatric procedures warrants further investigation.
New Findings.
What is the topic of this review?
This review describes how hyperglycaemia and attendant increases in proximal tubular growth and sodium reclamation form the mechanistic basis for the tubular hypothesis of hyperfiltration and nephropathy in the kidney of patients with diabetes.
The review highlights how signals arising from the gastrointestinal tract may be capable of modulating this response and how pharmacological enhancement of these signals may ameliorate the progression of diabetic kidney disease.
What advances does it highlight?
The review highlights the potential of GLP-1 analogues, GLP-1 receptor agonists and DPP-4 inhibitors to exercise renoprotective effects in diabetes independent of their classical effects on endocrine pancreas.
Acknowledgements
This work was supported by NIH grants R01DK56248, R01HL94728, the UAB/UCSD O'Brien Center of Acute Kidney Injury NIH-P30DK079337, and the Department of Veterans Affairs.
Disclosure Dr. Vallon has received within the past 12 months research grant support for basic science studies from Boehringer Ingelheim Pharma GmbH & Co.KG and Amylin/Brystol-Myers Squibb/Astra Zeneca.
Reference List
- Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St PW, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2008 Annual Data Report. Am J Kidney Dis. 2009;53:S1–374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301:F355–F363. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Forte LR., Jr. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104:137–162. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na(+)-H(+) exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J.Biol.Chem. 2001;276:46671–46677. doi: 10.1074/jbc.M106897200. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am.J.Physiol Renal Physiol. 2008;294:F414–F422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am.J.Physiol Cell Physiol. 2004;287:C1238–C1245. doi: 10.1152/ajpcell.00186.2004. [DOI] [PubMed] [Google Scholar]
- Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch.Biochem.Biophys. 2008;478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, Gutmann H, Stanga Z, Vogel D, Beglinger C. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion. 2006;73:142–150. doi: 10.1159/000094334. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin.Endocrinol.Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- Katagiri D, Hamasaki Y, Doi K, Okamoto K, Negishi K, Nangaku M, Noiri E. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J Am Soc Nephrol. 2013;24:2034–2043. doi: 10.1681/ASN.2013020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny AJ, Booth AG, George SG, Ingram J, Kershaw D, Wood EJ, Young AR. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem.J. 1976;157:169–182. doi: 10.1042/bj1570169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat.Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, Hirota D, Ono T, Usui HK, Makino H. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem.Biophys.Res.Commun. 2014;443:828–833. doi: 10.1016/j.bbrc.2013.12.049. [DOI] [PubMed] [Google Scholar]
- Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu.Rev.Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol. 2002;434:163–167. doi: 10.1016/s0014-2999(01)01542-4. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Matsui T, Takeuchi M, Yamagishi SI. Linagliptin Blocks Renal Damage in Type 1 Diabetic Rats by Suppressing Advanced Glycation End Products-Receptor Axis. Horm.Metab Res. 2014 doi: 10.1055/s-0034-1371892. [DOI] [PubMed] [Google Scholar]
- Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303:F963–F971. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul.Pept. 2007;141:120–128. doi: 10.1016/j.regpep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Skov J, Holst JJ, Gotze JP, Frokiaer J, Christiansen JS. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocr.Connect. 2014;3:11–16. doi: 10.1530/EC-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol. 2013;304:F137–F144. doi: 10.1152/ajprenal.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul.Integr.Comp Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194–F204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Huang DY, Deng A, Richter K, Blantz RC, Thomson S. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol. 2002;13:1865–1871. doi: 10.1097/01.asn.0000016441.41118.57. [DOI] [PubMed] [Google Scholar]
- Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu.Rev.Physiol. 2012;74:351–375. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Chang CH, Sun MF, Hsu SF, Weng CS. DPP-4 Inhibitor Attenuates Toxic Effects of Indoxyl Sulfate on Kidney Tubular Cells. PLoS.One. 2014;9:e93447. doi: 10.1371/journal.pone.0093447. [DOI] [PMC free article] [PubMed] [Google Scholar]