Abstract
Studies have reported that development of congestive heart failure (CHF) is associated with increased endoplasmic reticulum (ER) stress. Double stranded RNA activated protein kinase-like endoplasmic reticulum kinase (PERK) is a major transducer of the ER stress response and directly phosphorylates eIF2α, resulting in translational attenuation. However, the physiological effect of PERK on CHF development is unknown. In order to study the effect of PERK on ventricular structure and function, we generated inducible cardiac specific PERK knockout (KO) mice. Under unstressed conditions, cardiac PERK KO had no effect on left ventricular mass, or its ratio to body weight, cardiomyocyte size, fibrosis, or left ventricular function. However, in response to chronic transverse aortic constriction, PERK KO mice exhibited decreased ejection fraction, increased left ventricular fibrosis, enhanced cardiomyocyte apoptosis and exacerbated lung remodeling in comparison to wild type mice. PERK KO also dramatically attenuated cardiac sarcoplasmic reticulum Ca++-ATPase expression in response to aortic constriction. Our findings suggest that PERK is required to protect the heart from pressure overload-induced CHF.
Keywords: ER stress, translation regulation, ventricular hypertrophy
Introduction
Congestive heart failure (CHF) is a major cause of morbidity and mortality in developed countries and is a major threat to human health worldwide. CHF development is often associated with cardiomyocyte hypertrophy and increased protein synthesis. Protein synthesis and translation initiation are repressed during stress by phosphorylation of eukaryotic translation initiation factor 2 on the α subunit (eIF2α) of serine residue 51(Ser51) 1. Phosphorylation of eIF2αSer51 takes place via four known eIF2α protein kinases: Protein Kinase R (PKR), General Control Non-derepressible-2 (GCN2), Heme-Regulated Inhibitor kinase (HRI), and PKR like endoplasmic reticulum kinase (PERK). PERK is activated under conditions of endoplasmic reticulum stress. Phosphorylation of eIF2αSer51 is believed to be a protective mechanism to attenuate translation under stress conditions. Interestingly, we identified that the eIF2α protein kinase, PKR, is increased in myocardium of patients with CHF and in mice with CHF2. Deletion of the PKR gene (KO) markedly attenuated Transverse Aortic Constriction (TAC)-induced CHF in mice2. In addition, we demonstrated that genetic disruption of the eIF2α protein kinase GCN2 also significantly attenuated TAC-induced CHF in mice3. However, the effect of eIF2α protein kinase PERK on TAC-induced CHF is unknown.
Various cellular stresses, including ischemia, hypoxia, oxidative stress, inflammation, and protein synthesis overload, lead to impaired protein folding and accumulation of non-properly folded proteins in the lumen of the endoplasmic reticulum (ER). When unfolded protein levels exceed the protein folding capacity of the ER, an unfolded protein response (UPR) is activated, which induces expression of ER chaperones, activation of PERK and phosphorylation of eIF2αSer51 to transiently attenuate protein synthesis to decrease the burden on the ER and restore ER homeostasis. Extended activation of the UPR creates a state termed “ER stress” that can result in promotion of pro-apoptotic pathways mediated by proteins such as caspases and the C/EBP homologous protein (CHOP), etc.4,5. In mammalian cells, the UPR is also mediated by ER trans-membrane proteins inositol-requiring protein-1 (IRE1) and activating transcription factor 6 (ATF6) 6. Recently, several studies have demonstrated that development of CHF is associated with increased ER stress7-9, increased phosphorylation of PERK7-9, and increased phosphorylation of translation initiation factor eIF2α 3, 10, 11.
Because global PERK knockout causes growth retardation in mice12, we generated an inducible cardiomyocyte-specific PERK gene knockout model (designated PERK KO). We examined the effect of PERK KO on ventricular structure and function under control conditions (unstressed) and in response to pressure overload generated by TAC. In brief, under control conditions PERK KO had no detectable effect on LV structure and function in mice. In contrast, PERK KO profoundly exacerbated TAC-induced CHF and ventricular remodeling, indicating that PERK is dispensable for normal cardiac function under control conditions. However, PERK appears necessary for physiological adaptation to cardiac stress imposed by chronic pressure overload.
Materials and Methods
An extended Materials and Methods section can be found in the online-only Data Supplement.
Animals and Experimental Protocol
The experimental studies in mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Generation of inducible cardiomyocyte specific PERK KO strain
Adult PERKflox/flox mice12 and α-MHCMerCreMermice 13 were used for generating PERKflox/flox/α-MHCMerCreMer and PERKflox/flox(Figure 1A). PERKflox/flox/α-MHCMerCreMer mice have normal cardiac structure and function during unstressed conditions as compared with either DDAH1flox/flox or α-MHCMerCreMer mice. PERKflox/flox/α-MHCMerCreMer and PERKflox/flox were given 4-hydroxytamoxifen (Sigma) at 20mg/kg per day for 12 intra-peritoneal injections. TAC procedures were further performed in female wild type mice and PERK KO mice as previously described14-16.
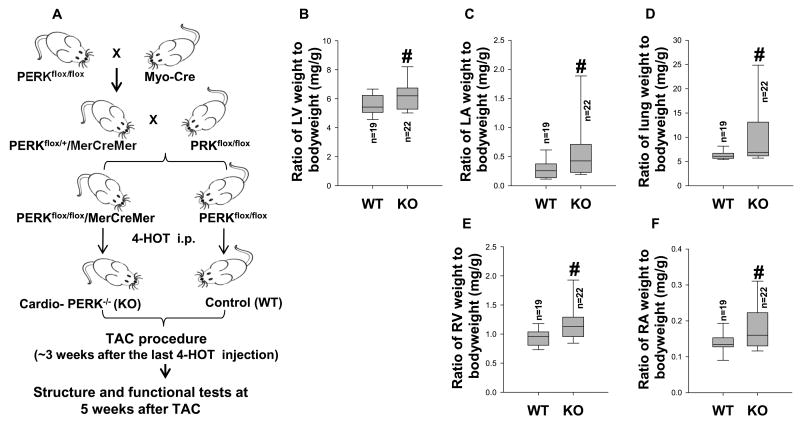
Figure 1. PERK KO exacerbated TAC-induced cardiac hypertrophy and increase of lung weight in mice.
Diagram shows the approach for generation of PERKflox/flox-Cre mice (A); TAC operations were performed on wild type (WT) and PERK KO mice. Tissue was collected from WT and PERK KO mice 5 weeks after TAC. Summarized left ventricular weight, left atrial weight, lung weight, right ventricular weight and right atrial weight in WT and PERK KO mice (B-F). #p<0.05 compared to the corresponding wild type group.
Results
Cardiomyocyte specific PERK KO had no effect on cardiac structure and function in mice under control conditions
Because global PERK gene knockout mice exhibit growth defects, an inducible cardiomyocyte specific PERK KO strain was generated in order to determine the role of PERK in cardiac function (Figure 1A, Figure S1A). To confirm the efficacy of cardiac specific PERK gene deletion, adult cardiomyocytes were isolated from cardiomyocyte PERK KO mice and control wild type mice ∼4 weeks after the last injection of 4-hydroxytamoxifen. Western blot demonstrated that PERK expression was largely abolished in cardiomyocytes isolated from cardiac specific PERK KO mice without affecting PERK expression in other organs such as lung and liver (Figure S1B-D), indicating that cardiomyocyte specific deletion of PERK was achieved. Echocardiography demonstrated that inducible cardiac specific PERK KO over 3 months had no detectable effect on heart rate, LV end diastolic diameter, LV end systolic diameter, LV wall thickness at both end diastole and end systole, and LV ejection fraction in mice (Figure S2A-C). PERK KO also had no detectable effect on LV weight, left atrial (LA) weight, lung weight, right ventricular (RV) weight, right atrial (RA) weight, and their ratios to body weight in both male and female mice (Figure S2D-H, Table S1). LV hemodynamics, measured in male PERK KO and wild type mice under control conditions, showed that PERK KO had no detectable effect on mean aortic pressure, LV pressure, LV dP/dtmax and LV dP/dtmin (Figure S3A-D).
PERK KO exacerbated TAC-induced cardiac remodeling, and increase of lung weight in female mice
As PERK KO had no detectable effect on LV structure and function in both male and female mice under control conditions, we further studied the effect of PERK KO on TAC-induced LV hypertrophy and dysfunction in a group of female mice. TAC induced increase in LV systolic pressure results in LV hypertrophy and dysfunction. The LV dysfunction causes an increase in LV diastolic pressure that leads to LA hypertrophy, lung remodeling and ultimately right ventricular hypertrophy or dysfunction14, 16. Therefore, TAC-induced CHF can be objectively evaluated by the progressive development of LA hypertrophy, pulmonary congestion and RV hypertrophy16. TAC-induced mortality rate was similar in wild type and PERK KO mice (Figure S4). PERK KO significantly exacerbated TAC-induced LV hypertrophy, LA hypertrophy and pulmonary congestion, as indicated by significant increases of LV weight, LA weight, lung weight and their ratios to body weight (Figure S5, Figure 1B-D, Table S2). TAC for 5 weeks caused a greater increase of right ventricular (RV) weight in PERK KO mice as compared with wild type mice (Figure 1E). TAC also caused a greater increase of right atrial (RA) weight and its ratios to body weight in PERK KO mice as compared with wild type mice (Figure 1F).
PERK KO exacerbated TAC-induced LV dysfunction in female mice
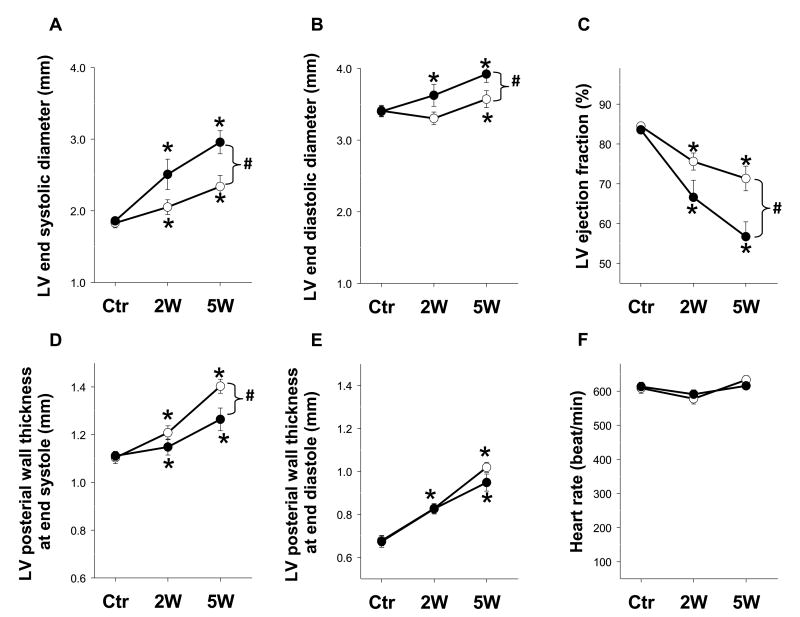
Serial echocardiography showed that PERK KO mice exhibited significantly greater LV end systolic diameter and LV end diastolic diameter in response to TAC compared to wild type mice. Serial echocardiography also revealed a significant decline in LV ejection fraction in PERK KO mice as compared with wild type littermates at 5 weeks after TAC (Figure 2A-C). TAC caused a significant increase of LV posterior wall thickness at end systole in both wild type and PERK KO mice (Figure 2D). This increase was significantly less in PERK KO mice (Figure 2D), consistent with the greater contractile dysfunction in these mice. TAC caused a similar increase of LV posterior wall thickness at end diastole in both cohorts, but had no effect on heart rate (Figure 2E, F). It should be mentioned that our pilot study in a small number of male mice also showed that PERK KO tended to exacerbate TAC-induced LV hypertrophy and dysfunction (data not shown).
Figure 2. PERK KO exacerbated TAC-induced LV dysfunction in mice.
LV ejection fraction, LV dimensions, LV wall thickness and heart rates were assessed using echocardiography before TAC, and after 2 and 5 weeks of TAC in mice. Echocardiography shows the differences in LV end systolic diameter (A), LV end diastolic diameter (B), LV ejection fraction (C), LV wall thickness at end systole (D), LV wall thickness at end diastole (E), and heart rates (F) of wild type and PERK KO mice following 5 weeks TAC. n=15 each group. *p<0.05 compared to before TAC; #p<0.05 compared KO mice to wild type mice.
PERK KO exacerbated TAC-induced LV fibrosis and cardiomyocyte hypertrophy in female mice
Histological examination demonstrated that PERK KO had no impact on LV cardiomyocyte size or LV fibrosis under control conditions. Morphological analysis showed evidence of LV fibrosis, LV perivascular fibrosis and cardiomyocyte hypertrophy in both wild type and PERK KO hearts in response to TAC. The increases in LV fibrosis and myocyte cross-sectional area were significantly greater in PERK KO hearts (Figure S6A-E).
PERK KO exacerbated TAC-induced increase of myocardial apoptosis in female mice
Cardiomyocyte death and replacement fibrosis are features of pathological hypertrophy that cause LV dysfunction. A recent study suggested that PERK activation attenuates apoptosis in endothelial cells exposed to ER stress17. To examine whether PERK influences cardiomyocyte susceptibility to apoptosis under pressure overload conditions, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed. While PERK KO had no effect on cardiac apoptosis under control conditions, the TAC-induced increase of apoptotic cells was significantly higher in PERK KO hearts (Figure S7A, B), a finding that is conceptually consistent with the previous report17. The increased apoptosis in PERK KO mice was associated with a significant increase of cleaved caspase-3 and total caspase-3 content in these hearts after TAC (Figure S8). Cleaved caspase-3 and total caspase-3 contents were also increased in PERK KO mice under control conditions (Figure S8).
PERK KO exacerbated TAC-induced decrease of Sarcoplasmic Reticulum Ca2+ ATPase (Serca2a)in female mice
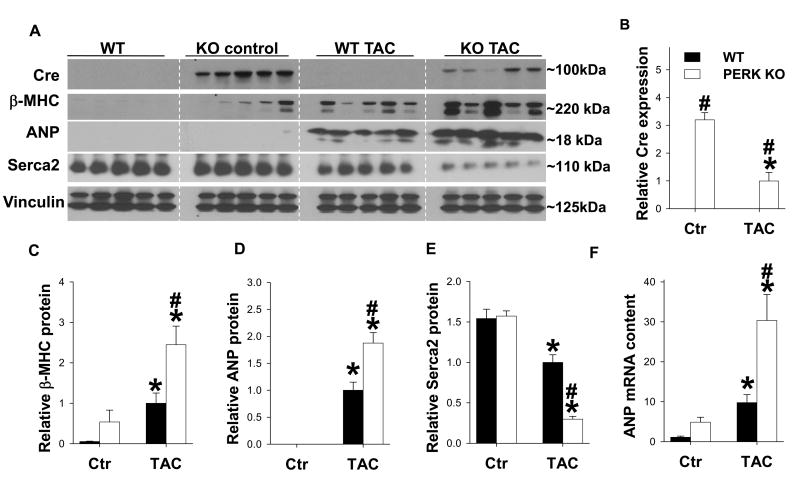
To further examine the impact of PERK KO in development of CHF, western blots were performed to examine expression of LV Serca2a, atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC), three commonly used biochemical markers for LV dysfunction. As anticipated, Cre is expressed only in PERK KO mice. The Cre expression was significantly reduced after TAC (Figure 3A, B). Under control conditions, PERK KO did not affect LV Serca2a, ANP or β-MHC expression (Figure 3A-E). In response to TAC, Serca2a (often reduced in CHF18, 14) was dramatically reduced in PERK KO mice compared with wild type mice (Figure 3A, E). ANP and β-MHC were elevated in both wild type and PERK KO mice after TAC, yet the increases were significantly greater in PERK KO mice (Figure 3). Expression of ANP mRNA also increased significantly more in PERK KO mice after TAC than in WT mice (Figure 3F). Serca2a function is controlled in part by the regulatory protein phospholamban (PLB). Thus we further examined protein levels of PLB as well as its phosphorylation on Ser16. PERK KO hearts exhibited increased phosphorylation of PLBSer16 (at 25KDa) under control conditions. TAC caused a significant increase of myocardial p-PLBSer16 in wild type mice, while the increase of p-PLBSer16 was largely diminished in PERK KO mice (Figure S9). PERK KO mice also exhibited significantly greater reduction of myocardial PLB content in response to TAC compared with wild type mice (Figure S9).
Figure 3. PERK KO exacerbated TAC-induced changes in myocardial Serca2a, ANP and β-myosin heavy chain (β-MHC) expression.
Tissue was collected from WT and PERK KO mice 5 weeks after TAC or control conditions, and lysates were examined by western blot for expression of Cre (A, B), atrial natrurietic peptide (ANP) (A, C), β-MHC (A, D) and Serca2a (A, E). Vinculin was used as a loading control. Relative ANP mRNA content in each group (F). * indicates p<0.05 comparing TAC to control. # indicates p<0.05 comparing WT to PERK KO.
PERK KO exacerbated TAC-induced lung remodeling in female mice
We previously reported that LV dysfunction causes severe lung fibrosis and lung vascular remodeling3, 16, which is anticipated to exacerbate the progression of CHF. Therefore, we examined TAC-induced lung fibrosis and lung vascular remodeling in wild type and PERK KO mice (Figure S10A-D). The percentage of non-muscularized (NM, with no apparent muscle), partially muscularized (PM, with only a crescent of muscle), and fully muscularized (FM, with a complete medial coat of muscle) small arteries in lung tissues were not different between wild type and PERK KO mice under control conditions. TAC caused an increase in FM small arteries in both wild type and PERK KO mice, but the number of FM small arteries was significantly greater in the PERK KO mice compared to wild type mice (Figure S10C). Similarly, both wild type and PERK KO mice exhibited a significant decrease in NM small arteries yet the number of NM small arteries was significantly reduced in PERK KO as compared to wild type mice (Figure S10C), indicating that PERK KO significantly exacerbated TAC-induced lung vascular remodeling. As anticipated, lung fibrosis was not affected in PERK KO mice under control conditions, but was significantly exacerbated in PERK KO lungs as compared to wild type lungs in response to TAC (Figure S10, Figure S11).
PERK KO attenuated TAC-induced phosphorylation of eIF2αSer51
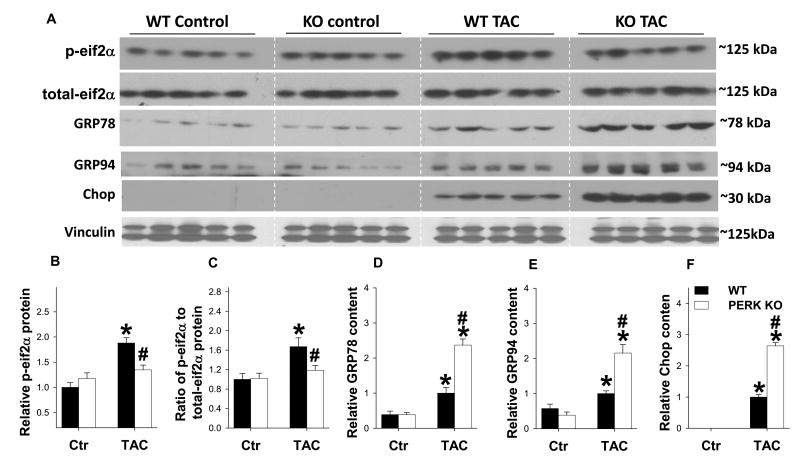
PERK is activated in response to elevated levels of misfolded proteins in the ER. Upon activation, PERK phosphorylates eIF2αSer51, which represses translation initiation to lessen the protein load in the ER. Therefore, we examined the impact of PERK KO on eIF2αSer51 phosphorylation in the heart. Interestingly, no difference was observed in eIF2αSer51 phosphorylation between WT and PERK KO mice under basal conditions. However, PERK KO significantly attenuated TAC-induced increase of eIF2αSer51 phosphorylation in the heart (Figure 4A, B), suggesting the ability to repress translation in response to cardiac ER stress was impaired.
Figure 4. Effects of PERK KO on LV eif2α phosphorylation and expression of GRP78, GRP94 and CHOP.
Tissue lysates were examined by western blot for expression of phospho-eif2αSer51(A, B) total-eif2α (C), GRP78 (A, D), GRP94 (A, E) and CHOP (A, F). n=5 each group.*indicates p<0.05 comparing TAC to control. # indicates p<0.05 comparing WT to PERK KO.
Expressions of GRP78, GRP94 and CHOP were increased in PERK KO heart after TAC
GRP78 and GRP94 are chaperones that promote protein folding and are up regulated under conditions of ER stress19. Expression levels of myocardial GRP78 and GRP94 were unchanged in PERK KO mice under control conditions (Figure 4). Interestingly, while expression levels of GRP78 and GRP94 were increased in both wild type and PERK KO hearts in response to TAC, expression of these chaperone proteins was significantly greater in PERK KO hearts. CHOP is a transcription factor up regulated by prolonged ER stress 20, and is believed to promote apoptosis in several disease models, including heart failure21. While CHOP is undetectable in wild type and KO hearts under control conditions, expression of CHOP was significantly elevated in PERK KO mice in response to TAC as compared to wild type mice. (Figure 4A, B). These data suggest that disruption of PERK exacerbates ER stress. TAC also caused significant increase of ATF4 in wild type hearts, but surprisingly, ATF4 expression was not attenuated by PERK KO (Figure S12) suggesting PERK is not required for induction of ATF4 in the overloaded heart. ATF6 expression was also not affected in PERK KO mice under control conditions or after TAC (Figure S12).
Discussion
The major finding in this study is that cardiomyocyte specific disruption of the eif2α kinase PERK exacerbated development of CHF in mice exposed to hemodynamic overload. Disruption of PERK expression in cardiomyocytes had no observable effect on cardiac structure or function under control conditions, but exacerbated pathological hypertrophy in response to TAC, as indicated by greater ventricular mass with decreased function and more ventricular dilation and fibrosis. This was accompanied by higher expression of cardiac stress markers ANP and β-MHC. PERK KO hearts also exhibited increased apoptosis and a dramatic reduction of Serca2a in response to TAC, suggesting PERK is important for cardiomyocyte survival and maintenance of intracellular calcium homeostasis during adaptation to pressure overload. Combining this work in the context of our previous findings showing that loss of theeIF2α protein kinases, GCN2, as well as PKR, also significantly attenuated TAC-induced CHF, our finding suggest that each of these three eIF2α kinases exerts unique effects on cardiac function in mice in response to systolic pressure overload.
Interestingly, we observed a significant reduction of Serca2a expression in PERK KO mice exposed to TAC. Serca2a is a well-defined therapeutic target for treating CHF and important regulator of calcium homeostasis in cardiomyocytes18. ER calcium depletion resulting from reduced Serca2a activity can induce ER stress22. Thapsigargin, an inhibitor of Serca2a activity23, is commonly used to induce ER stress. In the heart, exogenous expression of Serca2a has been shown to attenuate ER stress and CHF in mouse myocardial infarction or pressure overload models24, while selective Serca2a gene deletion in cardiomyocytes causes ER stress in cardiomyocytes and promotes CHF10. On the other hand, attenuation of ER stress was found to preserve Serca2a expression and cardiomyocyte function in an obesity model25 suggesting ER stress and impaired ER calcium handling aggravate one another. A recent study has also demonstrated that PERK KO disrupts intracellular calcium homeostasis and insulin secretion in beta cells through modulating Serca2a activity26, a finding that is consistent to the present study. The dramatic reduction in LV Serca2a protein expression in PERK KO after TAC is anticipated to further exacerbate ER stress in these mice. Because Serca2a activity is critical for maintaining cardiac function, the dramatic Serca2a reduction in PERK KO mice after TAC is likely an important mechanism for the exacerbated CHF in these mice.
PERK, ATF6, and Ire1 are the three most well recognized components of the ER stress sensing system, and together play a critical role in maintaining ER function during cell adaptation to stress. Under basal conditions, these transmembrane ER proteins are bound by the chaperone protein GRP78, which maintains them in an inactive state. Under conditions that increase misfolded protein content in the ER, GRP78 binds misfolded proteins and releases the ER stress sensors, resulting in their activation27. While ATF6 and Ire1 activation can directly upregulate UPR gene mRNA transcription28 or processing29, PERK helps relieve ER stress by inhibiting translation initiation through direct phosphorylation of eif2αSer51, thereby decreasing the protein load on the ER30, 31. The finding that cardiomyocyte specific PERK KO did not alter UPR gene expression or phosphorylation of eif2αSer51 under basal conditions, and had no observable effect on cardiac structure or function under basal conditions, suggests misfolded proteins do not accumulate under control conditions at levels sufficient to activate PERK. UPR genes GRP78, GRP94, and CHOP were significantly up-regulated in both wild type and PERK KO mice in response to TAC, consistent with previous studies demonstrating increased ER stress in the failing heart 9, 10, 21. The finding that UPR gene expression was higher in PERK KO hearts suggests that ER stress was exacerbated by PERK disruption. The somewhat surprising finding that ATF4 expression was not reduced, while CHOP expression was increased in PERK KO hearts suggests other components of the ER stress sensing machinery may be hyper-activated in absence of PERK. In support of a role for PERK dependent translational repression during cardiomyocyte ER stress, phosphorylation of eif2αSer51 was increased by TAC in wild type hearts, yet this increase was significantly blunted in PERK KO hearts. This reduction in eif2αSer51 phosphorylation may have contributed to ER stress by increasing protein overload in the ER. Together these findings indicate that ER stress signaling to UPR genes such as GRP78, GRP94, and CHOP can occur independent of PERK, and that cardiac ER stress is further elevated by PERK KO, possibly as a collective effect of reduced myocardial Serca2a expression and unabated translation during ER stress.
While GRP78 and GRP94 are markers of the UPR that protect against ER stress by improving protein folding19, CHOP is an ER stress induced transcription factor that is generally believed to promote apoptosis under conditions of prolonged ER stress32. CHOP expression is increased in the failing heart9, 10, 21, while CHOP gene deletion attenuates TAC- induced myocardial oxidative stress and heart failure21 and also reduces oxidative stress in beta cells in diabetes33. The increased CHOP expression and LV fibrosis in PERK KO hearts after TAC may contribute to the LV dysfunction observed in PERK KO mice by increasing both apoptosis and cardiomyocyte oxidative stress. The elevated TAC induced fibrosis observed after specific disruption of PERK in cardiomyocytes may be due to replacement fibrosis from increased cardiomyocyte death, as well as reactive fibrotic response to increased necrosis and inflammation resulting from Serca2a depletion. In addition to preserving Serca2a activity and limiting ER stress, PERK may also exert cardiac protective effects through modulating additional molecular pathways implicated in CHF development. A recent study demonstrated that PERK activation can induce cardiomyocyte autophagy34, an evolutionary stress response that is important for adaptation to cardiac stress 35, 36.
PERK is one of the four eIF2α kinases that phosphorylate eIF2α at Ser51 in response to different types of stress. PKR responds to viral infection, inflammation, ER stress, and nutrient signals. GCN2 is mainly activated during amino-acid starvation and nutrient deficiency, and Heme-regulated inhibitor kinase limits protein synthesis in heme-deficient cells. We have recently demonstrated that genetic knockout of eIF2α kinase GCN2 significantly attenuated TAC-induced cardiac dysfunction and cardiac eIF2α phosphorylation in mice3, while knockout of eIF2α kinase PKR markedly attenuated TAC-induced cardiac dysfunction without affecting cardiac eIF2α phosphorylation2. Interestingly, both GCN2 KO and PKR KO had no detectable effect on TAC-induced LV hypertrophy. In the present study, PERK KO significantly exacerbated TAC-induced LV hypertrophy and CHF but attenuated cardiac eIF2α phosphorylation in mice. These studies demonstrate that each of these three eIF2α kinases exerts unique effects on cardiac function in mice in response to systolic pressure overload. While inhibition of PKR and GCN2 may be effective in protecting the heart against CHF development in response to pressure overload, enhancing PERK signaling may be effective in attenuating CHF development.
Perspectives
eIF2α kinase PERK plays an important role in sensing ER stress. Studies have demonstrated that CHF is associated with increased ER stress, but the impact of PERK on CHF development was not known. Our study provides the first direct evidence that inhibition of PERK activity by PERK gene deletion in the heart exacerbates the development of CHF and lung remodeling, indicating that PERK sensing of ER stress in cardiomyocytes is important in protection against development of CHF.
Supplementary Material
Novelty and Significance.
What Is New?
We demonstrate for the first time that knock out of the ER stress sensor PERK in cardiomyocytes had no observable effect on cardiac structure and function under unstressed conditions, but profoundly exacerbated TAC-induced left ventricular hypertrophy and CHF in mice.
What Is Relevant?
This study demonstrates that proper ER stress sensing by PERK is important in protecting the heart against ventricular hypertrophy and dysfunction.
Summary
Our findings indicate that PERK is dispensable for cardiac function under unstressed conditions but it is necessary for physiological adaptation to cardiac stress imposed by chronic pressure overload.
Acknowledgments
None.
Sources of funding: This study was supported by U.S. Public Health Service Grants HL021872, HL098669, HL098719, HL102597, HL089249, R01HL105406 and T32HL069764 from the National Institutes of Health, and Research Grant 09GRNT2260175 from the American Heart Association.
Footnotes
Disclosures: None.
References
- 1.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Xu X, Fassett J, Kwak D, Liu X, MD, Hu X, Bell J, Bache R, Chen Y. Double stranded rna–dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure. Circulation. 2014;129:1397–1406. doi: 10.1161/CIRCULATIONAHA.113.002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Z, Xu X, Fassett J, Kwak D, Liu X, Hu X, Wang H, Guo H, Xu D, Yan S, McFalls EO, Lu F, Bache RJ, Chen Y. Loss of the eukaryotic initiation factor 2alpha kinase general control nonderepressible 2 protects mice from pressure overload-induced congestive heart failure without affecting ventricular hypertrophy. Hypertension. 2014;63:128–135. doi: 10.1161/HYPERTENSIONAHA.113.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida H. Er stress and diseases. Febs J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 6.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 7.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 8.Ni M, Lee AS. Er chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 10.Liu XH, Zhang ZY, Andersson KB, Husberg C, Enger UH, Raeder MG, Christensen G, Louch WE. Cardiomyocyte-specific disruption of serca2 in adult mice causes sarco(endo)plasmic reticulum stress and apoptosis. Cell Calcium. 2011;49:201–207. doi: 10.1016/j.ceca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The perk eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular pde5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of sarcolemmal atp-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: Heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase r-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- 18.Tilemann L, Ishikawa K, Weber T, Hajjar RJ. Gene therapy for heart failure. Circ Res. 2012;110:777–793. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (grp78 and grp94): Functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 20.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of c/ebp homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 22.Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum ca-atpase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 24.Xin W, Lu X, Li X, Niu K, Cai J. Attenuation of endoplasmic reticulum stress-related myocardial apoptosis by serca2a gene delivery in ischemic heart disease. Mol Med. 2011;17:201–210. doi: 10.2119/molmed.2010.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turdi S, Hu N, Ren J. Tauroursodeoxycholic acid mitigates high fat diet-induced cardiomyocyte contractile and intracellular ca2+ anomalies. PLoS One. 2013;8:e63615. doi: 10.1371/journal.pone.0063615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, McGrath BC, Kopp RF, Roe MW, Tang X, Chen G, Cavener DR. Insulin secretion and ca2+ dynamics in beta-cells are regulated by perk (eif2ak3) in concert with calcineurin. J Biol Chem. 2013;288:33824–33836. doi: 10.1074/jbc.M113.503664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian er quality control proteins is mediated by single or combined action of atf6alpha and xbp1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. Ire1-mediated unconventional mrna splicing and s2p-mediated atf6 cleavage merge to regulate xbp1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 31.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 32.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. Chop is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. Perk integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Hua Y, Nair S, Bucala R, Ren J. Macrophage migration inhibitory factor deletion exacerbates pressure overload-induced cardiac hypertrophy through mitigating autophagy. Hypertension. 2014;63:490–499. doi: 10.1161/HYPERTENSIONAHA.113.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Roe ND, Weiser-Evans MC, Ren J. Inhibition of mammalian target of rapamycin with rapamycin reverses hypertrophic cardiomyopathy in mice with cardiomyocyte-specific knockout of pten. Hypertension. 2014;63:729–739. doi: 10.1161/HYPERTENSIONAHA.113.02526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.