Abstract
Metronomic cyclophosphamide (CPA) treatment activates robust innate anti-tumor immunity and induces major regression of large, implanted brain tumor xenografts when administered on an intermittent, every 6-day schedule, but not on a daily low-dose or a maximum-tolerated dose CPA schedule. Here, we used an implanted GL261 glioma model to compare five intermittent metronomic CPA schedules to elucidate the kinetics and schedule dependence of innate immune cell recruitment and tumor regression. Tumor-recruited natural killer cells induced by two every 6-day treatment cycles were significantly ablated one day after a third CPA treatment, but largely recovered several days later. Natural killer and other tumor-infiltrating innate immune cells peaked 12 days after the last CPA treatment on the every 6-day schedule, suggesting that drug-free intervals longer than 6 days may show increased efficacy. Metronomic CPA treatments spaced 9 or 12 days apart, or on an alternating 6 and 9 day schedule, induced extensive tumor regression, similar to the 6-day schedule, however, the tumor-infiltrating natural killer cell responses were not sustained, leading to rapid resumption of tumor regrowth after day 24, despite ongoing metronomic CPA treatment. Increasing the CPA dose prolonged the period of tumor regression on the every 9-day schedule, but natural killer cell activation was markedly decreased. Thus, while several intermittent metronomic CPA treatment schedules can activate innate immune cell recruitment leading to major tumor regression, sustained immune and anti-tumor responses are only achieved on the 6-day schedule. However, even with this schedule, some tumors eventually relapse, indicating a need for further improvements in immunogenic metronomic therapies.
Keywords: metronomic chemotherapy, GL261 glioma, chemotherapy schedule, innate immune response, natural killer cells
1. Introduction
The alkylating agent cyclophosphamide (CPA; NSC 26271) was first approved by the FDA for cancer treatment in 1959 and is currently used to treat a variety of diseases, including breast and ovarian cancer, hematological malignancies, and autoimmune diseases [1]. CPA and other cytotoxic agents are commonly delivered to cancer patients on a maximum-tolerated dose schedule, but often show modest anti-cancer activity [2; 3]. This limited efficacy is in part due to high toxicity to the host, which necessitates a long drug-free break, during which chemotherapy-resistant tumor cell populations may emerge and residual tumors can neovascularize, leading to tumor regrowth [3; 4]. This limitation can be addressed, in part, by administration of CPA on a metronomic schedule [5; 6], which augments CPA cytotoxicity to tumor-associated endothelial cells and can increase overall therapeutic activity while reducing host toxicity [7; 8; 9]. Daily, oral low dose metronomic CPA schedules have shown promising results in preclinical models [10; 11] and are currently being tested in clinical trials [12; 13; 14].
CPA has substantial immunomodulatory activity [15; 16]. CPA can deplete immune suppressive regulatory T cells [17; 18], stimulate dendritic cell maturation [18], rescue tumor-driven CD4+ T-cell differentiation [19] and activate anti-tumor CD8+ T cell responses [20]. Furthermore, in studies from our laboratory, CPA was shown to activate a potent, innate anti-tumor immune response in several implanted glioma models grown in immune competent or immune deficient (scid) mice when delivered on an intermittent, 6-day repeating metronomic schedule, leading to strong innate immune-based tumor regression [21]. This regression is dependent on natural killer (NK) cells, as demonstrated by antibody depletion experiments and using mouse models deficient in NK cells or in the NK cell cytotoxic effector perforin 1 [21]. CPA administration on a more frequent metronomic schedule, e.g., daily low-dose CPA treatment, failed to activate a robust anti-tumor immune response or induce tumor regression [22]. Thus, an intermittent, 6-day repeating metronomic schedule of CPA is most effective in activating the anti-tumor innate immune response leading to tumor regression. Metronomic schedules that administer CPA or other cytotoxic drugs too frequently may hamper immune cell proliferation or suppress intratumoral accumulation of chemotherapy-sensitive immune cell populations. Consistent with these findings, bone marrow myelopoiesis suppressed by CPA requires ~10 days for recovery [23; 24], and in a myeloma model, CPA administered at long intervals elicited strong immune responses and better anti-tumor activity than CPA given at shorter intervals [18]. However, a drug-free break that is too long may increase the risk of tumor regrowth, as occurs with maximum-tolerated dose drug schedules [2]. Other studies indicate that a threshold level of tumor cell and/or stromal cell DNA damage may be required to activate cytokine and chemokine responses [21; 22], which can stimulate immune cell recruitment [25].
Although CPA is not typically used to treat gliomas, our prior findings indicate that CPA can be very active in multiple glioma models when administered on an intermittent metronomic schedule [21; 22; 26], suggesting it may have greater clinical potential for glioma treatment than recognized previously. Here, we investigate whether the efficacy of intermittent CPA treatment on an every 6-day schedule (CPA/6d) can be improved by extending the time between CPA treatments to allow for a longer drug-free interval. Four other schedules were investigated: CPA treatment every 9 days (CPA/9d) or every 12 days (CPA/12d), CPA treatment on a 6-day and 9-day alternating schedule (CPA/6-9d), and CPA treatment on an every 9 day schedule, but with an increase in CPA dose from the standard dose of 140 mg/kg/injection to 210 mg/kg/injection (CPA(210)/9d schedule). We found that all five intermittent CPA schedules initially induce strong tumor regression, which proceeds with strong momentum for ~24 days, after which many of the tumors treated at 9 or 12 day intervals rapidly regrow in association with a major decrease in the expression of NK cell activation markers. These findings are discussed in terms of the impact of metronomic CPA scheduling on the emergence of drug or immune cell resistant tumor cell populations.
2. Materials and Methods
2.1. Tumor cell lines and mouse xenografts
Mouse GL261 glioma cells were authenticated by and obtained from the Developmental Therapeutics Program Tumor Repository (National Cancer Institute, Frederick, MD). Cells were grown at 37°C in a humidified 5% CO2 atmosphere in RPMI-1640 culture medium containing 10% fetal bovine serum, 100 Units/ml penicillin and 100 μg/ml streptomycin. Six-wk-old (26-28 g) male ICR/Fox Chase immune deficient scid mice (Taconic Farms, Germantown, NY) were housed and treated under approved protocols and federal guidelines. GL261 glioma cells (4 × 106) were injected s.c. on each posterior flank in 0.2 ml serum-free RPMI using a U-100 insulin syringe with a 28.5 gauge needle (BD Biosciences, Cat.# 329461). Tumor areas (length x width) were measured twice weekly using Vernier calipers (VWR International, Cat# 62379-531) and tumor volumes were calculated based on the formula Vol = (π/6)*(L*W)3/2. Tumors were monitored and drug treatment was initiated when the mean tumor volumes reached ~500-700 mm3. The measured volume of each tumor was normalized to the treatment starting point (=100%) to obtain a normalized tumor volume. Mouse body weights were normalized in the same manner. Mice were treated with CPA given on the following intermittent metronomic schedules: CPA/6d (140 mg CPA/kg-body weight (BW), repeated every 6 days); CPA/6-9d (140 mg CPA/kg-BW, alternating between an every 6 day and an every 9 day schedule); CPA/9d (140 mg CPA/kg-BW, repeated every 9 days); CPA/12d (140 mg CPA/kg-BW, repeated every 12 days); and CPA(210)/9d (210 mg CPA/kg-BW, repeated every 9 days), on the days marked in each Figure using vertical arrows. CPA was administered as a monohydrate (Sigma, Cat. # C0768), with the CPA doses reported here based on the non-hydrated molecular weight of 261. Tumor sizes and mouse body weights were measured at least twice a week. Fisher’s exact test and Student’s t-test were used to assess the differential tumor responses (regression vs rebound) and tumor growth rate observed with each CPA schedule, respectively: *, p<0.05; **, p<0.01; and ***, p<0.001.
2.2. qPCR analysis of immune cell and other marker genes
Changes in tumor-infiltrating innate immune cells were monitored by changes in the expression of innate immune marker genes, as determined by qPCR analysis of tumor RNA. Changes in the immune cell marker genes used here are indicative of changes in the corresponding immune marker protein levels and immune cell numbers, as shown by immunohistochemistry and/or FACS analysis of metronomic CPA-treated 9L and U251 gliomas implanted in the same scid mouse model [21; 22; 26]. Further, NK cell marker gene expression levels show a close association with the extent of tumor regression induced by metronomic CPA treatment [22]. Isolation of total tumor RNA, reverse transcription, and qPCR were carried out as described [21]. Primers designed using Primer Express software (Applied Biosystems, Carlsbad, CA, USA) are described in [21; 22] or presented in Supplemental Table S1. qPCR data were analyzed using the comparative CT method and are presented as relative levels of each RNA compared to the RNA level in untreated tumors after normalization to the 18S RNA content of each sample. qPCR data are expressed as mean values ± S.E. for n= 4-6 tumors per time point for each treatment group unless indicated otherwise. Statistically significant differences between mean values of different treatment groups were determined by one-way ANOVA (for more than two group comparisons) or two-tailed Student’s t-test (for two group comparisons). Significance is indicated by: *, p<0.05; **, p<0.01; and ***, p<0.001.
3. Results
3.1 CPA/6d schedule induces GL261 glioma regression with strong momentum
GL261 gliomas were implanted subcutaneously in scid mice to investigate sensitivity to CPA treatment and innate immune cell recruitment using the same 6-day repeating metronomic schedule (CPA/6d schedule) shown to be efficacious in treating two other implanted brain tumors (9L gliosarcoma and U251 glioblastoma) [21]. A single CPA injection induced only a transient pause in GL261 tumor growth, from day 9 to day 12 after treatment, however, a prolonged period of regression, lasting 15-18 days, was induced by a second and also by a third CPA injection when given on the CPA/6d schedule (Fig. 1). Thus, CPA/6d treatment activates an anti-tumor response with strong momentum for continued regression once the second CPA injection is given. The time to initiate GL261 tumor regression was intermediate compared to two other gliomas investigated in the same mouse model: GL261 tumor regression began just after the second CPA/6d injection on day 6, whereas U251 tumor regression begins shortly after the first CPA injection, and 9L tumor regression begins after 3-4 CPA injections [21]. We used this experimental model to investigate the efficacy of other intermittent metronomic CPA schedules in which the drug-free interval is longer than 6 days.
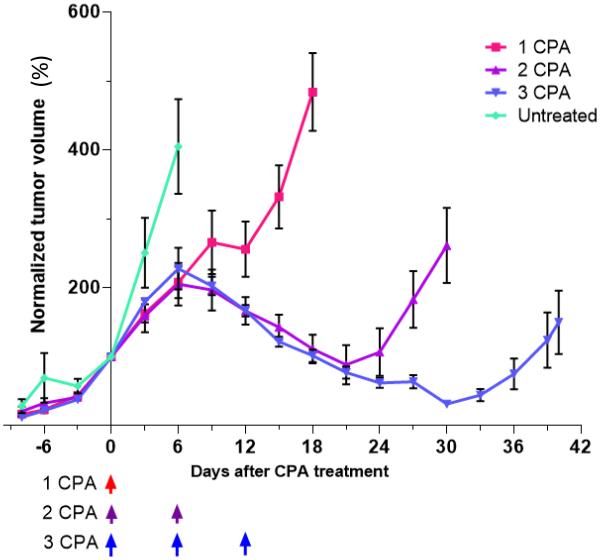
Fig. 1.
Growth curves of GL261 tumors that were untreated, or were given 1, 2, or 3 CPA injections on the CPA/6d schedule. Data shown are tumor volumes normalized to 100% on the first day of drug treatment (=day 0), mean ± SEM, for n = 6-8 tumors/group, except for 1 cycle CPA, where n=4. Arrows at bottom indicate days of CPA treatment at 140 mg/kg-BW. The measured tumor volumes on day 0 were 685 ± 55 mm3, 785 ± 370 mm3, 715 ± 105 mm3 (mean ± SEM) for the groups given 1, 2, and 3 CPA injections, respectively.
3.2 CPA ablation of tumor-infiltrating NK cells
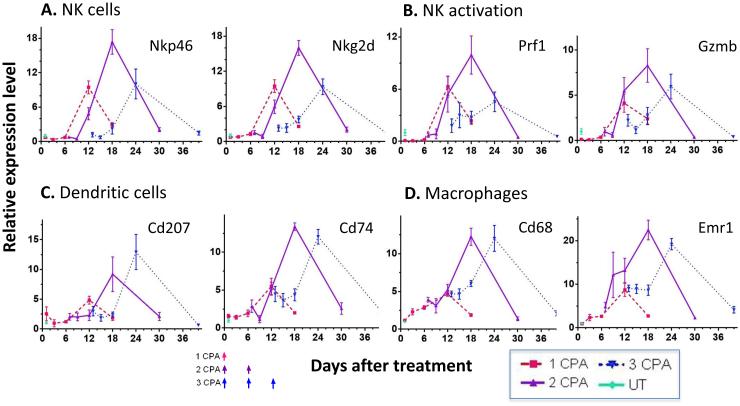
GL261 tumors treated with 1, 2 or 3 CPA injections on the CPA/6d schedule, as in Fig. 1, were sampled 1, 3, 6, and 12 days after the last CPA injection, and then again, once robust tumor growth resumed (days 18, 30 and 40, respectively, in Fig. 1). Tumor-infiltrating innate immune cells were assayed by qPCR analysis of innate immune cell marker genes, whose changes in expression are indicative of changes in the corresponding tumor-associated immune cell populations [21; 22; 26]. We examined the NK cell marker genes Nkp46 and Nkg2d, NK cell activation markers Prf1 and Gzmb, dendritic cell markers Cd207 and Cd74, and macrophage markers Cd68 and Emr1 (F4/80). All of the marker genes showed their highest expression 12 days after the last CPA injection (c.f., peaks of expression on days 12, 18 and 24) (Fig. 2). In addition, there were significant decreases in the NK cell markers one day after the third CPA injection (75% decrease in Nkp46 levels, and 62% decrease in Nkg2d levels from day 12 to day 13; Fig. 2A and Table 1), consistent with CPA cytotoxicity to the tumor-infiltrating NK cell population. Similar decreases were seen for the NK cell activation markers Prf1 and Gzmb (Fig. 2B and Table 1). Markers for the tumor-associated dendritic cells and macrophages did not show significant changes (Table 1), suggesting those cells are less sensitive to CPA cytotoxicity than the tumor-associated NK cells. The preferential loss of NK cells following CPA treatment on day 13 (Table 1) is consistent with reports that CPA is preferentially cytotoxic to NK cells [27; 28], depletes fewer granulocyte precursors than lymphocytes in mouse spleen and bone marrow [29], and does not affect peritoneal macrophage cytolytic activity [30]. Given the functional importance of NK cells for metronomic CPA-induced tumor regression [21], our findings further suggest that the efficacy of intermittent metronomic CPA might be improved by lengthening the 6 day interval between CPA treatments to decrease the frequency with which CPA ablates the tumor-associated NK cell population.
Fig. 2.
Time courses of changes in innate immune cell marker gene expression assayed by qPCR in GL261 tumors treated as in Fig. 1. Tumors were sampled 1, 3, 6 and 12 days after the last CPA injection, and at one additional time point, when strong tumor regrowth was evident (days 18, 30 and 40, for 1, 2 and 3 CPA injections, respectively; see Fig. 1). (A) NK cell markers Nkp46 and Nkg2d, (B) NK cell activation markers Prf1 and Gzmb, (C) dendritic cell markers Cd207 and Cd74, and (D) macrophage markers Cd68 and Emr1 (F4/80). The third CPA injection reduced NK cell marker expression compared to the level just prior to injection (day 13 vs. day 12; see Table 1). Gene expression levels relative to 18S rRNA over the time course (X-axis) were normalized to the mean of untreated tumors on day 1. Y-axis values are relative expression levels of each gene, mean ± SEM, for n = 4 to 8 tumors per time point.
Table 1.
Comparison of immune cell marker gene expression on day 13 vs. day 12. Changes in innate immune cell marker genes in CPA/6d-treated tumors on day 13 (i.e., one day after a third CPA injection) compared to day 12 (i.e., 6 days after 2 injections of CPA), based on data in Fig. 2. Values shown in columns 3 and 4 are fold-changes ± SE in the expression level of each immune marker gene compared to drug-free (untreated control) tumors. The percentage values shown in column 5 indicate the extent to which the immune marker gene declines within one day of a third CPA injection given on day 12, and is calculated using the formula: 100% × (day12 - day13)/day12. The significance of the change was assessed by 1-tailed t-test. NS, not significant (p>0.05).
| Cell type | Genes | Day 12 (2-CPA) fold-change |
Day 13 (3-CPA) fold-change |
Day 13 vs day 12 % decrease |
p-value |
|---|---|---|---|---|---|
| NK cells | Nkp46 | 4.8 ± 1.0 | 1.2 ± 0.4 | 75.4 | <0.01 |
| Nkg2d | 6.0 ± 1.1 | 2.3 ± 0.5 | 61.5 | <0.02 | |
| NK cell activation |
Prf1 | 4.1 ± 1.2 | 1.6 ± 0.6 | 60.1 | 0.06 |
| Gzmb | 5.5 ± 1.5 | 2.2 ± 0.6 | 59.3 | <0.05 | |
| Dendritic cells | Cd74 | 5.2 ± 0.8 | 4.5 ± 1.0 | 13.7 | NS |
| Cd207 | 2.3 ± 0.9 | 3.0 ± 0.8 | −29.9 | NS | |
| Macrophages | Cd68 | 5.2 ± 0.7 | 4.7 ± 0.4 | 10.9 | NS |
| Emr1 | 13.2 ± 2.8 | 9.1 ± 0.8 | 31.4 | NS |
3.3 CPA/6d-responsive cytokine, chemokine and adhesion molecule gene signatures
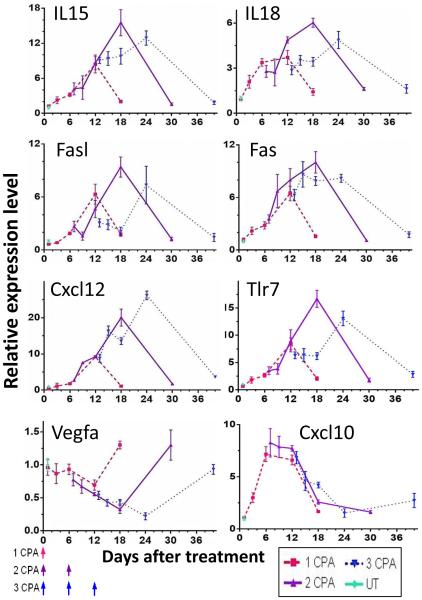
We investigated expression patterns for several other genes found to be responsive to intermittent CPA treatment to ascertain whether they also favor a metronomic CPA drug-free interval longer than 6 days. These genes include: IL15 and IL18, which are important for NK cell development, proliferation, and cytotoxicity [31; 32; 33; 34]; Fas and its ligand Fasl, which are expressed on tumor cells and NK cells, respectively, and together elicit NK cell-mediated tumor cell death [35; 36]; Cxcl12, a pleiotropic chemokine that recruits neutrophils and monocytic cells [37]; and Tlr7, which induces dendritic cell maturation and anti-tumor inflammatory immune responses [38; 39]. Gene responses were generally maximal 12 days after each CPA injection (Fig. 3). Similar patterns of response were seen for three other genes that may contribute to the strong innate immune responses seen in the metronomic CPA-treated tumors: Csf1, which induces macrophage and monocyte development and proliferation [40], and Icam2 and Vcam1, which are expressed on endothelial cells and immune cells and are important for immune cell trans-endothelial cell migration towards inflammation sites and tumors [41; 42] (Supplemental Fig. S1). Vegfa, which is a strong pro-angiogenic tumor factor with immune-suppressive and wound-healing activity [43; 44; 45; 46; 47], showed a distinct response pattern: Vegfa expression decreased progressively with each cycle of CPA treatment through at least day 24. This decrease in Vegfa could favor the activation of anti-tumor immunity [46; 47]. A distinct response pattern was seen for Icam1, as well as for the chemokines Cxcl9, Cxcl10 and Cxcl11 (Fig. 3, Supplemental Fig. S1), whose expression is driven by interferon-γ and is important for NK cell trafficking and recruitment into tumors [48]. These three chemokines were maximally expressed between day 6 and day 12 and thereafter declined, independent of the second or the third CPA treatment (Fig. 3, Supplemental Fig. S1). In the 9L glioma model, metronomic CPA treatment induces strong expression of Cxcl10 and Cxcl11, but those gene responses do not correlate with the efficacy of different metronomic CPA schedules or with the extent of activation of anti-tumor immune responses [22].
Fig. 3.
Time courses for changes in cytokine, chemokine and adhesion molecule gene expression in GL261 tumors treated as in Fig. 1. Tumor samples were the same as in Fig. 2. Error bars, mean expression values ± SEM, for n = 4 to 8 tumors per time point.
3.4 Impact of longer drug-free breaks on anti-tumor activity
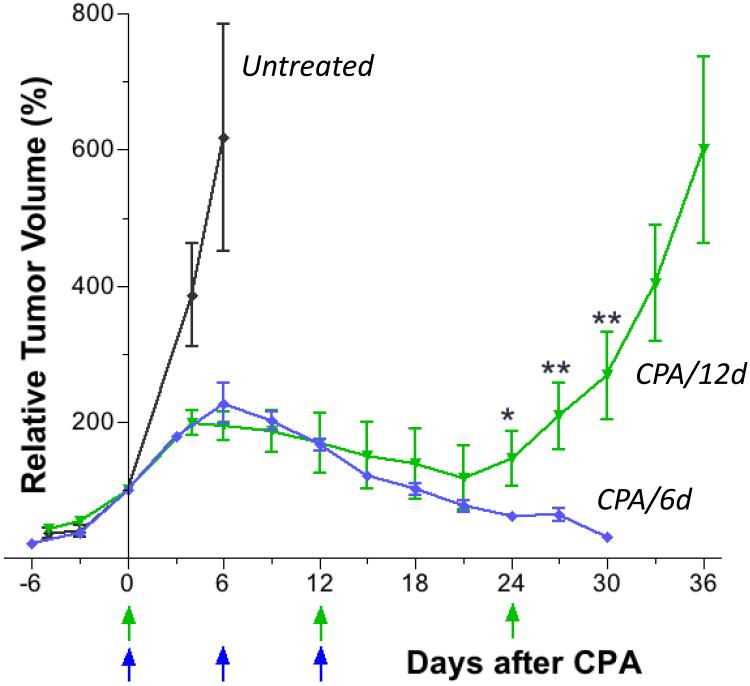
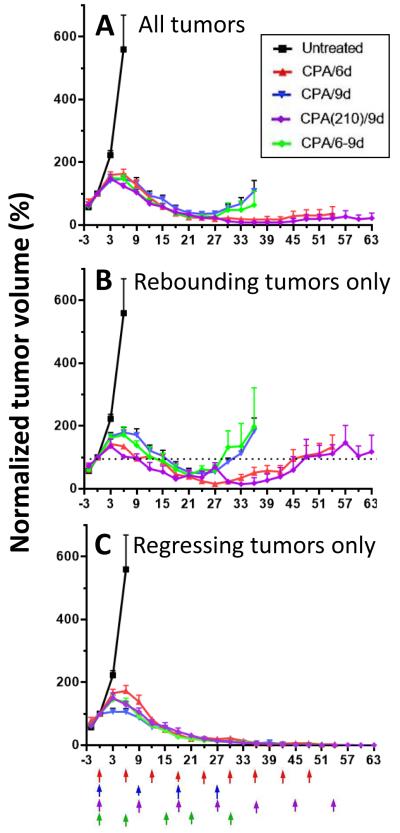
Based on the findings above, we investigated the efficacy of metronomic CPA treatment on an every 12-day schedule (CPA/12d). CPA/12d treatment induced tumor regression over an 18 to 21 day period that was indistinguishable from that of the CPA/6d schedule. However, by the third CPA treatment, on day 24, the ability of CPA to sustain tumor regression was lost (Fig. 4) and a rapid rebound in tumor growth was seen in 12 out of 14 tumors (Table 2).
Fig. 4.
Growth curves for GL261 tumors in mice given a total of 3 CPA injections on the CPA/12d schedule. Data are directly compared to the growth curve for 3 cycles of CPA/6d treatment, reproduced from Fig. 1, to highlight the divergence of responses beginning on day 24. Data shown are normalized tumor volumes, mean ± SEM, n = 8 for tumors in the CPA/6d group, n = 9 for tumors in the CPA/12d group. Tumor volume on the first day of CPA/12d treatment (day 0) = 610 + 130 mm3 (100%). Normalized tumor volumes in the CPA/12d group on days 24, 27, and 30 were significantly larger than in the CPA/6d group (*, p<0.05; **, p<0.01, by one-tailed t-test).
Table 2.
Impact of different metronomic CPA schedules on the number of rebounding and regressing tumors, the frequency of tumor rebound, and the rate of growth of rebounding tumors. Data shown are based on growth curves presented in Fig. 5. Rebounding tumor growth rates were estimated from Fig. 5B based on the number of days required for regressing tumors to return to the mean initial tumor volume prior to CPA treatment (day 0). Tumors in the CPA/6d and CPA(210)/9d groups were combined for these analyses; both groups were treated with the same CPA dose calculated on a per day basis, and they exhibited similar tumor rebound growth frequency (18-25%; mean value for combined group 21%) and rebound growth rate (45-48 days for rebound to initial tumor volume).
| CPA schedule | Rebounding tumors (number) |
Regressing tumors (number) |
p-value a | Rebound frequency % |
Time to rebound to initial tumor volume (number of days) |
|---|---|---|---|---|---|
| CPA/12d | 12 | 2 | 0.00030 b | 85.7 | 21-24 |
| CPA/9d | 8 | 6 | 0.033 | 57.6 | 27-33 |
| CPA/6d-9d | 3 | 7 | NS | 30 | 27-33 |
| CPA/6d + CPA(210)/9d |
4 | 15 | -- | 21.1 | 45-48 |
: p-value obtained by Fisher’s exact test comparing each group to the combined CPA/6d and CPA(210)/9d group. NS, not significant.
: p=0.008 and p=0.0011 for separate comparisons to the CPA/6d and CPA(210)/9d treatment groups, respectively.
Next, we considered three alternative schedules: CPA/9d (every 9 day dosing), CPA/6-9d (alternate between every 6 day and every 9 day CPA dosing), and CPA(210)/9d (every 9 day dosing, with an increase in the CPA dose from 140 to 210 mg/kg per injection). The CPA(210)/9d schedule delivers the same total CPA dose over time as the CPA/6d schedule when calculated on a per day basis. Fig. 5A shows that CPA/9d and CPA/6-9d treatment induced GL261 tumor regression comparable to the CPA/6d or CPA(210)/9d schedules until day 24, at which time several of the CPA/9d and CPA/6-9d treated tumors began to regrow rapidly (Fig. 5B). The CPA/6d and CPA(210)/9d schedules were significantly more efficacious than CPA/9d treatment (for both schedules, p<0.05 on days 30, 33, and 36; Fig. 5A). Schedule-dependent patterns of response were also evident when only the rebounding tumors were considered (Fig. 5B). Whereas the tumors treated on the CPA/6-9d and CPA/9d schedules rapidly regrew to their tumor volume prior to drug treatment (i.e., tumor volume on day 0; dashed line, Fig. 5B), the CPA/6d and CPA(210)/9d rebounding tumors required substantially longer time to return to their initial size (Fig. 5B and Table 2). No major difference between schedules was seen in the tumor regression curves when considering only the regressing tumors (Fig. 5C). However, the frequency of tumor rebound was substantially greater with the CPA/12d and CPA/9d schedules (Table 2; Supplemental Fig. S2). Thus, increasing the CPA treatment interval to longer than 6 days without a change in CPA dose (i.e. CPA/6-9d, CPA/9d, and CPA/12d schedules) does not alter the kinetics or the extent of tumor regression through day 24, but increases both the rate and the frequency of the subsequent rebound in tumor growth. Examination of changes in normalized mouse body weight as a marker for host toxicity did not reveal significant differences between the CPA schedules (Supplemental Fig. S3). In particular, body weights in the CPA/6d or CPA(210)/9d groups were stable through the end of each study, indicating that CPA is well tolerated on both schedules.
Fig. 5.
Growth curves for GL261 tumors in mice treated with CPA on four different metronomic schedules. Data shown are normalized tumor volumes, mean ± SEM. (A) Data shown are for all tumors included in the study: n = 4, 8, 14, 11, and 10 tumors for the untreated, CPA/6d, CPA/9d, CPA(210)/9d, and CPA/6-9d treatment groups, respectively. Normalized tumor volumes in the CPA/9d treatment group were significantly greater than in the CPA/6d and CPA(210)/9d treatment groups on days 30, 33, and 36 (p<0.05 by one tailed t-test). (B) Data are shown for untreated tumors (n=4) and for regrowing tumors only: n = 2, 8, 2, and 3, for CPA/6d, CPA/9d, CPA(210)/9d, and CAP/6-9d, respectively. Normalized tumor volumes in CPA/9d group were significantly greater than those in the CPA/6d group on days 27 and 30, and those in the CPA(210)/9d group on days 30, 33, and 36, respectively (p<0.05 by one tailed t-test). (C) Data are shown for untreated tumors (n=4) and for long-term regressing tumors only: n = 6, 6, 9, and 7, for CPA/6d, CPA/9d, CPA(210)/9d, and CAP/6-9d, respectively.
3.5 NK cell activation is maximally sustained by the CPA/6d schedule
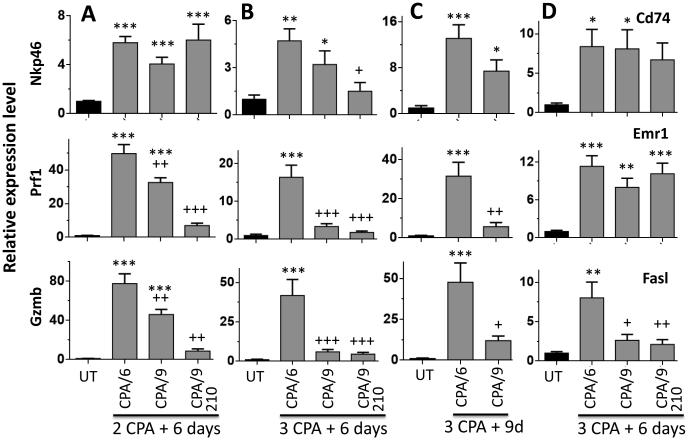
The NK cell responses stimulated by each metronomic CPA schedule were investigated to help understand the distinct regression profiles of each schedule. NK cell markers were analyzed in tumors excised 6 days after the second CPA injection (Fig. 5A: day 12 for CPA/6d; day 15 for CPA/9d and CPA(210)/9d), at which times all of the tumors in each group were actively regressing. The NK cell marker Nkp46 was significantly increased in all three CPA treatment groups (Fig. 6A). However, CPA induction of the NK cell activation markers Prf1 and Gzmb was significantly lower on the CPA/9d and CPA(210)/9d schedules than with CPA/6d treatment (Fig. 6A). This suggests that while all three schedules induce strong NK cell tumor recruitment, the CPA/6d schedule is significantly more efficacious at NK cell activation. This finding was confirmed in separate sets of analyses of NK cell activation markers 6 days and 9 days after three injections on each of metronomic CPA schedule. Again, induction of the NK cell activity markers Prf1 and Gzmb was significantly lower in tumors treated on the CPA/9d and CPA(210)/9d schedules compared to the CPA/6d schedule (Fig. 6B, 6C). A third NK cell activation marker, Fasl, showed the same schedule-dependent expression pattern as Prf1 and Gzmb (Fig. 6D). In contrast, dendritic cell marker Cd74 and macrophage marker Emr1 did not show differential expression between the CPA/6d, CPA/9d, and CPA(210)/9d treated tumors (Fig. 6D). Thus, NK cell activation is particularly sensitive to the metronomic schedule employed. In the case of the CPA(210)/9d schedule, the induction of Nkp46 seen after two treatment cycles (Fig. 6A) was not sustained through the third cycle (Fig. 6B), consistent with the sensitivity of NK cells to the higher circulating drug levels under this schedule.
Fig. 6.
NK cell recruitment and NK cell activation status in metronomic CPA-treated GL261 tumors. qPCR analysis of the indicated marker genes in tumors collected 6 days after two CPA injections (A), 6 days after three CPA injections (B), or 9 days after three injections (C) given on the indicated schedules: CPA/6d, CPA/9d and CPA(210)/9d. Data shown are relative expression levels, mean ± SEM: panel A, n = 18 (untreated), n = 20 (CPA/6d), n = 24 (CPA/9d), and n = 4 (CPA(210)/9d); panels B and C, n = 5 to 8 tumors per group. Significance when compared to untreated tumors: *, p<0.05; **, p<0.01; ***, p<0.001 (one-way ANOVA). Significance when compared to CPA/6d treatment: +, p<0.05; ++, p<0.01; +++, p<0.001 (one-way ANOVA for comparisons involving 3 or more groups; two-tailed t-test for comparisons limited to 2 groups).
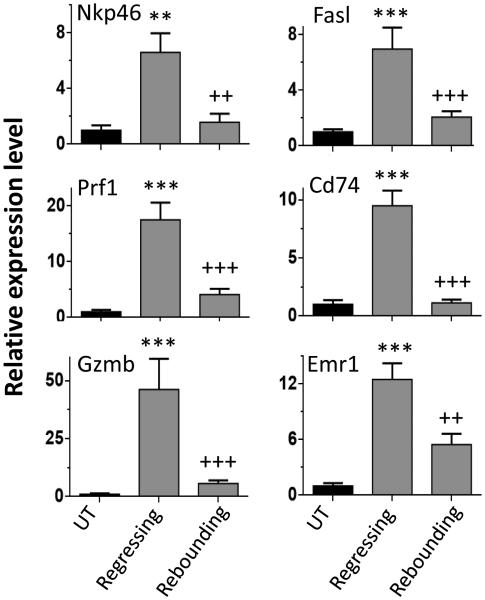
Finally, we compared immune responses between regressing tumors and rebounding tumors (Fig. 7). The expression of all of the innate immune cell markers examined, including Nkp46, Prf1, Gzmb, Cd74, Emr1, Fasl, was significantly lower in the rebounding tumors than in the regressing tumors (Fig. 7). Similar immune response profiles were seen 12 days after three CPA injections on the CPA/12d schedule (data not shown), at which point the tumors are rapidly regrowing (Fig. 4).
Fig. 7.
NK cell marker Nkp46, NK cell activation markers Prf1, Gzmb, and Fasl, dendritic cell marker Cd74, and macrophage marker Emr1 in regressing GL261 tumors compared to rebounding GL261 tumors. Data shown are relative expression levels normalized to the mean values for untreated tumors, mean ± SEM. n = 4 for untreated tumors, n = 5 regressing tumors, and n=18 rebounding tumors, as in Fig. 5B. Significance when compared to untreated tumors: *, p<0.05; **, p<0.01; ***, p<0.001. Significance when comparing regressing tumors vs. rebounding tumors: +, p<0.05; ++, p<0.01; +++, p<0.001 (one-way ANOVA).
4. Discussion
There is increasing clinical interest in metronomic chemotherapy as an alternative to traditional maximum tolerated dose cancer chemotherapy, however, there is little understanding of how the choice of metronomic schedule, in particular the dosing interval, impacts anti-tumor responses. Previous studies have shown that metronomic CPA treatment on an every 6-day schedule (CPA/6d) induces regression of large implanted gliomas by a mechanism that is at least in part dependent on tumor recruitment of anti-tumor NK cells [21]. Immune responses are substantially reduced and glioma regression is abolished by a moderate reduction of CPA dose, or by metronomic administration of CPA at more frequent intervals, including low-dose daily CPA scheduling [22]. Here, our investigation of the time-dependent changes in tumor-infiltrating NK cell, dendritic cell and macrophage markers revealed that the CPA/6d schedule is preferentially toxic to NK cells recruited to the treated tumors. Moreover, all of the immune cell and NK cell activation markers examined peaked on day 12, rather than on day 6 after each CPA treatment, as did several cytokines, chemokines and adhesion molecules that favor immune cell recruitment and activation. Tumor responses peaked much earlier for the chemokines Cxcl9, Cxcl10, and Cxcl11, whose induction by CPA does not correlate with anti-tumor immune responses and tumor regression [22]. In contrast, expression of the pro-angiogenic tumor factor VEGFA decreased progressively with each cycle of CPA treatment through at least day 24. These findings led us to compare the CPA/6d schedule to four metronomic schedules with longer intervals between CPA treatments, namely, CPA/12d, CPA/9d, CPA/6-9d, and CPA(210)/9d schedules.
We found that all five metronomic CPA schedules initially drive tumor regression with similar kinetics and to a similar maximal extent, even though the total dose of CPA administered over the first 21 days is substantially lower with the CPA/12d, CPA/9d and CPA/6-9d schedules than with the CPA/6d and CPA(210)/9d schedules. However, after day 24, many of the tumors treated on the CPA/12d, CPA/9d, and CPA/6-9d schedules showed robust regrowth, despite continued CPA treatment. In contrast, a large majority of the tumors treated on the CPA/6d and CPA(210)/9d schedules remained regressed long-term. The rapid rebound of tumors treated on the CPA/6-9d schedule, which has only one 9-day drug-free interval through day 24 (Fig. 5B), highlights the importance of the duration of the drug-free interval. We conclude that the efficacy of metronomic CPA treatment in this model is not dependent on the extent of initial tumor regression, which was similar for all five metronomic CPA schedules, but rather is dependent on the effectiveness of each schedule at inducing a strong and prolonged anti-tumor immune response. In particular, the ineffectiveness of every 9-day and every 12-day metronomic CPA schedules at sustaining tumor regression is associated with the inability of those schedules to sustain strong NK cell activity (Fig. 6). This, in turn, may facilitate the emergence of resistant tumor cell populations leading to the tumor relapse frequently seen after day 24.
Increasing the dose of CPA given on the every 9 day schedule from 140 to 210 mg/kg-BW (i.e., CPA/9d versus CPA(210)/9d treatment) did not augment immune responses, but nevertheless, was effective at reducing the frequency of tumor regrowth. NK cell activation markers were decreased in the CPA(210)/9d treated tumors, which may result from weak dendritic cell mobilization at high doses of CPA, which can decrease tumor NK cell activation [49; 50]. Alternatively, there might be increased DNA repair during drug-free intervals longer than 6 days, which may promote selection of resistance to chemotherapy and reduce tumor cell sensitivity to subsequent CPA injections. In this scenario, increasing the CPA dose per injection on an every 9-day schedule, as in the CPA(210)/9d treatment, may result in strong CPA cytotoxicity despite the longer drug-free break. These findings demonstrate that increasing the dose of CPA can at least in part compensate for the decreased anti-tumor immune response of the 9-day repeating CPA schedule. Increasing the CPA dose used in metronomic CPA regimens may be one option to improve efficacy, in particular in immune-deficient or immune-compromised patients.
NK cell marker levels are closely correlated with the extent of tumor regression induced by metronomic CPA treatment [22]. Here, we found a close association between metronomic CPA schedules with a 9-day or 12-day drug-free interval and a decrease in NK cell activation marker expression leading to rapid tumor regrowth after treatment day 24. A drug-free interval longer than 6 days could favor the expression of immunosuppressive factors compared to immune-stimulatory factors. Moreover, host immune reservoirs may become exhausted and immune resistance mutants may be selected by metronomic CPA treatment for prolonged periods of time [22]. Reduced NK cell recruitment was also seen in CPA(210)/9d-treated tumors, where the elevated CPA dose was able to compensate for the reduced innate immune response. Resistance to metronomic CPA has been associated with dormant stem-cell foci in hepatocellular carcinoma [51], ischemia-dependent K-ras mutations in colorectal carcinoma [52] and increased annexin A3 expression in prostate cancer [53; 54]. Different resistance mechanisms may be activated in different tumor models and by different CPA doses and schedules [55; 56]; these mechanisms could include immune-based resistance, as well as repopulation of quiescent tumor cells that are distal of blood vessel and deprived of oxygen and nutrients, selection of tumor cell populations with increased drug efflux and detoxification ability, increased DNA damage repair between metronomic CPA treatments, metabolic adaptation, and emergence of resistance to apoptosis [57; 58]. In preliminary studies of regressing GL261 tumors, we have seen ~2-fold higher expression of ALDH1A1 six days after two CPA injections on the CPA/9d compared to the CPA/6d schedule (unpublished results); ALDH1A1 inactivates CPA-derived aldophosphamide and can confer CPA resistance [59].
In summary, the innate immune stimulatory CPA/6d schedule described previously [21] is shown to be more efficacious than intermittent metronomic CPA schedules having longer drug-free breaks. CPA schedules with 9 day or 12 day drug-free intervals induced major regression of implanted gliomas through treatment day 24 in a manner very similar to the CPA/6d schedule, but this initial response period was followed by rapid tumor regrowth and a high frequency of rebounding tumors showing significantly reduced levels of NK cell activation. These findings complement our recent studies showing that more frequent CPA scheduling – either daily low dose or every 3 day CPA treatment with the same net drug exposure as the every 6 day schedule – is ineffective with regard to induction of robust NK cell recruitment and tumor regression [22]. Overall, these findings are consistent with the empirical finding of Browder et al that an every 6-day metronomic CPA schedule is more active than every 3, 4, 5, 7, or 8-day CPA scheduling in a CPA-resistant Lewis lung carcinoma model [7]. Additional studies are needed to establish the effectiveness of every 6-day metronomic CPA treatment in orthotopic glioma models, and in brain cancer patients, where metronomic CPA may potentially be combined with current standard of care treatments, such as temozolomide, as an associated immunotherapy. Further, our finding that increasing the CPA dose in the 9-day schedule substantially prolongs tumor regression suggests that while low dose metronomic drug treatment can be effective at inducing immune-based tumor regression, increases in drug doses may sometimes be needed for metronomic therapy to reduce the potential for relapse. Finally, further investigation is required to elucidate the mechanisms leading to the emergence of resistance to immunogenic metronomic scheduling, as well as to translate these findings to improve the efficacy of CPA-based metronomic immunotherapies in the clinic.
Supplementary Material
Highlights.
Five metronomic drug schedules are compared for immune response and tumor regression
Tumor natural killer (NK) cells are highly sensitive to metronomic dosing interval
Drug-free breaks > 6-days do not sustain NK cell activation, leading to tumor escape
Long-term remission requires a strong, sustained anti-tumor innate immune response
Increasing the metronomic drug dose may compensate for decreased NK cell responses
Acknowledgements
Supported in part by NIH grant CA49248 (to DJW).
Abbreviations
- BW
body weight
- CPA
cyclophosphamide
- CPA/6d, CPA/9d, and CPA/12d
CPA treatment every 6, 9 and 12 days, respectively
- qPCR
quantitative real-time polymerase chain reaction
- NK cell
natural killer cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
References
- [1].Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- [2].Crawford S. Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front Pharmacol. 2013;4:68. doi: 10.3389/fphar.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- [4].Noronha V, Krishna MV, Patil V, Joshi A, Banavali SD, Prabhash K. Metronomic therapy: chemotherapy revisited. Indian J Cancer. 2013;50:142–148. doi: 10.4103/0019-509X.117027. [DOI] [PubMed] [Google Scholar]
- [5].Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- [6].Hahnfeldt P, Hlatky L, Klement GL. Center of cancer systems biology second annual workshop--tumor metronomics: timing and dose level dynamics. Cancer Res. 2013;73:2949–2954. doi: 10.1158/0008-5472.CAN-12-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- [8].Emmenegger U, Man S, Shaked Y, Francia G, Wong JW, Hicklin DJ, Kerbel RS. A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004;64:3994–4000. doi: 10.1158/0008-5472.CAN-04-0580. [DOI] [PubMed] [Google Scholar]
- [9].Pasquier E, Tuset MP, Street J, Sinnappan S, MacKenzie KL, Braguer D, Andre N, Kavallaris M. Concentration- and schedule-dependent effects of chemotherapy on the angiogenic potential and drug sensitivity of vascular endothelial cells. Angiogenesis. 2013;16:373–386. doi: 10.1007/s10456-012-9321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- [12].Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- [13].Malik PS, Raina V, Andre N. Metronomics as Maintenance Treatment in Oncology: Time for Chemo-Switch. Front Oncol. 2014;4:76. doi: 10.3389/fonc.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49:3387–3395. doi: 10.1016/j.ejca.2013.06.038. [DOI] [PubMed] [Google Scholar]
- [15].Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- [16].Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, Crawford HC, Zong WX. DNA alkylating therapy induces tumor regression through an HMGB1-mediated activation of innate immunity. J Immunol. 2011;186:3517–3526. doi: 10.4049/jimmunol.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maglioco A, Machuca D, Mundinano J, Cabrera G, Camicia G, Bruzzo J, Camerano G, Costa H, Ruggiero RA, Dran GI. Lymphadenectomy exacerbates tumor growth while lymphadenectomy plus the adoptive transfer of autologous cytotoxic cells and low-dose cyclophosphamide induces regression of an established murine fibrosarcoma. Cancer Immunol Immunother. 2011;60:389–399. doi: 10.1007/s00262-010-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sharabi A, Laronne-Bar-On A, Meshorer A, Haran-Ghera N. Chemoimmunotherapy reduces the progression of multiple myeloma in a mouse model. Cancer Prev Res (Phila) 2010;3:1265–1276. doi: 10.1158/1940-6207.CAPR-10-0138. [DOI] [PubMed] [Google Scholar]
- [19].Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010;115:2397–2406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S, Larma I, Prosser A, Robinson BW, Smyth MJ, Scalzo AA, Degli-Esposti MA, Lake RA. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72:1103–1115. doi: 10.1158/0008-5472.CAN-11-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen CS, Doloff JC, Waxman DJ. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84–96. doi: 10.1593/neo.131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schlick E, Ruffmann R, Chirigos MA, Welker RD, Herberman RB. In vivo modulation of myelopoiesis and immune functions by maleic anhydride divinyl ether copolymer (MVE-2) in tumor-free and MBL-2 tumor-bearing mice treated with cyclophosphamide. Cancer Res. 1985;45:1108–1114. [PubMed] [Google Scholar]
- [24].Mackova N, Suliova J. Repair processes of hemopoiesis after applying cyclophosphamide. I. Morphological changes in the bone marrow, spleen and thymus. Folia Haematol Int Mag Klin Morphol Blutforsch. 1986;113:596–604. [PubMed] [Google Scholar]
- [25].Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Doloff JC, Chen CS, Waxman DJ. Anti-tumor innate immunity activated by intermittent metronomic cyclophosphamide treatment of 9L brain tumor xenografts is preserved by anti-angiogenic drugs that spare VEGF receptor 2. Mol Cancer. 2014;13:158. doi: 10.1186/1476-4598-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shih WW, Baumhefner RW, Tourtellotte WW, Haskell CM, Korn EL, Fahey JL. Difference in effect of single immunosuppressive agents (cyclophosphamide, CCNU, 5-FU) on peripheral blood immune cell parameters and central nervous system immunoglobulin synthesis rate in patients with multiple sclerosis. Clin Exp Immunol. 1983;53:122–132. [PMC free article] [PubMed] [Google Scholar]
- [28].Sakurai T, Misawa E, Yamada M, Hayasawa H, Motoyoshi K. Effects of macrophage-colony-stimulating factor on cyclophosphamide-injected mouse NK1.1+ cell activity. Cancer Immunol Immunother. 2000;49:94–100. doi: 10.1007/s002620050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sefc L, Psenak O, Sykora V, Sulc K, Necas E. Response of hematopoiesis to cyclophosphamide follows highly specific patterns in bone marrow and spleen. J Hematother Stem Cell Res. 2003;12:47–61. doi: 10.1089/152581603321210136. [DOI] [PubMed] [Google Scholar]
- [30].Mantovani A, Luini W, Candiani GP, Spreafico F. Effect of chemotherapeutic agents on natural and BCG-stimulated macrophage cytotoxicity in mice. Int J Immunopharmacol. 1980;2:333–339. doi: 10.1016/0192-0561(80)90033-8. [DOI] [PubMed] [Google Scholar]
- [31].Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- [32].Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- [33].Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996;157:2909–2915. [PubMed] [Google Scholar]
- [36].Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88:463–473. doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- [38].Hong X, Dong T, Hu J, Yi T, Li W, Zhang Z, Lin S, Niu W. Synergistical toll-like receptors activated dendritic cells induce antitumor effects against carcinoembryonic antigen-expressing colon cancer. Int J Colorectal Dis. 2013;28:25–33. doi: 10.1007/s00384-012-1530-7. [DOI] [PubMed] [Google Scholar]
- [39].Tel J, Sittig SP, Blom RA, Cruz LJ, Schreibelt G, Figdor CG, de Vries IJ. Targeting uptake receptors on human plasmacytoid dendritic cells triggers antigen cross-presentation and robust type I IFN secretion. J Immunol. 2013;191:5005–5012. doi: 10.4049/jimmunol.1300787. [DOI] [PubMed] [Google Scholar]
- [40].Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274:168–170. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- [41].Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- [42].Radi ZA, Kehrli ME, Jr., Ackermann MR. Cell adhesion molecules, leukocyte trafficking, and strategies to reduce leukocyte infiltration. J Vet Intern Med. 2001;15:516–529. doi: 10.1892/0891-6640(2001)015<0516:camlta>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- [43].Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- [44].Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- [45].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- [46].Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- [47].Johnson BF, Clay TM, Hobeika AC, Lyerly HK, Morse MA. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Expert Opin Biol Ther. 2007;7:449–460. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- [48].Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- [49].Ferrari S, Rovati B, Porta C, Alessandrino PE, Bertolini A, Collova E, Riccardi A, Danova M. Lack of dendritic cell mobilization into the peripheral blood of cancer patients following standard- or high-dose chemotherapy plus granulocyte-colony stimulating factor. Cancer Immunol Immunother. 2003;52:359–366. doi: 10.1007/s00262-002-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, Luznik L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59:137–148. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Martin-Padura I, Marighetti P, Agliano A, Colombo F, Larzabal L, Redrado M, Bleau AM, Prior C, Bertolini F, Calvo A. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Invest. 2012;92:952–966. doi: 10.1038/labinvest.2012.65. [DOI] [PubMed] [Google Scholar]
- [52].Shahrzad S, Shirasawa S, Sasazuki T, Rak JW, Coomber BL. Low-dose metronomic cyclophosphamide treatment mediates ischemia-dependent K-ras mutation in colorectal carcinoma xenografts. Oncogene. 2008;27:3729–3738. doi: 10.1038/sj.onc.1211031. [DOI] [PubMed] [Google Scholar]
- [53].Kubisch R, Meissner L, Krebs S, Blum H, Gunther M, Roidl A, Wagner E. A Comprehensive Gene Expression Analysis of Resistance Formation upon Metronomic Cyclophosphamide Therapy. Transl Oncol. 2013;6:1–9. doi: 10.1593/tlo.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Thoenes L, Hoehn M, Kashirin R, Ogris M, Arnold GJ, Wagner E, Guenther M. In vivo chemoresistance of prostate cancer in metronomic cyclophosphamide therapy. J Proteomics. 2010;73:1342–1354. doi: 10.1016/j.jprot.2010.02.019. [DOI] [PubMed] [Google Scholar]
- [55].Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, Kerbel RS. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia. 2011;13:40–48. doi: 10.1593/neo.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL, Engelman JA. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- [58].O'Connor R. A review of mechanisms of circumvention and modulation of chemotherapeutic drug resistance. Curr Cancer Drug Targets. 2009;9:273–280. doi: 10.2174/156800909788166583. [DOI] [PubMed] [Google Scholar]
- [59].Moreb JS, Mohuczy D, Ostmark B, Zucali JR. RNAi-mediated knockdown of aldehyde dehydrogenase class-1A1 and class-3A1 is specific and reveals that each contributes equally to the resistance against 4-hydroperoxycyclophosphamide. Cancer Chemother Pharmacol. 2007;59:127–136. doi: 10.1007/s00280-006-0233-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.