Abstract
Multidrug-resistant (MDR) Enterobacteriaceae infections are associated with increased morbidity. We describe a 20-year-old hematopoietic cell transplantation recipient with recurrent MDR Klebsiella pneumoniae infection, prolonged intestinal colonization, and subsequent intestinal decontamination. Further study should evaluate stool surveillance, molecular typing, and fecal microbiota transplantation for patients with intestinal MDR Enterobacteriaceae carriage.
Introduction
Intestinal carriage of multidrug-resistant (MDR) Enterobacteriaceae has been increasingly reported among patients with both community- and healthcare-associated infections. Broad-spectrum antibiotic exposure is a well-documented risk factor for the subsequent identification of resistant isolates (de Lastours et al., 2012, Gijon et al., 2012, Han et al., 2012, 2013). An estimated 10-50% of patients colonized with MDR Enterobacteriaceae subsequently develop infection (Calfee and Jenkins, 2008, Tzouvelekis et al., 2012); receipt of antipseudomonal penicillins among colonized patients is associated with developing infection (Borer et al., 2012). Specifically, receipt of a solid organ or hematopoietic cell transplantation (HCT) is associated with higher risk of carbapenem-resistant Klebsiella pneumoniae (CRKP) infection, and CRKP infection is furthermore associated with increased in-hospital mortality (Patel et al., 2008).
While MDR Enterobacteriaceae infections are challenging to treat, even CRKP infections have been successfully treated using combination antibiotic regimens such as colistin plus tigecycline (Humphries et al., 2010). Selective intestinal decontamination using enteral aminoglycosides, alone or in combination with colistin, may eradicate CRKP carriage (Saidel-Odes et al., 2012, Zuckerman et al., 2011).
We present a 20 year-old immunocompromised male with persistent colonization and recurrent infection with MDR K. pneumoniae, who underwent successful eradication of MDR Klebsiella intestinal carriage. The Seattle Children’s Hospital Institutional Review Board approved this report.
Case report
A 20-year-old male was diagnosed with high-risk acute lymphoblastic leukemia and received four days of empiric meropenem for neutropenic fever (Figure 1). One month later, he developed a superficial paraspinous infection with MDR, but carbapenem-susceptible K. pneumoniae and received 16 days of meropenem. Subsequent surveillance stool cultures revealed presence and persistence of MDR K. pneumoniae, including two K. pneumoniae colony morphologies with distinct antibiotic resistance phenotypes (Figure 1). No antibiotic therapy targeting these isolates was administered.
Figure 1.
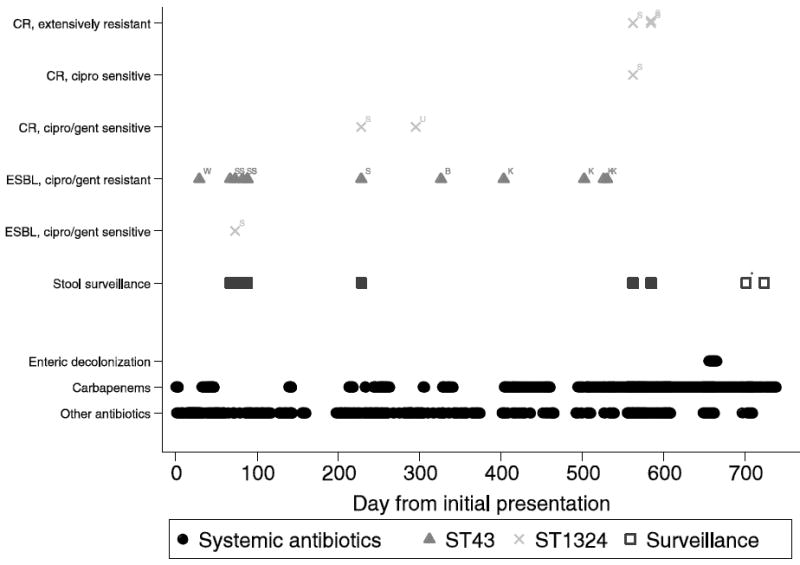
Timeline of Antibiotic Exposures and K. pneumoniae Clones by Resistance and Body Site, through day 750.
B – blood, CR – carbapenem resistance phenotype, ESBL – Extended-spectrum beta-lactam resistance phenotype, K – knee, S – stool, U – urine, W – wound. Solid squares represent surveillance stool cultures positive for resistant K. pneumoniae; open squares represent surveillance stool cultures negative for resistant K. pneumoniae. Resistance profiles for each surveillance isolate are provided above the surveillance isolates, by clone.
* - This surveillance culture was negative for resistant K. pneumoniae but positive for carbapenem-resistant E. coli.
On day 192 after initial clinical presentation with leukemia, the patient received a matched, unrelated donor HCT. Surveillance stool culture on day 228 again demonstrated two K. pneumoniae phenotypes, one carbapenem-susceptible and one carbapenem-resistant. On day 295 the patient received three days of empiric imipenem for a urinary tract infection ultimately found to be caused by CRKP, and on day 326 he received 14 days of meropenem for carbapenem-susceptible K. pneumoniae bacteremia. He developed carbapenem-susceptible K. pneumoniae left knee septic arthritis and received carbapenem therapy from days 403 to 460. His knee pain recurred on day 494 and carbapenem therapy was restarted; diagnostic knee tissue and joint fluid sampling on days 526 and 533 again demonstrated carbapenem-susceptible K. pneumoniae. The patient developed chronic osteomyelitis, and received 6 total months of therapy with meropenem, amikacin (first 3 weeks only), and tigecycline (first 15 weeks only). Extensive local tissue destruction necessitated total knee arthroplasty on day 696. Intervening surveillance stool cultures (days 562 and 584) revealed growth of CRKP sensitive only to colistin and amikacin.
To prevent further infectious complications associated with CRKP intestinal carriage, the patient received an intestinal decontamination regimen including oral colistin and amikacin between days 655 and 665. Follow-up surveillance stool cultures for MDR and CRKP were performed at 36 and 58 days after intestinal decontamination (days 701 and 723) and were negative. However, the day 701 (but not day 723) surveillance culture demonstrated carbapenem-resistant, carbapenemase-negative E. coli. The patient’s HCT has remained successful without leukemia relapse.
Post hoc K. pneumoniae isolate molecular typing was performed using MLST and PFGE (Brisse et al., 2009, Tenover et al., 1995). Next, monoplex PCR was carried out for detection of bla genes encoding common carbapenemases (KPC, IMP, NDM, VIM, and OXA-48), common extended-spectrum cephalosporinases (CTX-M, SHV-12, CMY and DHA), and the K. pneumoniae-intrinsic narrow-spectrum ampicillinases SHV-1 and SHV-11. Then, PCR amplification and sequencing of the major outer membrane porin-encoding loci, ompK35 and ompK36, was performed (Supplemental Table 1).
Molecular typing revealed two ST profiles (Figure 2): ST43, encompassing the index wound isolate, and isolates from blood, knee and stool, which remained carbapenem-susceptible (Figure 1, clone A); and ST1324, encompassing the urine isolate and multiple stool isolates, which became carbapenem-resistant over the surveillance period (Figure 1, clone B). Discrete major outer membrane porin locus lesions occurred serially among the ST1324 isolates, first in ompK36 (insertion of IS5 in the promoter region, detected day 228 [data not shown]), concurrent with development of carbapenem non-susceptibility, and subsequently in ompK35 (truncation, detected day 562). Of interest, two pulsotypes were observed among the ST1324 isolates, corresponding to earlier and later periods of the clinical course (days 73-295 and days 562-584, respectively).
Figure 2.
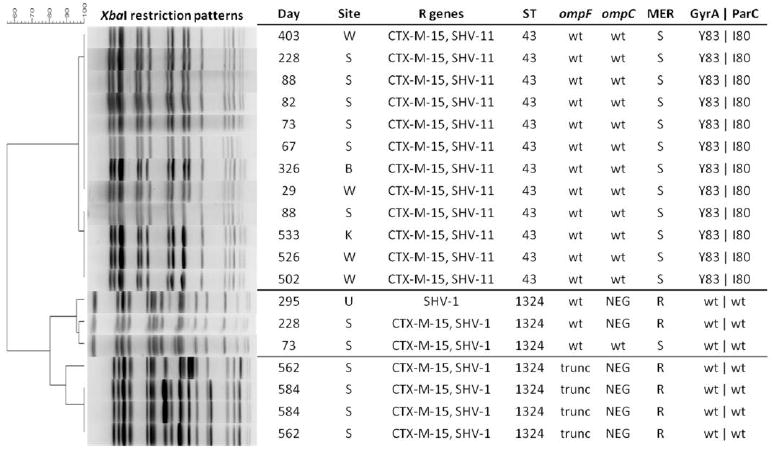
Clinical and molecular features of sterile and non-sterile site isolates of K. pneumoniae recovered from a single patient.
Day, day of clinical course (as described in text). Site, clinical specimen: W, wound; S, stool; B, blood; K, knee; U, urine. R genes, resistance genes (includes plasmid-associated cephalosporinases and chromosome-associated ampicillinases). ST, sequence type. wt, wild type proteins or residues. For GyrA, ParC, wild type amino acid residues in Quinolone Resistance Determining Regions are S83 (GyrA) and S80 (ParC). NEG, negative by PCR. trunc, truncated due to sequence polymorphism. MER, meropenem susceptibility. S, susceptible. R, resistant.
Discussion
We present the successful intestinal decontamination of MDR K. pneumoniae in an immunosuppressed patient using combination colistin and amikacin and document a series of resistance-conferring porin mutations that occurred under antibiotic selection pressure. This patient’s antimicrobial therapy likely promoted overgrowth of two MDR K. pneumoniae clones, one exhibiting invasive capacity and the other a capacity for developing antibiotic resistance in situ. While intestinal decontamination eradicated MDR K. pneumoniae carriage in this immunocompromised HCT patient, we hypothesize the still-damaged intestinal microbiota allowed for overgrowth and intestinal carriage of carbapenem-resistant E. coli.
The combination of an aminoglycoside and a polymyxin as selective digestive decontamination has been shown to reduce rectal carriage of CRKP up to 6 weeks after treatment in a randomized study, and eradication of CRKP using oral gentamicin has been shown in the HCT population (Saidel-Odes et al., 2012, Zuckerman et al., 2011). Likewise, a combination of enteral tobramycin, colistin, and amphotericin B can decrease rectal colonization with cephalosporin- and aminoglycoside-resistant Enterobacteriaceae in an intensive care unit setting (Oostdijk et al., 2012). Systematic surveillance for MDR Enterobacteriaceae among high-risk patients might identify those who could benefit from decontamination, and early decontamination in our patient might have averted subsequent infections.
While decontamination may be successful, the results are often short-lived (Saidel-Odes et al., 2012). Success in curing refractory Clostridium difficile infection with fecal microbiota transplantation (FMT) raises the question whether FMT could be effective in patients with MDR Enterobacteriaceae carriage both in stable eradication of the carriage and in preventing subsequent, difficult-to-treat infections due to these organisms (van Nood et al., 2013). Though recent reports of FMT for recurrent C. difficile infection in solid-organ transplantation recipients exist, FMT has not yet been proven safe in severely immunocompromised hosts, and has the theoretical potential to precipitate invasive disease caused by the FMT microflora (Friedman-Moraco et al., 2014).
Surveillance stool cultures and molecular typing may help inform antibiotic therapy for at-risk patients infected or colonized by MDR Enterobacteriaceae. To manage the considerable potential risks associated with MDR Enterobacteriaceae carriage in immunocompromised patients with intestinal dysbiosis, further studies of the safety and efficacy of intestinal decontamination and FMT are warranted.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [award number R01AI083413 to DMZ and SJW]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
SJW is party to a patent application describing a point-of-care diagnostic test to optimize selection of initial antibiotics in urinary tract infection. The method remains in the developmental stage, and SJW has received no financial compensation for or benefit from the patented entity. He has also received grant funding from Pfizer to study Antimicrobial Stewardship Program effectiveness at US children’s hospitals. None of the other authors reports a significant financial conflict of interest relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Vital signs: carbapenem-resistant enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–70. [PMC free article] [PubMed] [Google Scholar]
- BORER A, SAIDEL-ODES L, ESKIRA S, NATIV R, RIESENBERG K, LIVSHIZ-RIVEN I, SCHLAEFFER F, SHERF M, PELED N. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40:421–5. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- BRISSE S, FEVRE C, PASSET V, ISSENHUTH-JEANJEAN S, TOURNEBIZE R, DIANCOURT L, GRIMONT P. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALFEE D, JENKINS SG. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect Control Hosp Epidemiol. 2008;29:966–8. doi: 10.1086/590661. [DOI] [PubMed] [Google Scholar]
- DE LASTOURS V, CAMBAU E, GUILLARD T, MARCADE G, CHAU F, FANTIN B. Diversity of individual dynamic patterns of emergence of resistance to quinolones in Escherichia coli from the fecal flora of healthy volunteers exposed to ciprofloxacin. J Infect Dis. 2012;206:1399–406. doi: 10.1093/infdis/jis511. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN-MORACO RJ, MEHTA AK, LYON GM, KRAFT CS. Fecal Microbiota Transplantation for Refractory Clostridium difficile Colitis in Solid Organ Transplant Recipients. Am J Transplant. 2014 doi: 10.1111/ajt.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIJON D, CURIAO T, BAQUERO F, COQUE TM, CANTON R. Fecal carriage of carbapenemase-producing Enterobacteriaceae: a hidden reservoir in hospitalized and nonhospitalized patients. J Clin Microbiol. 2012;50:1558–63. doi: 10.1128/JCM.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN JH, KASAHARA K, EDELSTEIN PH, BILKER WB, LAUTENBACH E. Risk factors for infection or colonization with CTX-M extended-spectrum-beta-lactamase-positive Escherichia coli. Antimicrob Agents Chemother. 2012;56:5575–80. doi: 10.1128/AAC.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHRIES RM, KELESIDIS T, DIEN BARD J, WARD KW, BHATTACHARYA D, LEWINSKI MA. Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose tigecycline and colistin. J Med Microbiol. 2010;59:1383–6. doi: 10.1099/jmm.0.023010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OOSTDIJK EA, DE SMET AM, KESECIOGLU J, BONTEN MJ. Decontamination of cephalosporin-resistant Enterobacteriaceae during selective digestive tract decontamination in intensive care units. J Antimicrob Chemother. 2012;67:2250–3. doi: 10.1093/jac/dks187. [DOI] [PubMed] [Google Scholar]
- PATEL G, HUPRIKAR S, FACTOR SH, JENKINS SG, CALFEE DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- SAIDEL-ODES L, POLACHEK H, PELED N, RIESENBERG K, SCHLAEFFER F, TRABELSI Y, ESKIRA S, YOUSEF B, SMOLYKOV R, CODISH S, BORER A. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33:14–9. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- TENOVER FC, ARBEIT RD, GOERING RV, MICKELSEN PA, MURRAY BE, PERSING DH, SWAMINATHAN B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZOUVELEKIS LS, MARKOGIANNAKIS A, PSICHOGIOU M, TASSIOS PT, DAIKOS GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN NOOD E, VRIEZE A, NIEUWDORP M, FUENTES S, ZOETENDAL EG, DE VOS WM, VISSER CE, KUIJPER EJ, BARTELSMAN JF, TIJSSEN JG, SPEELMAN P, DIJKGRAAF MG, KELLER JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN T, BENYAMINI N, SPRECHER H, FINEMAN R, FINKELSTEIN R, ROWE JM, OREN I. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011;46:1226–30. doi: 10.1038/bmt.2010.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


