Abstract
Previously, we found that transferring 6.1 Mb of SS chromosome 12 (13.4-19.5 Mb) onto the consomic SS-12BN background significantly elevated mean arterial pressure in response to an 8% NaCl diet (178±7 vs. 144±2 mmHg; P<0.001). Using congenic mapping, we have now narrowed the blood pressure locus by 86% from a 6.1 Mb region containing 133 genes to an 830 kb region (chr12:14.36-15.19 Mb) with 14 genes. Compared with the SS-12BN consomic, the 830 kb blood pressure locus was associated with a Δ+15 mmHg (P<0.01) increase in blood pressure, which coincided with elevated albuminuria (Δ+32 mg/day; P<0.001), proteinuria (Δ+48 mg/day; P<0.01), protein casting (Δ+154%; P<0.05), and renal fibrosis (Δ+79%; P<0.05). Of the 14 genes residing in the 830 kb locus, 8 were differentially expressed and among these, Chst12 (carbohydrate chondroitin 4 sulfotransferase 12) was the most consistently down-regulated by 2.6 to 4.5-fold (P<0.05) in both the renal medulla and cortex under normotensive and hypertensive conditions. Moreover, whole genome sequence analysis of overlapping blood pressure loci revealed an ∼86 kb region (chr12:14,541,567-14,627,442 bp) containing single nucleotide variants near Chst12 that are unique to the hypertensive SS strain when compared to the normotensive BN, SR, and WKY strains. Finally, the 830 kb interval is syntenic to a region on human chromosome 7 that has been genetically linked to blood pressure, suggesting that insight gained from our SS-12BN congenic strain may be translated to a better understanding of human hypertension.
Keywords: Dahl salt-sensitive, Brown Norway, hypertension, genetic, congenic
Introduction
Hypertension is a major risk factor for multiple cardiovascular diseases (e.g., stroke, atherosclerosis, renal failure, and heart failure) and contributed to 362,895 U.S. deaths in 2010.1 Multiple genetic and environmental factors contribute to hypertension risk,2 posing a significant challenge to identifying the individual risk variants that underlie human hypertension. One method to identify hypertension risk genes is by congenic mapping to narrow genomic regions that modify blood pressure (BP) and renal damage. We previously used congenic mapping to isolate a 6.1 Mb region of SS/Mcwi (Dahl salt-sensitive) rat chromosome 12 (13.4-19.5 Mb) that was associated with elevated BP (Δ+34 mmHg; P<0.001) and proteinuria (Δ+152 mg/day; P<0.001) compared with the SS-12BN consomic strain.3 This 6.1 Mb congenic interval on chromosome 12 (13.4-19.5 Mb) contained 133 candidate genes and 12,673 single nucleotide variants, of which 5 were predicted to damage to protein function according to PolyPhen.3, 4 Syntenic regions of human chromosome 7 were also linked with BP5-10 and renal diseases,11-13 suggesting that the pathogenic elements in this region might be shared across species. However, due to the large number of candidate genes in the original 6.1 Mb congenic interval, the causative gene(s) underlying these disease phenotypes within the rat and human loci are presently unknown.
In the present study, we generated and phenotyped three new congenic strains that narrowed the 6.1 Mb (chr12:13.4-19.5 Mb) candidate region by 86% to ∼830 kb (chr12:14,365,649-15,194,843 bp), which reduced the list of candidate genes to 14 and the number of single nucleotide variants to 1,585. The 830 kb candidate region (chr12:14,365,649-15,194,843 bp) has significantly increased BP and urinary protein and albumin excretion that approximates the levels observed in the larger 6.1 Mb (chr12:13.4-19.5 Mb) congenic interval.3 Expression analysis of renal medulla and cortex identified Chst12 (carbohydrate chondroitin 4 sulfotransferase 12) as a differentially expressed candidate that co-segregated in hypertensive rat strains and was suggestively linked to BP by human GWAS. Seven other genes were also differentially expressed, but lacked additional co-segregation and comparative genetic evidence. Collectively, this study has physically reduced the list of candidate genes to 14 and provides rationale for functional testing of Chst12, a novel candidate gene for BP regulation, in future studies.
Materials and Methods
Generation of SS-12BN Congenic Rats
All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin. Congenic strains were generated by backcrossing line C3 to SS-12BN/Mcwi. Genomic boundaries for the 3 new congenic strains are shown in Figure 1.
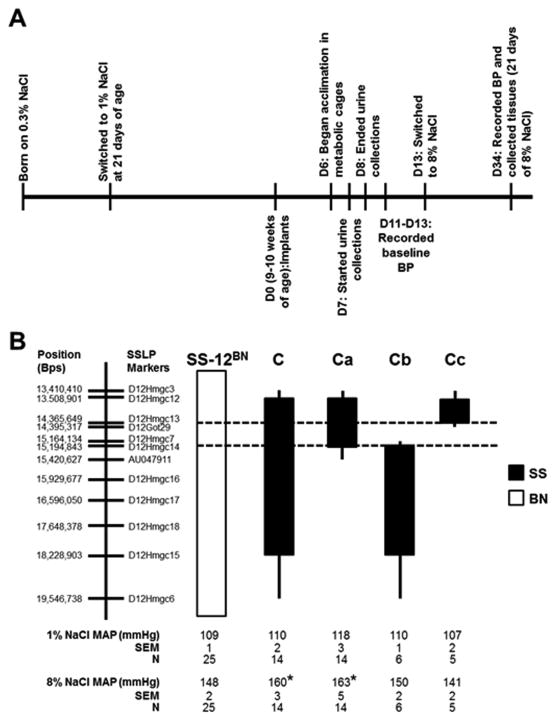
Figure 1.
Experimental timeline for diet switches, urine collection, blood pressure measurements, and tissue collections (A). D0, day of transmitter implants; D#, number of days post-implant. B, Schematic representation of the overlapping salt-sensitive (SS)-12BN congenic strains that were generated by introgressing segments of the SS chromosome 12 (black) into the genetic background of the SS-12BN consomic rat (white) by marker-assisted breeding. Mean arterial pressure (MAP) of the congenic strains while on 1% NaCl diets and stressed for 3 weeks on 8% NaCl diets. Data were analyzed by 1-way ANOVA on ranks followed by Dunn's multiple comparison test. The sample size for each group is shown in the figure. The dashed lines indicate the boundaries (markers D12Hmgc13 to D12Hmgc14) of the narrowed candidate region after excluding the Cb and Cc genetic regions. The thin black bars represent chromosomal regions that could be either BN or SS. There were no statistical differences between the strains on 1% NaCl.*P<0.05 vs. SS-12BN on 8% salt diet.
BP Measurements
Male rats were born to mothers on 0.3% NaCl diet (7034 Teklad, Harlan, Indianapolis, IN), weaned onto 1% NaCl diet (5001 Purina, LabDiet, St. Louis, MO) at 21 days of age, and switched to an 8% NaCl (Dyets) diet at 11 weeks-of-age for phenotyping (Figure 1A). Mean arterial pressure (MAP) was measured by telemetry transmitter implantation with a catheter inserted into the abdominal aorta of 9-10 week old rats, as described previously.3
Protein and Albumin Excretion Measurements
Total protein and albumin was quantified using Weichselbaum biuret reagent (Henry Schein, Melville, NY) and Albumin Blue 580 (Sigma-Aldrich, St. Louis, MO), respectively.3, 14
Renal Histology
Renal histological analysis was described previously.15 Briefly, kidneys were isolated after 21 days on 8% NaCl diet (AIN-76, Dyets, Bethlehem, PA) and fixed in 10% buffered formalin, sectioned at 4 μm thickness, and stained with Masson's trichrome and H&E.
Genomic Sequence Analysis
Whole genome sequence was accessed from the Rat Genome Database (http://rgd.mcw.edu). Functional consequences of single nucleotide variants and insertions/deletions were predicted using PolyPhen-216 and Ensembl's Variant Effect Predictor17 v2.6 based on the Ensembl v68 transcripts. Protein models were generated using either YASARA homology modeling18 or I-TASSER hybrid modeling.19 Multiple species protein sequences were obtained from UniProt, aligned with ClustalW,20 and sequence conservation was mapped onto the protein model using ConSurf.21 Protein structures for human, rat, and mouse were structurally aligned with the MUSTANG algorithm.22
RT-qPCR
Transcript expression in the renal cortex and medulla of 9-10 week old SS-12BN and Ca strains on 1% NaCl diets and 14-15 week old rats after being on 8% NaCl diets for 21 days were analyzed by RT-qPCR as described previously.23
Statistical Analysis
Statistical analyses were performed using SigmaPlot 12.0 software. All data are presented as mean ± standard error of the mean (SEM). Since the MAP data failed the normality test, these data were analyzed by Kruskal-Wallis 1-way ANOVA on ranks followed by Dunn's multiple comparison test vs. the SS-12BN control group. Proteinuria, albuminuria, and histology data were analyzed by 1-way ANOVA followed by the Holm-Sidak test for multiple comparisons. Gene expression data were analyzed by Student's t-test. Since protein casting measurements were not normally distributed, the data were log-transformed prior to analysis by t-test.
Results
Blood Pressure
In our previous BP mapping study,3 rats were maintained on a 1% NaCl (Purina) diet before switching to 8% NaCl diet at ∼10 weeks-of-age. However, elevated BP (Δ+19 mmHg) in the 6.1 Mb (chr12:13.4-19.5 Mb) line C congenic on 1% NaCl diet complicated further congenic mapping due to the shortened lifespan of the breeders (Supplementary Figure S1). Consequently, we modified our protocol for the current study by maintaining breeders on 0.3% NaCl (Teklad) diet. As described below, this minor change in diet (0.3% NaCl vs. 1% NaCl), during gestation and 21 days after birth, blunted the increase in BP levels, but still enabled congenic mapping on 8% NaCl diets.
To narrow the 6.1 Mb region on chromosome 12 (line C), we generated 3 overlapping SS-12BN congenic rat strains as depicted in Figure 1B. On 1% NaCl diets, lines C (110±2; n=14), Ca (118±3 mmHg; n=14), Cb (110±1; n=6), and Cc (107±2; n=5) had no differences in MAP compared to the SS-12BN consomic (109±1 mmHg; n=25) (Figure 1). After 21 days on 8% NaCl diet, the MAP of line C (160±3 mmHg; P<0.05; n=14) and line Ca (163±5 mmHg; P<0.01; n=14) were significantly elevated compared to SS-12BN (148±2 mmHg; n=25). However, lines Cb (150±2 mmHg; n=6) and Cc (141±2 mmHg; n=5) were not significantly different compared with SS-12BN (Figure 1). Since MAP was elevated in lines C and Ca compared with SS-12BN, but not in the flanking lines Cb or Cc regions, these data suggest that the causative genetic variant(s) reside within the 830 kb (chr12:14.36-15.19 Mb) congenic interval of line Ca (Figure 1).
Renal Damage and Histology
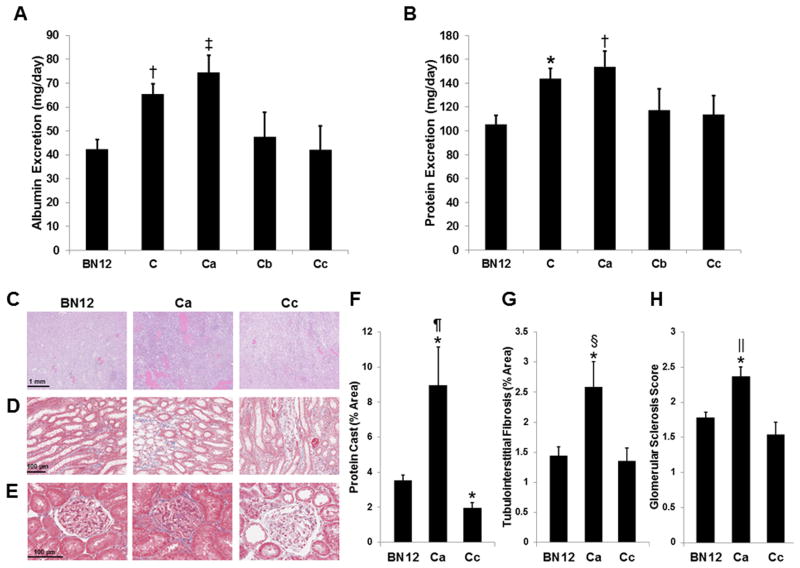
Renal damage was assessed by urinary albumin and protein measurements from 9-10 week old SS-12BN consomic and congenic rats on 1% NaCl diets. Compared with urinary albumin excretion in the SS-12BN consomic (43±4 mg/day), albuminuria was significantly elevated in line C (65±4 mg/day; P<0.01) and line Ca (74±7 mg/day; P<0.001) (Figure 2A). Likewise, urinary protein excretion was significantly elevated in line C (144±8; P<0.05) and line Ca (154±13 mg/day; P<0.01) compared with the SS-12BN consomic (105±7 mg/day) (Figure 2B). Lines Cb and Cc had no differences in albumin and protein excretion compared with the SS-12BN consomic. We also evaluated renal damage after 21 days of 8% NaCl diet using H&E and trichrome staining of SS-12BN, line Ca, and line Cc kidneys. Compared with SS-12BN and Cc kidneys, line Ca had significantly increased protein casting (Figure 2C, F), tubulointerstitial fibrosis (Figure 2D, G), and glomerular sclerosis (Figure 2E, H). Line Cc kidneys had significantly lower protein casting (1.98±0.30 vs. 3.54±0.30%; P<0.05; n=5), and no difference in tubulointerstitial fibrosis and glomerular sclerosis compared to SS-12BN kidneys. Collectively, these data confirm that the causative variant(s) driving elevated BP (Figure 1) reside within the 830 kb (chr12:14.36-15.19 Mb) region of line Ca.
Figure 2.
Urinary albumin (A) and protein (B) excretion on a 1% NaCl diet (n=26 SS-12BN, 20 C, 14 Ca, 6 Cb, and 5 Cc animals). H&E-stained (C) SS-12BN (BN12), Ca, and Cc kidneys for examining protein casting after an 8% salt load. Trichrome-stained SS-12BN (BN12), Ca, and Cc kidneys for examining tubulointerstitial fibrosis (D) and glomerular sclerosis (E). F, Quantification of percent area of protein casting in the outer medulla (n=5 per group). G, Quantification of percent area of tubulointerstitial fibrosis (n=5 per group). H, Quantification of glomerular sclerosis scores (n=5 per group). Data are presented as mean ± SEM. *P<0.05, †P<0.01, and ‡P<0.001 vs. BN12. §P<0.05, ‖P<0.01, and ¶P<0.001 vs. Cc.
Candidate Gene Expression
We used RT-qPCR to examine expression of the 14 genes within the 830 kb region in the renal cortex and medulla of SS-12BN consomic and line Ca rats on 1% and 8% NaCl diets (Tables 1, 2, and S2). Compared with SS-12BN, Chst12 expression was significantly down-regulated in line Ca rats in all conditions tested: renal cortex on 1% NaCl diet (-4.4±1.0-fold; P<0.01), renal cortex on 8% NaCl diet (-2.6±0.4-fold; P<0.001), renal medulla on 1% NaCl diet (-4.5±0.7-fold; P<0.01), and renal medulla on 8% NaCl diet (-2.7±0.2-fold; P<0.001) (Table 1).In comparison, the only other expression changes were 6 genes in the renal medulla on either 1% or 8% NaCl diets: Brat1 (BRCA1-associated ATM activator 1), Ttyh3 (tweety family member 3), Lfng (LFNG O-fucosylpeptide 3-beta-N-acetylglucosaminyltransferase), LOC679924, Mad1l1 (MAD1 mitotic arrest deficient-like 1 [yeast]), and Elfn1 (extracellular leucine-rich repeat and fibronectin type III domain containing 1). Iqce (IQ motif/domain containing E) differed in the renal medulla on both 1% and 8% NaCl diets, but in opposite directions (Table 2). Collectively, these data prioritize Chst12 as a more likely positional candidate, but do not preclude the potential role(s) of the other differentially expressed genes.
Table 1.
Candidate Gene Expression in the Renal Cortex of Ca and SS-12BN Rats on 1% and 8% NaCl Diets.
| 1% NaCl | 8% NaCl | |||
|---|---|---|---|---|
|
| ||||
| Gene | SS-12BN | Ca | SS-12BN | Ca |
| Amz1 | 1.00±0.14 | 1.08±0.18 | 1.00±0.36 | 1.06±0.19 |
| Brat1 | 1.00±0.24 | 0.78±0.06 | 1.00±0.10 | 0.95±0.08 |
| Iqce | 1.00±0.10 | 0.88±0.10 | 1.00±0.15 | 0.95±0.08 |
| Ttyh3 | 1.00±0.11 | 0.84±0.09 | 1.00±0.07 | 0.96±0.11 |
| Lfng | 1.00±0.26 | 0.81±0.22 | 1.00±0.32 | 1.06±0.17 |
| Grifin | - | - | - | - |
| Chst12 | 1.00±0.15 | 0.32±0.09* | 1.00±0.10 | 0.47±0.07† |
| LOC679924 | 1.00±0.31 | 1.09±0.41 | 1.00±0.17 | 0.94±0.12 |
| Eif3b | 1.00±0.12 | 0.88±0.10 | 1.00±0.08 | 1.01±0.05 |
| Snx8 | 1.00±0.13 | 0.95±0.12 | 1.00±0.12 | 0.86±0.06 |
| Nudt1 | 1.00±0.29 | 1.14±0.42 | 1.00±0.14 | 1.14±0.18 |
| Ftsj2 | 1.00±0.22 | 1.06±0.38 | 1.00±0.13 | 0.96±0.17 |
| Mad1l1 | 1.00±0.13 | 0.85±0.06 | 1.00±0.11 | 1.05±0.07 |
| Elfn1 | 1.00±0.13 | 0.91±0.07 | 1.00±0.13 | 0.75±0.11 |
Data are presented as mean fold-expression ± SEM. Statistical significance was determined by t-test. n=5-6 Ca and 6 SS-12BN on 1% NaCl and 8-9 Ca and 7-8 SS-12BN on 8% NaCl diets. (-) below detection.
P<0.01 and
P<0.001 vs. SS-12BN.
Table 2.
Candidate Gene Expression in the Renal Medulla of Ca and SS-12BN Rats on 1% and 8% NaCl Diets.
| 1% NaCl | 8% NaCl | |||
|---|---|---|---|---|
|
| ||||
| Gene | SS-12BN | Ca | SS-12BN | Ca |
| Amz1 | 1.00±0.12 | 0.99±0.22 | 1.00±0.16 | 0.89±0.07 |
| Brat1 | 1.00±0.08 | 0.60±0.11* | 1.00±0.05 | 1.15±0.06 |
| Iqce | 1.00±0.06 | 0.61±0.09† | 1.00±0.06 | 1.21±0.04* |
| Ttyh3 | 1.00±0.16 | 0.49±0.09† | 1.00±0.07 | 1.09±0.07 |
| Lfng | 1.00±0.17 | 0.61±0.09* | 1.00±0.20 | 0.75±0.05 |
| Grifin | - | - | - | - |
| Chst12 | 1.00±0.19 | 0.29±0.05† | 1.00±0.13 | 0.39±0.03† |
| LOC679924 | 1.00±0.36 | 2.20±1.28 | 1.00±0.08 | 0.70±0.05† |
| Eif3b | 1.00±0.06 | 0.86±0.14 | 1.00±0.10 | 0.82±0.05 |
| Snx8 | 1.00±0.08 | 1.66±0.48 | 1.00±0.17 | 0.66±0.05 |
| Nudt1 | 1.00±0.46 | 1.85±1.26 | 1.00±0.04 | 0.88±0.07 |
| Ftsj2 | 1.00±0.26 | 0.97±0.33 | 1.00±0.08 | 1.00±0.05 |
| Mad1l1 | 1.00±0.05 | 0.72±0.09* | 1.00±0.05 | 1.01±0.04 |
| Elfn1 | 1.00±0.20 | 0.68±0.16 | 1.00±0.17 | 0.43±0.06* |
Data are presented as mean fold-expression ± SEM. Statistical significance was determined by t-test. n=10-11 Ca and 7 SS-12BN on 1% NaCl and 9 Ca and 8 SS-12BN on 8% NaCl diets. (-) below detection.
P<0.05 and
P<0.01 vs. SS-12BN.
Sequence Analysis
Our data suggest that causative variant(s) reside in the 830 kb BP QTL (Figures 1 and 2), prompting us to prioritize candidate(s) within this region that were most likely causative. Sequence analysis of the SS/JrHsDMcwi and BN/NHsdMcwi genomes in the line C region (chr12:13.4-19.5 Mb) was previously performed by Flister et al.3 Consequently, we focused our analysis on detailed annotation of the variants predicted to cause non-synonymous changes (Table 3) or alter transcriptional regulatory regions of the differentially expressed candidate genes (Tables 1 and 2). In total, the 830 kb interval (chr12:14,365,649-15,194,843 bp) contains 1,585 single nucleotide variants (SNVs) between SS/JrHsDMcwi and BN/NHsdMcwi, of which 17 reside in coding regions and 3 result in non-synonymous changes. In addition, 398 insertions/deletions (indels) reside within the 830 kb candidate region (Table S4), none of which were within coding regions.
Table 3.
Non-synonymous Variants in Genes Within the 830 kb Candidate Region.
| Position | Reference Allele | Alternate Allele | Gene | Amino Acid Change | PolyPhen-2 Prediction |
|---|---|---|---|---|---|
| 14,406,110 | A | G | Brat1 | V188A | Benign |
| 14,480,874 | A | C | Ttyh3 | N331T | Benign |
| 15,139,510 | C | T | Elfn1 | M287I | Benign |
Analysis of Non-synonymous SNVs
We used PolyPhen-216 and a protein modeling approach to predict whether non-synonymous changes in Brat1, Ttyh3, and Elfn1 were likely to damage protein function. Polyphen-2 predicted all 3 non-synonymous variants to be benign (Table 3). Likewise, protein modeling showed that the variants in Brat1 and Elfn1 are located in non-conserved disordered loops and are unlikely to alter protein packing (Supplementary Figure S2). The variant in Ttyh3 is in a conserved site; however, conservation of the surface exposed hydroxyl group in both Asn (N) and Thr (T) likely maintain protein function (Supplementary Figure S2). Based on these analyses, we predict that the 3 non-synonymous variants within the 830 kb region are most likely not causative.
Analysis of Sequence Segregation
BP QTLs overlapping the 830 kb interval have been reported for SS×WKY24 and SS×SR25 linkage analyses, in addition to SS×BN,26 enabling us to use co-segregating analysis to further narrow the list of potentially causative variants. To do so, we plotted the total unique and common variants per 50 kb bin in SS/JrHsdMcwi versus BN/NHsdMcwi, SR/JrHsd, and WKY/NHsd. Across the 830 kb interval, only one 86 kb region (chr12: chr12:14,541,567-14,627,442 bp) had alleles that were unique to SS, and not seen in BN, SR, and WKY (Figure 3), suggesting that SS-derived variant(s) within this region are most likely to be causative. Of note, Chst12 and Grifin are the only two genes residing in the 86 kb region.
Figure 3.
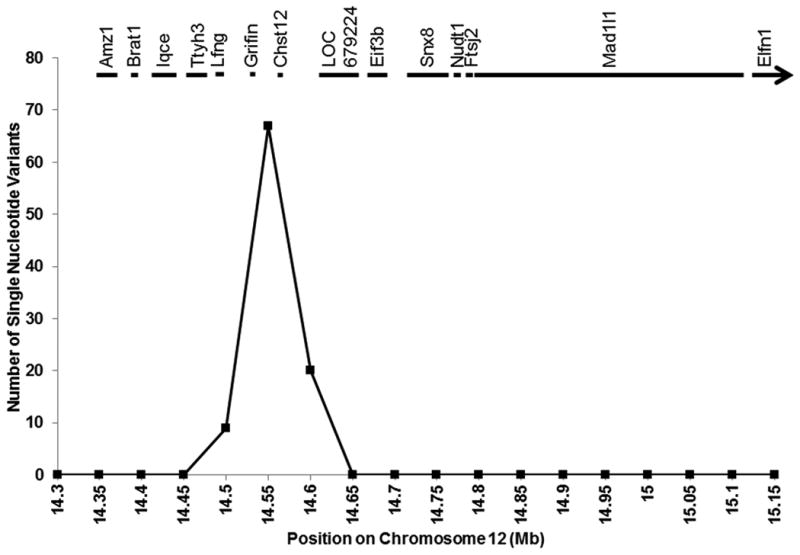
Comparison of Dahl salt-sensitive (SS/JrHsdMcwi), Dahl salt-resistant (SR/JrHsd), Brown Norway (BN/NHsdMcwi), and Wistar-Kyoto (WKY/NHsd) sequences over the 830 kb interval that is uniquely SS in the Ca congenic strain. Data are presented as number of single nucleotide variants per 0.05 Mb bin where SS has a different allele than SR, BN, and WKY. The locations of the 14 genes within this region are also shown.
Analysis of Variants and Indels within Transcriptional Regulatory Motifs
We used in silico analysis (TRANSFAC27) to predict whether variants that segregated when comparing SS/JrHsdMcwi to BN/NHsdMcwi, SR/JrHsd, and WKY/NHsd altered potential regulatory motifs in close proximity (<5 kb) to differentially expressed candidate genes (Chst12, Brat1, Iqce, Ttyh3, Lfng, LOC679924, Mad1l1, and/or Elfn1). This analysis revealed 6 variants and 2 indels, all of which were located near Chst12, that were predicted to alter a total of 10 transcription factor (TF) binding motifs residing in the 5 kb regulatory regions of the differentially candidate genes (either gain or loss of TF binding) (Table 4). Of note, the 5 variants predicted to alter binding sites for ER, TBP, NF-κB, Sp1, C/EBP alpha, Olf-1, MBP-2, and c-Rel reside in the 5 kb regulatory regions of Chst12, suggesting that these variants may play a role in the differences of Chst12 expression observed between the Ca congenic and SS-12BN consomic strains.
Table 4.
Predicted Altered Transcription Factor Binding Sites 5 kb Upstream and Downstream of Differentially Expressed Candidate Genes.
| Start Position | End Position | Reference Allele | Alternate Allele | Gene | Consequence | Transcription Factors |
|---|---|---|---|---|---|---|
| 14,572,855 | 14,572,855 | - | A | Chst12 | Downstream | none |
| 14,572,856 | 14,572,857 | - | A | Chst12 | Downstream | none |
| 14,574,713 | 14,574,713 | C | T | Chst12 | Downstream | none |
| 14,576,414 | 14,576,414 | G | T | Chst12 | Downstream | ER |
| 14,576,471 | 14,576,471 | G | A | Chst12 | Downstream | TBP, NF-kappaB |
| 14,579,265 | 14,579,265 | T | A | Chst12 | Upstream | C/EBP alpha |
| 14,579,975 | 14,579,975 | C | T | Chst12 | Upstream | Sp1 |
| 14,583,805 | 14,583,805 | G | A | Chst12 | Upstream | C/EBP alpha, NF-kappaB, Olf-1, MBP-2, c-Rel |
Examined single nucleotide variants and insertions/deletions where SS/JrHsdMcwi was homozygous for a different allele than BN/NHsdMcwi, SR/JrHsd, and WKY/NHsd.
Discussion
We previously demonstrated that genetic elements within the line C congenic (6.1 Mb) region on rat chromosome 12 increased BP, proteinuria, and albuminuria.3 The goal of the present study was to further narrow the candidate region on chromosome 12 to a manageable number of genes, which are now prioritized for future mechanistic analyses. Using a series of overlapping congenic rat strains, we reduced the candidate region by ∼86% from the 6.1 Mb region previously described3 to an 830 kb region (chr12:14,365,649-15,194,843 bp) containing 14 genes. We also analyzed candidate gene expression (Tables 1 and 2) and sequence variants (Figures 3 and S2, Tables 3, 4, S3, and S4), all of which point to Chst12 as a likely candidate gene for hypertension. Finally, since this region overlaps with a syntenic human hypertension and kidney disease locus,9, 10, 13discovering the causative gene(s) may provide insights into human disease pathophysiology.
Genetic Modifier(s) of Hypertension on Rat Chromosome 12
We found that significantly increased proteinuria and albuminuria in line C and Ca rats on 1% NaCl diet (Figure 2A and B) preceded elevation of BP, which was not apparent until rats were placed on 8% NaCl diet (Figure 1). These data suggest that functional element(s) in the 830 kb locus (chr12:14,365,649-15,194,843 bp) potentially increase hypertension risk by elevating susceptibility to renal damage. This fits with prior evidence that renal damage precedes elevated BP in the SS rat28 and that the genetic risk of hypertension is transferrable by transplantation of SS kidneys into normotensive SR rats.29 It is also possible that multiple causative variants co-segregate at the 830 kb locus, as we have demonstrated previously for other hypertension loci.15, 23, 30 However, future studies targeting specific genes or further reducing the congenic interval will be necessary to localize the causative variant(s).
The 830 kb BP locus contains 3 non-synonymous variants (Table 3) and 8 differentially expressed genes (Tables 1 and 2). Of the non-synonymous variants, none were predicted to damage protein function (Table 3), and only Elfn1 was differentially expressed, specifically in the renal medulla on 8% NaCl diet (Table 2). Brat1, Iqce, Ttyh3, Lfng, LOC679924, and Mad1l1 were differentially expressed, but only in one renal tissue (i.e., medulla or cortex) or under only one condition (i.e., 1% or 8% NaCl diet). In comparison, Chst12 was consistently down-regulated by 53-71% in renal medulla and cortex of line Ca rats on both 1% and 8% NaCl diets (Tables 1 and 2). Grifin was the only gene in the 830 kb region that was not detectable in the kidney, suggesting that it is unlikely to affect renal-dependent BP regulation. Chst12 and Grifin reside in an 86 kb region (chr12: chr12:14,541,567-14,627,442 bp) that was unique to the hypertensive SS strain compared to normotensive BN, SR, and WKY strains (Figure 3). Within this region, 4 variants unique to the SS altered binding sites in the proximal Chst12 regulatory regions for multiple TF have been previously implicated in the pathogenesis of hypertension (e.g., NF-kB, 31, 32 Sp1,33-35 and TBP36).
Candidate Genes
Chst12 was the only gene that was differentially expressed in the renal cortex and/or medulla on 1% and 8% NaCl diets (Tables 1 and 2) and co-segregated in the 86 kb chromosome 12 region that was unique to SS (Figure 3), suggesting that Chst12 is the most likely positional candidate gene for hypertension and renal disease in the SS rat. Despite this, a role for Chst12 in the pathogenesis of hypertension was not previously reported. Chst12 (also known as C4ST-2 in humans) is a ubiquitously expressed37 member of the sulfotransferase 2 family that mediates synthesis of the extracellular matrix proteoglycan, chondroitin sulfate.38 The role of chondroitin sulfate in systemic hypertension is also unclear, but the contribution of chondroitin sulfate to disease-associated fibrosis has been suggested in the literature. For example, (1) systemic injection of chondroitin sulfate attenuated renal fibrosis in mice undergoing unilateral ureteral obstruction;39 (2) injection of chondroitin sulfate into the eye elevated intraocular BP;40 and (3) chondroitin sulfate levels were elevated by 70% in the aortas of hypertensive SS rats compared with normotensive WKY rats.41 Additionally, multiple GWAS suggestively linked an intergenic variant (rs2969070 [G]) in close proximity to CHST12 with hypertension (P<10-6 for MAP and pulse pressure).42, 43 Collectively, these data suggest that Chst12 might indirectly influence hypertension risk via chondroitin sulfate synthesis. Future studies will be necessary to directly test the role of Chst12 in the pathogenesis of hypertension and determine whether this Chst12-dependent susceptibility is conveyed by chondroitin sulfate or mediated by another substrate of Chst12.
Multiple other candidates in the 830 kb interval were differentially expressed (Tables 1 and 2), precluding us from definitively claiming Chst12 as the causative gene. Rather, these genes (including Chst12) will need to be validated in future studies by protein expression, gene manipulation, and/or further congenic mapping. Of the other genes, Elfn1 and Brat1 are the next likeliest candidates based on prior evidence. Elfn1 (PPP1R28) inhibits the phosphatase activity of protein phosphatase 1 (PP1),44 which was implicated in hypertension45 and heart failure.46 BRAT1 regulates cell-cycle checkpoints by interacting with BRCA1, which was implicated in hypertension risk.47 Of the remaining candidates (Ttyh3, Lfng, LOC679924, Mad1l1, Iqce), none were directly or indirectly implicated in hypertension or renal failure.
Perspectives
By generating overlapping congenic strains and exclusion mapping, we reduced the candidate region for hypertension on chromosome 12 by ∼86% (from a 6.1 Mb region with 133 genes that we previously published to an 830 kb candidate region with 14 genes). With the use of whole genome sequencing, expression analyses, protein modeling, and bioinformatics approaches, we have prioritized Chst12 as a likely candidate for hypertension risk, followed by Elfn1 and Brat1 as the next best candidates. Narrowing the congenic interval or functional testing by gene targeting approaches will be necessary to exclude/include these candidates. Additionally, future studies will be needed to determine which of these candidates are causative and which are secondary to renal damage.
Supplementary Material
Novelty and Significance: 1) What is New, 2) What is Relevant, 3) Summary.
-
What is New?
We reduced the candidate region for hypertension and renal disease on rat chromosome 12 by ∼86% with the development of overlapping congenic strains, which reduces the number of candidate genes from 133 to 14.
This is the first study to utilize the recently published rat whole genome sequencing data to help accelerate the nomination of candidate genes for complex diseases in rat.
By sequence and expression analyses, we have nominated Chst12 as a novel gene involved in blood pressure regulation.
-
What is Relevant?
Since several human and mouse studies have implicated regions homologous to rat chromosome 12 in cardiovascular disease and related traits, dissecting the genetic basis of hypertension and renal disease on rat chromosome 12 may provide insights into the genetic components that increase susceptibility to cardiovascular disease in humans.
Summary
By combining data from overlapping congenic rat models and sequence and expression studies, we have prioritized Chst12 as a gene that may be involved in BP regulation and renal disease, which can be tested in the future.
Acknowledgments
We thank Rebecca Schilling, Angela Lemke, Allison Zappa, Elizabeth Schneider, and the RGD team for their excellent technical support.
Sources of Funding: This study was supported by NHLBI (5R01HL089930 and 5R01HL069321) to HJJ. SZP is a member of the Medical Scientist Training Program at MCW, which is partially supported by NIGMS (5T32GM080202). MJF is supported by a NHLBI training grant (5T32HL007792).
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement – None
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flister MJ, Prisco SZ, Sarkis AB, O'Meara CC, Hoffman M, Wendt-Andrae J, Moreno C, Lazar J, Jacob HJ. Identification of hypertension susceptibility loci on rat chromosome 12. Hypertension. 2012;60:942–948. doi: 10.1161/HYPERTENSIONAHA.112.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramensky V, Bork P, Sunyaev S. Human non-synonymous snps: Server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atwood LD, Samollow PB, Hixson JE, Stern MP, MacCluer JW. Genome-wide linkage analysis of pulse pressure in mexican americans. Hypertension. 2001;37:425–428. doi: 10.1161/01.hyp.37.2.425. [DOI] [PubMed] [Google Scholar]
- 6.Atwood LD, Samollow PB, Hixson JE, Stern MP, MacCluer JW. Genome-wide linkage analysis of blood pressure in mexican americans. Genet Epidemiol. 2001;20:373–382. doi: 10.1002/gepi.7. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SC, Ellison RC, Atwood LD, Pankow JS, Province MA, Leppert MF. Genome scans for blood pressure and hypertension: The national heart, lung, and blood institute family heart study. Hypertension. 2002;40:1–6. doi: 10.1161/01.hyp.0000022660.28915.b1. [DOI] [PubMed] [Google Scholar]
- 8.Cheng LS, Davis RC, Raffel LJ, et al. Coincident linkage of fasting plasma insulin and blood pressure to chromosome 7q in hypertensive hispanic families. Circulation. 2001;104:1255–1260. doi: 10.1161/hc3601.096729. [DOI] [PubMed] [Google Scholar]
- 9.Tayo BO, Luke A, Zhu X, Adeyemo A, Cooper RS. Association of regions on chromosomes 6 and 7 with blood pressure in nigerian families. Circ Cardiovasc Genet. 2009;2:38–45. doi: 10.1161/CIRCGENETICS.108.817064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adeyemo A, Luke A, Wu X, Cooper RS, Kan D, Omotade O, Zhu X. Genetic effects on blood pressure localized to chromosomes 6 and 7. J Hypertens. 2005;23:1367–1373. doi: 10.1097/01.hjh.0000173519.06353.8b. [DOI] [PubMed] [Google Scholar]
- 11.McDonough CW, Palmer ND, Hicks PJ, et al. A genome-wide association study for diabetic nephropathy genes in african americans. Kidney Int. 2011;79:563–572. doi: 10.1038/ki.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner ST, Kardia SL, Mosley TH, Rule AD, Boerwinkle E, de Andrade M. Influence of genomic loci on measures of chronic kidney disease in hypertensive sibships. J Am Soc Nephrol. 2006;17:2048–2055. doi: 10.1681/ASN.2005121254. [DOI] [PubMed] [Google Scholar]
- 13.Bowden DW, Colicigno CJ, Langefeld CD, Sale MM, Williams A, Anderson PJ, Rich SS, Freedman BI. A genome scan for diabetic nephropathy in african americans. Kidney Int. 2004;66:1517–1526. doi: 10.1111/j.1523-1755.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 14.O'Meara CC, Lazar J, Hoffman M, Moreno C, Jacob HJ. Refined mapping of the renal failure rf-3 quantitative trait locus. J Am Soc Nephrol. 2011;22:518–525. doi: 10.1681/ASN.2010060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flister MJ, Hoffman MJ, Reddy P, Jacob HJ, Moreno C. Congenic mapping and sequence analysis of the renin locus. Hypertension. 2013;61:850–856. doi: 10.1161/HYPERTENSIONAHA.111.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the ensembl api and snp effect predictor. Bioinformatics. 2010;26:2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger E, Koraimann G, Vriend G. Increasing the precision of comparative models with yasara nova--a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- 19.Roy A, Kucukural A, Zhang Y. I-tasser: A unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal w and clustal x version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. Consurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konagurthu AS, Whisstock JC, Stuckey PJ, Lesk AM. Mustang: A multiple structural alignment algorithm. Proteins. 2006;64:559–574. doi: 10.1002/prot.20921. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman MJ, Flister MJ, Nunez L, Xiao B, Greene AS, Jacob HJ, Moreno C. Female-specific hypertension loci on rat chromosome 13. Hypertension. 2013;62:557–563. doi: 10.1161/HYPERTENSIONAHA.113.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato N, Hyne G, Bihoreau MT, Gauguier D, Lathrop GM, Rapp JP. Complete genome searches for quantitative trait loci controlling blood pressure and related traits in four segregating populations derived from dahl hypertensive rats. Mamm Genome. 1999;10:259–265. doi: 10.1007/s003359900983. [DOI] [PubMed] [Google Scholar]
- 25.Herrera VL, Tsikoudakis A, Ponce LR, Matsubara Y, Ruiz-Opazo N. Sex-specific qtls and interacting loci underlie salt-sensitive hypertension and target organ complications in dahl s/jrhs hypertensive rats. Physiol Genomics. 2006;26:172–179. doi: 10.1152/physiolgenomics.00285.2005. [DOI] [PubMed] [Google Scholar]
- 26.Stoll M, Cowley AW, Jr, Tonellato PJ, Greene AS, Kaldunski ML, Roman RJ, Dumas P, Schork NJ, Wang Z, Jacob HJ. A genomic-systems biology map for cardiovascular function. Science. 2001;294:1723–1726. doi: 10.1126/science.1062117. [DOI] [PubMed] [Google Scholar]
- 27.Matys V, Kel-Margoulis OV, Fricke E, et al. Transfac and its module transcompel: Transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–110. doi: 10.1093/nar/gkj143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW., Jr Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the dahl-ss rat. J Hypertens. 2000;18:1497–1505. doi: 10.1097/00004872-200018100-00019. [DOI] [PubMed] [Google Scholar]
- 29.Dahl LK, Heine M, Thompson K. Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circ Res. 1974;40:94–101. doi: 10.1161/01.res.40.4.94. [DOI] [PubMed] [Google Scholar]
- 30.Flister MJ, Tsaih SW, O'Meara CC, Endres B, Hoffman MJ, Geurts AM, Dwinell MR, Lazar J, Jacob HJ, Moreno C. Identifying multiple causative genes at a single gwas locus. Genome Res. 2013;23:1996–2002. doi: 10.1101/gr.160283.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, Haefliger JA. An angiotensin ii- and nf-kappab-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res. 2010;87:166–176. doi: 10.1093/cvr/cvq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luft FC. Workshop: Mechanisms and cardiovascular damage in hypertension. Hypertension. 2001;37:594–598. doi: 10.1161/01.hyp.37.2.594. [DOI] [PubMed] [Google Scholar]
- 33.Kubo T, Kinjyo N, Ikezawa A, Kambe T, Fukumori R. Sp1 decoy oligodeoxynucleotide decreases angiotensin receptor expression and blood pressure in spontaneously hypertensive rats. Brain Res. 2003;992:1–8. doi: 10.1016/s0006-8993(03)03534-0. [DOI] [PubMed] [Google Scholar]
- 34.Menzaghi C, Paroni G, De Bonis C, Soccio T, Marucci A, Bacci S, Trischitta V. The -318 c>g single-nucleotide polymorphism in gnai2 gene promoter region impairs transcriptional activity through specific binding of sp1 transcription factor and is associated with high blood pressure in caucasians from italy. J Am Soc Nephrol. 2006;17:S115–119. doi: 10.1681/ASN.2005121340. [DOI] [PubMed] [Google Scholar]
- 35.Negoro N, Kanayama Y, Haraguchi M, Umetani N, Nishimura M, Konishi Y, Iwai J, Okamura M, Inoue T, Takeda T. Blood pressure regulates platelet-derived growth factor a-chain gene expression in vascular smooth muscle cells in vivo. An autocrine mechanism promoting hypertensive vascular hypertrophy. J Clin Invest. 1995;95:1140–1150. doi: 10.1172/JCI117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savinkova LK, Ponomarenko MP, Ponomarenko PM, Drachkova IA, Lysova MV, Arshinova TV, Kolchanov NA. Tata box polymorphisms in human gene promoters and associated hereditary pathologies. Biochemistry (Mosc) 2009;74:117–129. doi: 10.1134/s0006297909020011. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka N, Nakagawa H, Ong E, Akama TO, Fukuda MN, Fukuda M. Molecular cloning and expression of two distinct human chondroitin 4-o-sulfotransferases that belong to the hnk-1 sulfotransferase gene family. J Biol Chem. 2000;275:20188–20196. doi: 10.1074/jbc.M002443200. [DOI] [PubMed] [Google Scholar]
- 38.Izumikawa T, Koike T, Kitagawa H. Chondroitin 4-o-sulfotransferase-2 regulates the number of chondroitin sulfate chains initiated by chondroitin n-acetylgalactosaminyltransferase-1. Biochem J. 2012;441:697–705. doi: 10.1042/BJ20111472. [DOI] [PubMed] [Google Scholar]
- 39.Melo-Filho NM, Belmiro CL, Goncalves RG, Takiya CM, Leite M, Jr, Pavao MS, Mourao PA. Fucosylated chondroitin sulfate attenuates renal fibrosis in animals submitted to unilateral ureteral obstruction: A p-selectin-mediated event? Am J Physiol Renal Physiol. 2010;299:F1299–1307. doi: 10.1152/ajprenal.00217.2010. [DOI] [PubMed] [Google Scholar]
- 40.Belforte N, Sande P, de Zavalia N, Knepper PA, Rosenstein RE. Effect of chondroitin sulfate on intraocular pressure in rats. Invest Ophthalmol Vis Sci. 2010;51:5768–5775. doi: 10.1167/iovs.10-5660. [DOI] [PubMed] [Google Scholar]
- 41.Reynertson RH, Oparil S, Roden L. Proteoglycans and hypertension: Iii: Aorta proteoglycans in dahl salt-sensitive hypertensive rats. Am J Med Sci. 1987;293:171–176. doi: 10.1097/00000441-198703000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wain LV, Verwoert GC, O'Reilly PF, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendrickx A, Beullens M, Ceulemans H, Den Abt T, Van Eynde A, Nicolaescu E, Lesage B, Bollen M. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol. 2009;16:365–371. doi: 10.1016/j.chembiol.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Shen GQ, Ikegami H, Fujisawa T, Hamada Y, Kamide K, Rakugi H, Higaki J, Murakami H, Shimamoto K, Ogihara T. Asp905tyr polymorphism of protein phosphatase 1 g subunit gene in hypertension. Hypertension. 1997;30:236–239. doi: 10.1161/01.hyp.30.2.236. [DOI] [PubMed] [Google Scholar]
- 46.Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac sr-coupled pp1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 47.Lovren F, Pan Y, Quan A, Singh KK, Khan R, Gupta N, Brezden-Masley C, Teoh H, Wheatcroft MD, Al-Omran M, Verma S. Brca1 shields vascular smooth muscle cells from oxidative stress. J Thorac Cardiovasc Surg. 2014;147:1946–1955. doi: 10.1016/j.jtcvs.2013.09.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.