Abstract
Obesity is an important risk factor for the development of insulin resistance. Initial compensatory mechanisms include an increase in insulin levels, which are thought to induce sympathetic activation in an attempt to restore energy balance. We have previously shown, however, that sympathetic activity has no beneficial effect on resting energy expenditure in obesity. On the contrary, we hypothesize that sympathetic activation contributes to insulin resistance. To test this hypothesis, we determined insulin sensitivity using a standard hyperinsulinemic euglycemic clamp protocol in obese subjects randomly assigned in a crossover design one month apart to receive saline (intact day) or trimetaphan (4 mg/min IV, autonomic blocked day). Whole body glucose uptake (MBW in mg/kg/min) was used as index of maximal muscle glucose utilization. During autonomic blockade we clamped blood pressure with a concomitant titrated IV infusion of the nitric oxide synthase inhibitor L-NMMA. Of the 21 obese subjects (43±2 years of age, 35±2 kg/m2 BMI) studied fourteen were insulin resistant; they were more obese, had higher plasma glucose and insulin, and higher muscle sympathetic nerve activity (23.3±1.5 vs. 17.2±2.1 burst/min, p=0.03) compared to insulin sensitive subjects. Glucose utilization improved during autonomic blockade in insulin resistant subjects (MBW 3.8±0.3 blocked vs. 3.1±0.3 mg/kg/min intact; p=0.025), with no effect in the insulin sensitive group. These findings support the concept that sympathetic activation contributes to insulin resistance in obesity and may result in a feedback loop whereby the compensatory increase in insulin levels contributes to greater sympathetic activation.
Keywords: Autonomic Nervous System, Autonomic Blockade, Insulin Resistance, Obesity, Insulin Clamp, Hyperinsulinemic Euglycemic Clamp, Insulin Sensitivity
INTRODUCTION
The prevalence of obesity and associated morbidities are increasing worldwide,1 making obesity the second most preventable cause of death in the United States 2, 3. The association between obesity and insulin resistance has been known for more than four decades 4; overweight and obese subjects have a greater risk of developing insulin resistance and, therefore, of developing diabetes and other complications of obesity. However, not all obese subjects develop insulin resistance 5 and this study explored the possibility that the presence of sympathetic activation is associated with insulin resistance in obese individuals, and contributes to this phenomenon.
Obesity is also associated with an increase in sympathetic activity. Fat mass, and more specifically visceral fat, is positively correlated with muscle sympathetic nerve activity (MSNA), which is arguably the most accurate measurement of central sympathetic outflow coupled to blood pressure (BP) regulation 6. The sympathetic nervous system plays an important role in metabolic regulation, influencing key processes such as glucose and lipid metabolism, and all components of daily energy expenditure, including resting metabolic rate, the thermic effect of food and energy expenditure from physical activity. Furthermore, increased sympathetic activity has been associated with the development of insulin resistance 7, and activation of the sympathetic nervous system with lower body negative pressure acutely decreases forearm muscle glucose uptake in healthy subjects 8. Animal studies suggest that sympathetic activation contributes to insulin resistance 9. In humans, there is an inverse relationship between insulin sensitivity and whole body NE spillover in obese individuals, and MSNA is higher in those with insulin resistance compared to those who are insulin sensitive 10. Not all studies, however, have found that sympathetic activity is associated with decreased insulin sensitivity 11, 12. Furthermore, a direct causal link between the sympathetic nervous system and insulin sensitivity has not been established in obesity.
We hypothesized that sympathetic activation associated with obesity contributes to insulin resistance. To test this hypothesis we used the hyperinsulinemic euglycemic clamp to measure insulin sensitivity in obese subjects suspected to have both increased sympathetic activity and insulin resistance. We studied them under intact conditions and following acute autonomic blockade induced by the ganglionic blocker trimethaphan. If our hypothesis is correct, we would expect that autonomic blockade would result in improved insulin sensitivity, and that this effect would be apparent in obese subjects with increased sympathetic activity and insulin resistance.
METHODS
Subjects
The study was reviewed and approved by the Vanderbilt University Institutional Review Board and written informed consent was obtained from each subject before initiating the study.
Obese volunteers were recruited from the Vanderbilt University Clinical Research Center volunteer database and Research Match.13 Subjects 18 to 60 years old of either gender with a BMI between 30–45 kg/m2 were considered eligible for the study. Current smokers, subjects with diabetes and pregnant women were excluded. Hypertension was not a criterion for exclusion but all medications including antihypertensives were withdrawn at least 7 days before each study day with the exception of oral contraceptives. Subjects abstained from caffeine and other substances that are known to have an effect on the autonomic nervous system for ≥72 hours before testing. Screening included clinical examination, ECG, urinalysis, and routine laboratory testing (i.e. blood count and chemistry).
Study Design
Insulin sensitivity was assessed on two separate days, one month apart, using a randomized single-blind crossover design with a computer generated randomization code. A hyperinsulinemic euglycemic clamp was performed on both occasions as previously described 14 using identical instrumentation; once with intact autonomic function and once during autonomic blockade (Figure 1). Subjects were classified as insulin resistant or sensitive based on the glucose infusion rate on the intact day. Muscle sympathetic nerve activity was measured on a separate occasion, at least 1 week after either insulin clamp.
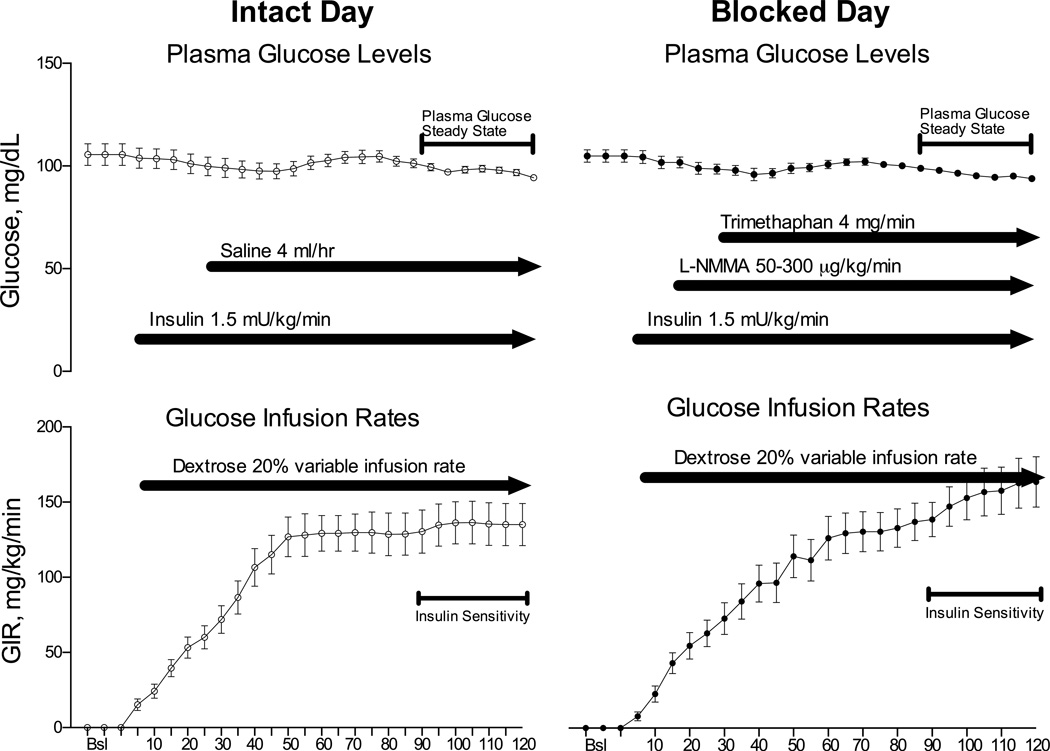
Figure 1. Schematic Protocol Description.
A standard hyperinsulinemic euglycemic clamp was performed during the intact study day (left panels) with insulin sensitivity measured from 90–120 minutes. During the blocked day trimethaphan was infused starting at 30 minutes, and blood pressure was clamped with a titrated infusion of the nitric oxide inhibitor L-NMMA starting 15 minutes before the trimethaphan infusion. Values of plasma glucose (mg/dL, upper panels) and the glucose infusion rate (GIR, mg/kg/min, lower panels) are the average data obtained in the insulin resistant group.
Study procedures
Hyperinsulinemic euglycemic clamp
Participants were admitted to the Vanderbilt Clinical Research Center the evening before the study and given a standard meal. After an overnight fast, subjects were studied the next morning in the supine position. A catheter was inserted into a forearm vein for drug infusion. A second catheter was inserted into a contralateral superficial forearm or hand vein, which was heated to obtain arterialized blood samples15. A primed continuous insulin infusion (4 mU/kg/min for 8 min, then 2 mU/kg/min) was maintained for two hours. Plasma glucose levels were measured every 5 minutes for the duration of the study (120 minutes). Potassium chloride was infused at 10 mmol/hr to prevent insulin-induced hypokalemia. Plasma samples were taken at baseline and at the middle of the clamp. Euglycemia (4.99-5.55 mmol/L or 90–100 mg/dL) was maintained by a variable infusion of 20% dextrose, and the glucose infusion rate (GIR) during minutes 90–120 of the clamp was used as a measure of insulin sensitivity (Figure 1).
Autonomic Blockade Study Day
Continuous blood pressure was measured through the volume clamp method (BMEYE, Nexfin, Edwards Lifesciences), and also every five minutes with an automated oscillometric brachial cuff (Vital-Guard 450C, Ivy Biomedical Systems). Heart rate (HR) was determined with continuous ECG monitoring. Thirty minutes after the insulin infusion was started, intravenous infusion of trimethaphan was started and maintained throughout the study at a dose (4 mg/min) shown to induce complete autonomic blockade 16.Insulin induces nitric oxide (NO) mediated vasodilation and this will lower blood pressure in the presence of autonomic blockade. We prevented a drop in blood pressure using a variable infusion rate of the NO synthase inhibitor L-NMMA, started ten minutes prior to trimethaphan at a dose of 50 mcg/kg/min and adjusted to restore the individual subjects’ baseline blood pressure (up to a maximum systolic blood pressure of 140 mm Hg in hypertensive subjects). Similar blood pressure levels were achieved on both days at baseline and at the end of the hyperinsulinemic clamp (Table 2).
Table 2.
Differences in hemodynamic parameters between days and between baseline and hyperinsulinemic euglycemic clamp.
| Parameters | Saline | Autonomic Blockade | p (clamp) |
||
|---|---|---|---|---|---|
| Baseline | Clamp | Baseline | Clamp | ||
| Insulin Resistant Subjects (n=14) | |||||
| SBP, mm Hg | 147 ± 4.8 | 142 ± 5.8 | 146 ± 4.3 | 136 ± 4.8 | 0.404 |
| DBP, mm Hg | 79 ± 3.1 | 74 ± 3.4 | 80 ± 2.6 | 79 ± 2.2 | 0.263 |
| HR, bpm | 68 ± 2.1 | 70 ± 2.1 * | 68 ± 2.2 | 80 ± 2.6 * | 0.0001 |
| CO, L/min | 6.4 ± 0.3 | 7.5 ± 0.7 | 7.3 ± 0.81 | 6.8 ± 0.56 | 0.272 |
| SV, mL | 94 ± 4.7 | 103 ± 7.9 | 106 ± 11.5 | 87 ± 6.5 | 0.095 |
| TPR, dyn×sec×cm−5 | 1262 ± 70.8 | 1134 ± 113.7 | 1243 ± 105.6 | 1159 ± 92.5 | 0.808 |
| Epinephrine, ρg/mL | 14 ± 2.4 | 40 ± 15.1 * | 15 ± 2.9 | 14 ± 5.3 | 0.039 |
| Norepinephrine, ρg/mL | 185 ± 15.8 | 207 ± 19.0 | 171 ± 22.5 | 55 ± 11.4 * | 0.0001 |
| LFSYS, mm Hg2 | 8.5 ± 2.26 | 6.18 ± 0.91 | 7.42 2.01 | 2.24 ± 1.04 * | 0.245 |
| LFRRI, msec2 | 447.4 ± 87.7 | 387.9 ± 81.22 | 398.5 ± 68.8 | 1.97 ± 0.45 * | 0.001 |
| HFRRI, msec2 | 363.1 ± 109.4 | 302.0 ± 136.97 | 346.3 ± 131.6 | 7.74 ± 1.99 * | 0.001 |
| Insulin Sensitive Subjects (n=7) | |||||
| SBP, mm Hg | 142 ± 7.1 | 142 ± 7.7 | 143 ± 7.5 | 138 ± 5.5 | 0.509 |
| DBP, mm Hg | 74 ± 3.0 | 70 ± 4.29 | 73 ± 4.6 | 73 ± 3.6 | 0.545 |
| HR, bpm | 66 ± 3.8 | 70 ± 3.46 * | 66 ± 4.0 | 77 ± 4.7 * | 0.117 |
| CO, L/min | 6.6 ± 0.63 | 7.5 ± 1.0 | 6.4 ± 0.69 | 7.0 ± 0.72 | 0.317 |
| SV, mL | 98 ± 11.9 | 98 ± 12.3 | 96 ± 9.8 | 91 ± 10.1 | 0.216 |
| TPR, dyn×sec×cm−5 | 1202 ± 135.7 | 1159 ± 206.4 | 1265 ± 173.1 | 1182 ± 137.9 | 0.287 |
| Epinephrine, ρg/mL | 16 ± 3.9 | 24 ± 6.99 | 16 ± 3.8 | 8.3 ± 2.9 | 0.073 |
| Norepinephrine, ρg/mL | 177 ± 14.0 | 227 ± 21.24 | 189 ± 26.0 | 67 ± 26.2 * | 0.0001 |
| LFSYS, mm Hg2 | 11.2 ± 3.10 | 14.06 ± 4.08 | 11.49 ± 4.04 | 2.91 ± 1.35 * | 0.249 |
| LFRRI, msec2 | 335.8 ± 85.44 | 441.2 ± 130.19 | 409.2 ± 99.5 | 3.00 ± 0.45 * | 0.028 |
| HFRRI, msec2 | 158.5 ± 68.38 | 130.0 ± 51.63 | 144.4 ± 25.6 | 7.8 ± 1.56 * | 0.028 |
Values are expressed as mean ± S.E.M. The “p (clamp)” column reflects differences between study days (saline vs. blocked) during the clamp
p <0.05 for the comparison between baseline and clamp for each study day.
No significant differences were observed between study days at baseline.
Intact Autonomic Study Day
All procedures were identical to the blocked study day; normal saline was infused instead of trimethaphan and L-NMMA at a rate of 48 mL/hr to mimic the rate of trimethaphan infused on the autonomic blockade day.
Muscle Sympathetic Nerve Activity (MSNA)
Resting MSNA was measured as previously described 17. MSNA bursts were identified after filtering the integrated signal and artifacts were eliminated using an automated detection algorithm. MSNA bursts were accepted if the signal-to-noise ratio was greater than 2:1 and synchronization to a previous cardiac event was found in an interval between 1.2 and 1.6 seconds. All detections were visually verified.
Spectral Analysis
ECG and blood pressure were digitized with 14-bit resolution and 500 Hz sample frequency, recorded (WINDAQ data acquisition system, DATAQ), and analyzed using a customized analysis program written in PV-Wave by one of the authors (AD). Spectral analysis was performed as previously described 18, and according to Task Force recommendations 19. Linear trends were removed and power spectral density was estimated with the FFT-based Welch algorithm using segments of 256 data points with 50% overlapping and Hanning window 20. The power in the frequency range of low frequencies (LF: 0.04–0.15 Hz) and high frequencies (HF: 0.15–0.40 Hz) were calculated. Abolition of HR rate and BP variability was used to document autonomic blockade. In addition, low frequency variability of systolic blood pressure (LFSYS) was used as an indirect measure of sympathetic modulation 21, while high frequency variability of HR (HFRRI) was used as an indirect measure of cardiac parasympathetic tone 22, 23.
Analytical Measurements
Screening glucose, total cholesterol, triglycerides, high density lipoprotein (HDL) and low density lipoprotein (LDL) levels were measured by the Vanderbilt University Medical Center Clinical Laboratory. During the clamps plasma glucose was measured in duplicate every five minutes using a YSI 2300 STAT plus device (YSI Inc. Life Sciences, Yellow Spring USA). Plasma insulin, glucagon and leptin were measured by double-antibody/PEG RIA by Vanderbilt Hormone Assay Core Laboratory. Plasma adiponectin and resistin were measured using Lincoplex® Multiplex Immunoassay (Millipore). Free fatty acids (FFA), were measured using an enzymatic assay (HR series NEFA-HR(2) assay, Wako Diagnostics, Richmond, VA, USA) by Vanderbilt DRTC lipid core. Catecholamines were measured using HPLC separation with electrochemical detection 24.
Calculations
Whole body glucose uptake (MBW in mg/kg/min), an index of maximal muscle glucose utilization, was calculated as the average amount of glucose infused during the last 30 minutes of the clamp14. Lean body mass (LBM, in kg), obtained from DEXA scans were used to adjust M (MLBM). A cutoff value of 5.2mg/kg/min during the intact day was used to define insulin sensitive (>5.2 mg/kg/min) or resistant subjects (<5.2mg/kg/min) 25.
Statistical Analysis
Data are presented as mean±S.E.M. Comparisons of outcomes by intervention within subjects were performed using Wilcoxon signed-rank non-parametric tests. The main outcome was insulin sensitivity measured as the GIR corrected by body weight (MBW, mg/kg/min, higher number indicating greater insulin sensitivity) needed to maintain euglycemia during the last 30 minutes of the hyperinsulinemic clamp. The null hypothesis was that insulin sensitivity would not be different between intact and blocked study days among insulin resistant subjects. All of the tests were 2-tailed, and a P value of <0.05 was considered significant. Analyses were performed with SPSS statistical software (Version 22.0.0, SPSS Inc.). Power calculations estimated that 13 subjects were needed to detect a difference in glucose utilization of 1.7 mg/kg/min between the intact and blocked days, with 90% power, assuming a standard deviation of 1.7, and a type I error probability of 5%.
RESULTS
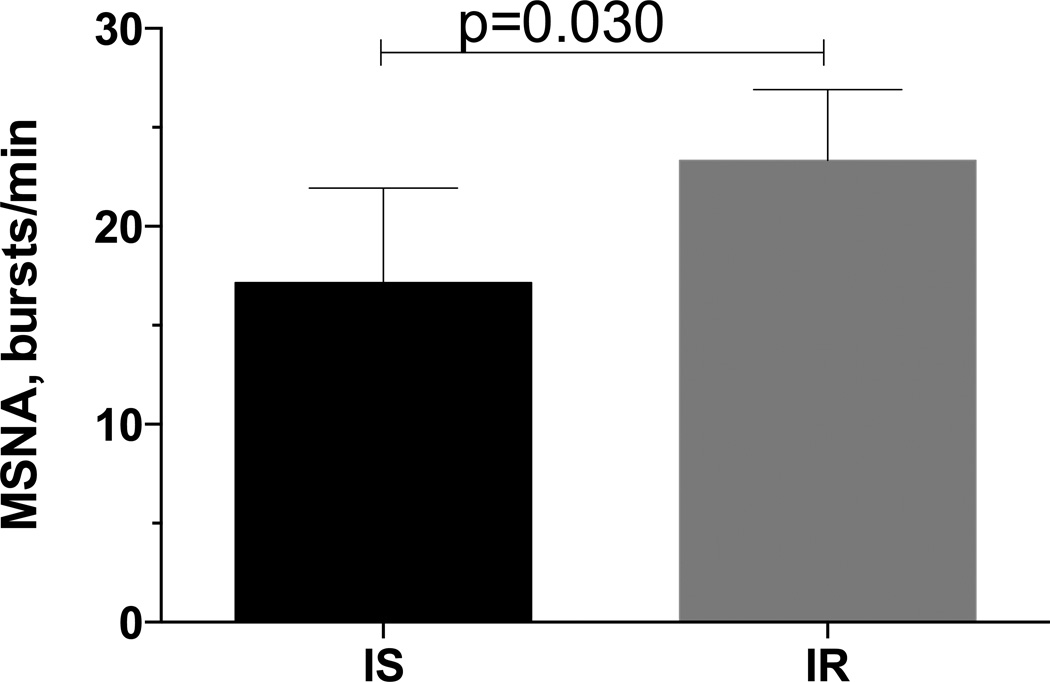
We enrolled 21 subjects with a mean age of 43±2.3 years. As expected, they were obese with a BMI of 35±2.2 kg/m2, and 41.5±1.7 % of body fat. Most of them were also pre-hypertensive or had hypertension (139/85±4/3 mm Hg). Based on the euglycemic clamp data from the saline day, subjects were divided into insulin sensitive subjects (MBW≥5, n=7) or resistant (MBW<5, n=14). Baseline data obtained during the screening visit and during the study days are presented in Table 1. Insulin resistant subjects were more obese, and had higher plasma glucose and insulin levels. Figure 2 shows basal MSNA for the 5 insulin sensitive and 6 insulin resistant subjects in whom this data was available. The remaining subjects declined to participate in the MSNA portion of the study (n=7) or a satisfactory recording could not be obtained (n=3). MSNA was higher among insulin resistant subjects than in insulin sensitive subjects (23.3±1.5 vs.17.2±2.1 burst/min, p=0.03).
Table 1.
Demographic and baseline characteristics of all patients studied
| Parameters | Insulin Sensitive (n, 7) |
Insulin Resistant (n, 14) |
p value |
|---|---|---|---|
| Gender, F/T (%) | 4/7 (57) | 5/14 (36) | |
| Age, years | 44 ± 4 | 42 ± 3 | 0.689 |
| Weight, kg | 92 ± 4 | 115 ± 4 | 0.003 |
| BMI, kg/m2 | 33.0 ± 0.6 | 36.3 ± 1.1 | 0.067 |
| Body Fat, % | 41.9 ± 3.9 | 41.4 ± 2.1 | 0.775 |
| Body Fat, kg | 37.2 ± 0.13 | 46.9 ± 3.11 | 0.075 |
| Fat Free Mass, kg | 52.0 ± 5.1 | 66.5 ± 3.4 | 0.059 |
| Android/Gynecoid ratio | 1.0 ± 0.1 | 1.3 ± 0.1 | 0.026 |
| Waist/Hip ratio | 0.9 ± 0.14 | 1.2 ± 0.02 | 0.046 |
| Systolic BP, mm Hg | 133 ± 7 | 141 ± 5 | 0.636 |
| Diastolic BP, mm Hg | 86 ± 4 | 85 ± 3 | 0.913 |
| Heart rate, bpm | 70 ± 6 | 72 ± 3 | 0.689 |
| Glucose, mmol/L | 4.7 ± 0.19 | 5.6 ± 0.20 | 0.012 |
| Insulin, mU/dL | 8.8 ± 1.8 | 23.3 ± 3.9 | 0.006 |
| Triglycerides, mg/dL | 71.8 ± 10.2 | 129.1 ± 16.2 | 0.020 |
| Cholesterol, mg/dL | 167.3 ± 6.4 | 165.2 ± 6.1 | 0.467 |
| HDL Cholesterol, mg/dL | 47.3 ± 4.4 | 40.4 ± 2.4 | 0.153 |
| LDL Cholesterol, mg/dL | 105.7 ± 5.8 | 109.1 ± 8.9 | 0.639 |
| hsCRP, µg/ml | 5.2 ± 2.7 | 4.3 ± 1.2 | 0.902 |
| HOMA2 %B | 121.4 ± 21.7 | 160.9 ± 17.8 | 0.197 |
| HOMA2 %S | 133.5 ± 44.3 | 61.9 ± 22.3 | 0.003 |
| HOMA2 IR | 1.1 ± 0.2 | 3.0 ± 0.5 | 0.003 |
| Epinephrine, pg/mL * | 19 ± 5 | 28 ± 8 | 0.913 |
| Norepinephrine, pg/mL * | 183 ± 17 | 178 ± 18 | 0.689 |
| LFSYS, mm Hg2* | 10.8 ± 3.3 | 8.0 ± 1.9 | 0.360 |
| LFRRI, msec2* | 372 ± 81 | 423 ± 72 | 0.743 |
| HFRRI, msec2* | 151 ± 28 | 355 ± 115 | 0.689 |
| Glucagon, pg/mL* | 77 ± 7 | 91 ± 7 | 0.197 |
| Leptin, ng/mL* | 25 ± 5 | 24 ± 4 | 0.913 |
| Adiponectin, µg/mL* | 17 ± 4 | 7 ± 1 | 0.025 |
| Resistin, ng/mL* | 27 ± 4 | 27 ± 2 | 0.585 |
| Free Fatty Acids mmol/L* | 0.41 ± 0.06 | 0.51 ± 0.03 | 0.689 |
Values are expressed as mean ± S.E.M and were obtained during the screening visit, except for those marked with an asterisk (*), which were the average at baseline of the two study days
Figure 2.
Basal Muscle Sympathetic Nerve Activity (MSNA) was significantly higher in obese insulin resistant subjects (IR, n=6) compared to obese insulin sensitive subjects (IS, n=5).
Hemodynamic data obtained on both study days is shown in Table 2. During the saline study day (intact autonomic function), insulin produced a small increase in HR in both insulin sensitive and resistant subjects; it also increased plasma epinephrine levels but this increase only reached statistical significance in insulin resistant subjects. None of the subjects developed hypoglycemia (plasma glucose>70mg/dL) at any point during any of the clamps. Furthermore, during the last 30 minutes of the clamp, when blood was drawn for catecholamines, plasma glucose levels were ≥90 mg/dL in all subjects. During the autonomic blockade study day, HR increased significantly as expected, reflecting net vagal withdrawal, and all indices of autonomic function decreased, including plasma norepinephrine, LFSYS and HFRRI, in both insulin sensitive and resistant subjects. Ganglionic blockade also prevented the increase in plasma epinephrine induced by insulin in insulin resistant subjects.
Metabolic data obtained on both study days is shown in Table 3. During the saline study day (intact autonomic function), hyperinsulinemia resulted in a decrease in glucagon and in FFA, in both insulin sensitive and resistant subjects. These changes were also apparent during the autonomic blockade study day. When comparing values during the clamp between autonomic blockade and saline days, autonomic blockade resulted in higher FFA plasma values but only in the insulin sensitive subjects (0.06±0.01 vs. 0.11±0.06 mmol/L, p=0.039).
Table 3.
Differences in metabolic parameters between days and between baseline and hyperinsulinemic euglycemic clamp.
| Parameters | Saline | Autonomic Blockade | p (Clamp) |
||
|---|---|---|---|---|---|
| Baseline | Clamp | Base line |
Clamp | ||
| Insulin Resistant Subjects (n=14) | |||||
| Glucose, mmol/L | 6.2 ± 0.4 | 5.5 ± 0.07 | 5.9 ± 0.2 | 5.3 ± 0.06 * | 0.198 |
| Insulin, µU/mL | 24.6 ± 5.7 | 156 ± 10.5 * | 18.5 ± 2.8 | 160.9 ± 9.7 * | 0.209 |
| Glucagon, ρg/mL | 91.7 ± 7.5 | 74.8 ± 6.6 * | 90.8 ± 7.2 | 69.0 ± 5.9 * | 0.198 |
| Leptin, ηg/mL | 23.1 ± 3.7 | 22.7 ± 3.6 | 23.9 ± 3.9 | 25.0 ± 4.0 | 0.124 |
| Adiponectin, µg/mL | 6.9 ± 0.9 | 6.9 ± 0.9 | 7.7 ± 1.0 | 7.3 ± 1.0 | 0.594 |
| Resistin, ηg/mL | 27.0 ± 2.6 | 29.8 ± 2.7 | 27.9 ± 2.2 | 29.8 ± 2.6 * | 0.826 |
| Free Fatty Acids, mmol/L | 0.50 ± 0.03 | 0.12 ± 0.02 * | 0.53 ± 0.04 | 0.11 ± 0.014 * | 0.304 |
| Insulin Sensitive Subjects (n=7) | |||||
| Glucose, mmol/L | 5.3 ± 0.1 | 5.2 ± 0.07 | 5.6 ± 0.3 | 5.0 ± 0.1 | 0.499 |
| Insulin, µU/mL | 13.3 ± 1.2 | 130.5 ± 6.9 * | 11.8 ± 1.1 | 127.6 ± 6.1 * | 0.866 |
| Glucagon, ρg/mL | 77.1 ± 6.5 | 55.0 ± 4.8 * | 77.0 ± 8.6 | 51.0 ± 3.8 * | 0.075 |
| Leptin, ηg/mL | 25.4 ± 5.4 | 25.0 ± 5.2 | 24.3 ± 5.6 | 25.1 ± 5.5 | 0.345 |
| Adiponectin, µg/mL | 17.2 ± 3.3 | 15.5 ± 2.9 | 16.7 ± 3.9* | 14.7 ± 3.0 | 0.600 |
| Resistin, ηg/mL | 27.3 ± 4.3 | 28.0 ± 3.9 | 25.9 ± 4.2 | 27.1 ± 5.5 | 0.500 |
| Free Fatty Acids, mmol/L | 0.42 ± 0.06 | 0.11 ± 0.06 * | 0.39 ± 0.07 | 0.06 ± 0.01 * | 0.039 |
Values are expressed as mean ± S.E.M. The “p (clamp)” column reflects differences between study days (saline vs. blocked) during the clamp.
p <0.05 for the comparison between baseline and clamp for each study day. No significant differences were observed between study days at baseline, but insulin was significantly lower in the insulin resistant subjects at baseline on the blockade day (p=0.048)
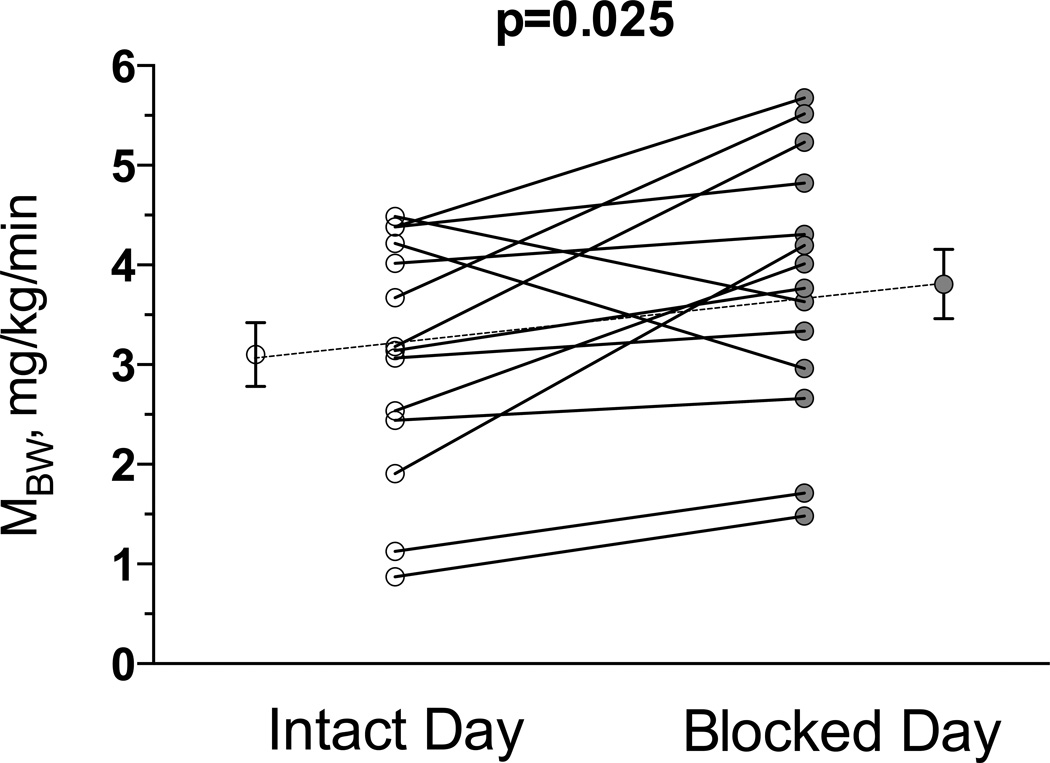
There were no differences in insulin sensitivity between intact and blocked study days in insulin sensitive subjects, (MBW, 6.9±0.8 and 6.5±0.9 mg/kg/min for the intact and blocked days respectively, p=0.310). In contrast, in insulin resistant subjects, autonomic blockade significantly improved insulin sensitivity (MBW from 3.1±0.3 to 3.8±0.3 mg/kg/min, p=0.025, Figure 3). A similar result was obtained if values were adjusted by lean body mass (MLBM, from 5.4±0.6 to 6.6±0.6 mg/kg of lean body mass/min, p=0.030).
Figure 3.
Individual differences in insulin sensitivity were measured on the intact and blocked autonomic study days in the insulin resistant obese subjects. MBW, a measure of the glucose infusion rate needed to maintain euglycemia corrected by plasma glucose and body weight was significantly higher on the blocked compared to intact study day suggesting increased insulin sensitivity following acute autonomic blockade.
DISCUSSION
The main finding in this study is that sympathetic activity was higher in insulin resistant obese subjects compared to insulin sensitive subjects, and that autonomic withdrawal acutely improved insulin sensitivity in insulin resistant patients, as determined by the hyperinsulinemic euglycemic clamp method. We interpret these findings as supporting the notion that sympathetic activation contributes to the metabolic abnormalities associated with obesity. They also raise the possibility of a negative feedback loop, whereby the increased sympathetic activity contributes to insulin resistance, leading to a compensatory increase in insulin levels, which then contribute to grater sympathetic activation. Consistent with this possibility, the improvement in insulin sensitivity produced by pioglitazone is associated with lowering of sympathetic activity 26.
Most studies have shown that obesity is accompanied by an increase in sympathetic tone. Furthermore, total fat mass and more specifically visceral fat mass are positively correlated with measurements of sympathetic activity.27 Our results are in agreement with these reports because our insulin resistant subjects, who had increased sympathetic activity, also had higher levels of fat mass and a greater waist/hip ratios suggesting greater visceral fat. They also had higher insulin and lower adiponectin levels two pathways proposed to contribute to sympathetic activation in obesity 27, 28.
The sympathetic activation in obesity, however, is not homogenous. It is often not reflected in forearm venous plasma norepinephrine, but is preferentially increased in sympathetic fibers relevant to blood pressure regulation, as evidenced by an increase MSNA, which reflects central sympathetic outflow coupled to baroreflex regulation.29 There is increasing evidence this sympathetic activation contributes to hypertension in obesity. We have previously shown, using the same ganglionic blockade approach employed in this study, that autonomic withdrawal normalizes blood pressure in obese hypertensives.30 Intrinsic systolic blood pressure (in the absence of autonomic influences) decreased to normal levels in obese hypertensives (106±4.6 mm Hg), consistent with the hypothesis that sympathetic activation contributes to hypertension in obesity. However, sympathetic activation is unlikely the sole determinant of the increase in blood pressure associated with obesity, because intrinsic blood pressure was still 15 mm Hg higher than normotensive controls (91±2.2 mm Hg) 30.
Less is know about the contribution of sympathetic activation to the metabolic abnormalities of obesity. It has been proposed that sympathetic activation is a compensatory homeostatic attempt to increase resting metabolic rate and restore energy balance. Resting metabolic rate is indeed consistently higher in obese individuals, but we previously found that ganglionic blockade decreased resting energy expenditure by the same percentage (about 3%) in in obese and lean individuals, so that resting energy expenditure in obese individuals remained elevated after ganglionic blockade.31 This suggests that sympathetic activation failed to provide the expected beneficial metabolic effect on resting energy expenditure. Furthermore, the increase in resting energy expenditure seen in obesity could be explained completely by the increase in fat free mass that accompanies weight gain.31 Collectively, these findings suggest that the sympathetic activation in obesity contributes to hypertension, but has no benefit on metabolic function. On the contrary, we hypothesized that sympathetic activation has a negative metabolic effect, contributing to insulin resistance.
Indeed, we found that ganglionic blockade selectively improves insulin sensitivity in patients that are insulin resistant, but has no effect in those who are insulin sensitive. Insulin sensitivity was determined using the hyperinsulinemic euglycemic clamp, which is considered the gold standard. Based on these findings, we propose that sympathetic activation contributes to the insulin resistance of obesity. In agreement with our findings, previous studies showed that an acute increase in sympathetic nerve activity results in reduced forearm glucose uptake in healthy lean controls.8 We propose that the improvement in glucose utilization with autonomic blockade is best explained by an improvement in muscle glucose uptake, rather than changes in hepatic glucose production. This interpretation is based on our use of an insulin infusion rate that is high enough to suppress hepatic glucose production. Definitive proof will require the use of isotopes to confirm suppression of glucose production and to measure endogenous glucose production, which is particularly important in insulin resistant subjects, and even in subjects with impaired fasting glucose 32.
The mechanism by which sympathetic activation would impair insulin-mediated glucose uptake 8 is not completely understood. Adrenergic receptors can inhibit insulin-mediated glucose uptake in target tissue like skeletal muscle 33 although it is not clear how important this mechanism is during normal situations or during the conditions of our study (hyperinsulinemic clamps). Insulin sensitivity is also modulated by glucagon and hormonal factors, but we did not observe significant differences between intact and blocked days in a variety of metabolic indices measured during the euglycemic clamp (Table 3, insulin resistant group). Therefore, it is unlikely that changes in any of these hormones explain the improvement in insulin sensitivity. Insulin sensitivity is also affected by blood flow, and sympathetically-mediated vasoconstriction may impair insulin sensitivity through this mechanism 34. Conversely, insulin induces nitric-oxide mediated vasodilation, which increases substrate delivery to the muscle, to promote glucose uptake and thus improve insulin sensitivity 35. This effect is more important in the microvasculature, and insulin-induced vasodilation is hemodynamically not apparent in subjects with an intact autonomic nervous system because of baroreflex buffering of blood pressure. In contrast, insulin administration substantially lowers blood pressure in autonomic failure patients 36. We were, therefore, concerned that insulin would lower blood pressure when infused during ganglionic blockade. This could create both a safety concern, and would also complicate the interpretation of our results. For this reason we decided to clamp blood pressure with the NOS inhibitor L-NMMA while infusing insulin during autonomic blockade. Using this approach we were successful in matching blood pressure on each study day during the last 30 minutes of the clamp, when insulin sensitivity was measured. It is noteworthy that we observed an improvement of insulin sensitivity even in the presence of NOS inhibition, which can reduce glucose uptake. 35,37 In the present study however, we did not try to achieve complete NOS inhibition but only to counteract any decrease in blood pressure produced by insulin in the presence of ganglionic blockade. Even though we effectively clamped blood pressure during our studies, it is possible that sympathetic withdrawal improved blood flow at the level of the microcirculation, and improved insulin sensitivity through this mechanism 38.
We should note that ganglionic blockade produces complete suppression of both sympathetic and parasympathetic activities. Parasympathetic stimulation has beneficial metabolic effects 39, so parasympathetic suppression is less likely to explain the improved sensitivity to insulin during ganglionic blockade. A direct vasodilatory effect of ganglionic blockade has been reported in animal studies 40–42, but in the absence of other vasodilators trimethaphan does not lower blood pressure in normal subjects below the “intrinsic blood pressure” range, and does not lower blood pressure in patients with pure autonomic failure 43. It is unlikely, therefore, that a direct vasodilating action of trimethaphan played a role in the responses observed in the present study.
In summary, these findings suggest that sympathetic activation contributes to impaired glucose utilization and insulin resistance in obesity, thus supporting the concept that sympathetic activation contributes to the cardiovascular and metabolic derangements associated with obesity. This is also consistent with the existence of a negative feedback loop whereby the increase in insulin levels that accompanies insulin resistance contributes to sympathetic activation, leading to further insulin resistance.
Perspectives
Obesity has become the most common underlying condition associated with hypertension. Our findings would imply that therapies that lower sympathetic tone would be preferable in the treatment of obesity hypertension. Uncontrolled studies suggest that newer sympatholytics (i.e., moxonidine) improve insulin sensitivity 44 but are not widely used or available. It has been suggested that renal denervation improved insulin sensitivity 45, but its overall effectiveness in reducing sympathetic tone 46, or treating hypertension47, remains unclear. On the other hand thiazides, which are recommended as first line therapy for hypertension, increase in sympathetic activity 48 and increase the risk of developing diabetes. These recommendations, however, are based on outcome trials that enrolled patients that were older and less obese than the vast majority of obese hypertensives seen in clinical practice49. It is not clear, therefore, that thiazides should be the initial choice for starting antihypertensive therapy in such patients.
Novelty and Significance.
What is New?
We found that obese insulin resistant subjects have higher sympathetic activity than obese insulin sensitive subjects.
Acute pharmacologic blockade of the autonomic nervous system improved whole body glucose uptake in obese insulin resistant subjects.
What is Relevant?
Obesity and its complications are expected to keep increasing, therefore, finding novel therapeutic targets are of the utmost importance to fight this condition.
The association between increased sympathetic activity; obesity and insulin resistance is very well recognized. The role of increased sympathetic activity on insulin resistance, however, is not fully understood and not many studies have focused on this interaction.
Summary
Sympathetic activation contributes to impaired glucose utilization and insulin resistance in obesity, thus supporting the concept that sympathetic activation contributes to the cardiovascular and metabolic derangements associated with obesity.
Acknowledgements
We are very grateful to all the volunteers, the nurses form Vanderbilt clinical trial center and the Lipid Core of the Vanderbilt Diabetes Research Training Center (DRTC) (DK20593).
Sources of Support: This work was supported in part by National Institutes of Health grants P01 HL056693, U54 NS065736 and UL1 TR000445 from NCATS and DK 020593 (NNA). A.G is supported by NIH Grant K23 HL-95905.
Footnotes
Conflict(s) of Interest/Disclosure(s): None
CLINICAL TRIAL REGISTRATION
Please refer to this study by ClinicalTrials.gov: NCT00580957
Other Study ID Numbers: 060085, CRC-1522
Disclosures: None
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA : the journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the united states, 2000. JAMA : the journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: Actual causes of death in the united states, 2000. JAMA : the journal of the American Medical Association. 2005;293:293–294. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- 4.Olefsky J, REAVEN GM, Farquhar JW. Effects of weight reduction on obesity. Studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. The Journal of clinical investigation. 1974;53:64–76. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blüher M. Are there still healthy obese patients? Current opinion in endocrinology, diabetes, and obesity. 2012;19:341–346. doi: 10.1097/MED.0b013e328357f0a3. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Esler M. How to assess sympathetic activity in humans. Journal of Hypertension. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Flaa A, Aksnes TA, Kjeldsen SE, Eide I, Rostrup M. Increased sympathetic reactivity may predict insulin resistance: An 18-year follow-up study. Metabolism. 2008;57:1422–1427. doi: 10.1016/j.metabol.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R, Trimarco B, Saccá L. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. The American journal of physiology. 1994;266:E242–247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- 9.Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999;33:548–553. doi: 10.1161/01.hyp.33.1.548. [DOI] [PubMed] [Google Scholar]
- 10.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 11.Lind L, Eriksson M. Influence of nervous blockade on insulin-mediated glucose uptake in the human forearm. Metabolism: clinical and experimental. 2003;52:413–417. doi: 10.1053/meta.2003.50069. [DOI] [PubMed] [Google Scholar]
- 12.May M, Ahrens J, Menne J, Haller H, Beige J, Eckert S, Jordan J, Engeli S. Limited acute influences of electrical baroreceptor activation on insulin sensitivity and glucose delivery: A randomized, double-blind, cross-over clinical study. Diabetes. doi: 10.2337/db13-1651. Published ahead of print March 19, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: The volunteer for vanderbilt research program http://www.Volunteer.Mc.Vanderbilt.Edu. Journal of the American Medical Informatics Association : JAMIA. 2005;12:608–613. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. The American journal of physiology. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism: clinical and experimental. 1981;30:936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 16.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: Assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Biaggioni I, Killian TJ, Mosqueda-Garcia R, Robertson RM, Robertson D. Adenosine increases sympathetic nerve traffic in humans. Circulation. 1991;83:1668–1675. doi: 10.1161/01.cir.83.5.1668. [DOI] [PubMed] [Google Scholar]
- 18.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape SY, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension. 2007;49:170–177. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 19.Task-Force S. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Oppenheim AV, Schafer RW-ja. Digital signal processing. Englewood Cliffs, N.J.: Prentice-Hall; 1996. [Google Scholar]
- 21.Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 22.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: Investigation by spectral analysis. Am.J.Physiol. 1985;249:H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 23.Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- 24.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl A, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance. Role of the renin-angiotensin system. AJM. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 25.Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes care. 2012;35:1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoe H, Yuasa F, Yuyama R, Murakawa K, Miyasaka Y, Yoshida S, Tsujimoto S, Sugiura T, Iwasaka T. Effect of pioglitazone on arterial baroreflex sensitivity and sympathetic nerve activity in patients with acute myocardial infarction and type 2 diabetes mellitus. Journal of cardiovascular pharmacology. 2012;59:563–569. doi: 10.1097/FJC.0b013e31824f91a7. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 28.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. The Journal of clinical investigation. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 30.Gamboa A, Okamoto LE, Diedrich A, Choi L, Robertson D, Farley G, Paranjape S, Biaggioni I. Sympathetic activation and nitric oxide function in early hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302:H1438–1443. doi: 10.1152/ajpheart.01020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 32.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: Role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–1711. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- 33.Hunt DG, Ivy JL. Epinephrine inhibits insulin-stimulated muscle glucose transport. Journal of applied physiology (Bethesda, Md : 1985) 2002;93:1638–1643. doi: 10.1152/japplphysiol.00445.2002. [DOI] [PubMed] [Google Scholar]
- 34.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. The Journal of clinical investigation. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. The Journal of clinical investigation. 1995;96:786–792. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathias CJ, da Costa DF, Fosbraey P, Christensen NJ, Bannister R. Hypotensive and sedative effects of insulin in autonomic failure. British medical journal (Clinical research ed.) 1987;295:161–163. doi: 10.1136/bmj.295.6591.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natali A, Ribeiro R, Baldi S, Tulipani A, Rossi M, Venturi E, Mari A, Macedo MP, Ferrannini E. Systemic inhibition of nitric oxide synthesis in non-diabetic individuals produces a significant deterioration in glucose tolerance by increasing insulin clearance and inhibiting insulin secretion. Diabetologia. 2013;56:1183–1191. doi: 10.1007/s00125-013-2836-x. [DOI] [PubMed] [Google Scholar]
- 38.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 39.Straznicky NE, Nestel PJ, Esler MD. Autonomic nervous system: Metabolic function. Elsevier; 2009. pp. 951–959. [Google Scholar]
- 40.Mccubbin JW, Page IH. Nature of the hypotensive action of a thiophanium derivative (ro 2–2222) in dogs. The Journal of Pharmacology and Experimental Therapeutics. 1952;105:437–442. [PubMed] [Google Scholar]
- 41.Page IH, McCubbin JW. Increased resistance to autonomic ganglionic blockade by tetraethylammonium chloride and pentamethonium iodide in experimental neurogenic hypertension. The American journal of physiology. 1952;168:208–217. doi: 10.1152/ajplegacy.1951.168.1.208. [DOI] [PubMed] [Google Scholar]
- 42.Castro-Tavares J. Direct effects of trimetaphan on the dog mesenteric artery and saphenous vein. British journal of anaesthesia. 1980;52:769–771. doi: 10.1093/bja/52.8.769. [DOI] [PubMed] [Google Scholar]
- 43.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 44.Chazova I, Almazov VA, Shlyakhto E. Moxonidine improves glycaemic control in mildly hypertensive, overweight patients: A comparison with metformin. Diabetes, Obesity and Metabolism. 2006;8:456–465. doi: 10.1111/j.1463-1326.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- 45.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: A pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 46.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: Prospective case series. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. The New England journal of medicine. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 48.Okada Y, Jarvis SS, Best SA, Bivens TB, Adams-Huet B, Levine BD, Fu Q. Chronic renin inhibition lowers blood pressure and reduces upright muscle sympathetic nerve activity in hypertensive seniors. The Journal of Physiology. 2013;591:5913–5922. doi: 10.1113/jphysiol.2013.261362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biaggioni I. Should we target the sympathetic nervous system in the treatment of obesity-associated hypertension? Hypertension. 2008;51:168–171. doi: 10.1161/HYPERTENSIONAHA.107.090514. [DOI] [PubMed] [Google Scholar]