Abstract
Background
Cognitive impairment, including dementia, is common in Parkinson disease (PD). The Mini-Mental State Examination (MMSE) has been recommended as a screening tool for PDD, with values below 26 indicative of possible dementia. Using a detailed neuropsychological battery, we examined the range of cognitive impairment in PD patients with a MMSE score ≥ 26.
Methods
In this multi-center, cross-sectional, observational study, we performed neuropsychological testing in a sample of 788 PD patients with MMSE ≥ 26. Evaluation included tests of global cognition, executive function, language, memory, and visuospatial skills. A consensus panel reviewed results for 342 subjects and assigned a diagnosis of no cognitive impairment, mild cognitive impairment, or dementia.
Results
67% of the 788 subjects performed 1.5 standard deviations below the normative mean on at least one test. On eight of the 15 tests, more than 20% of subjects scored 1.5 standard deviations or more below the normative mean. Greatest impairments were found on Hopkins Verbal Learning and Digit Symbol Coding tests. The sensitivity of the MMSE to detect dementia was 45% in a subset of participants who underwent clinical diagnostic procedures.
Conclusions
A remarkably wide range of cognitive impairment can be found in PD patients with a relatively high score on the MMSE, including a level of cognitive impairment consistent with dementia. Given these findings, clinicians must be aware of the limitations of the MMSE in detecting cognitive impairment, including dementia, in PD.
Keywords: Parkinson disease, Parkinson disease with dementia, Cognition, Assessment of cognitive disorders/dementia, mild cognitive impairment
INTRODUCTION
In the last decade, clinicians and researchers have become increasingly aware of the non-motor aspects of Parkinson disease (PD), including cognitive impairment (CI). CI, which includes both dementia and mild cognitive impairment, is now recognized as a common component of PD. Indeed, point prevalence estimates of Parkinson’s disease dementia (PDD) range from 22% to 48%1 and prevalence estimates for cognitive impairment without dementia (PD-MCI) range from 19% to 55%.2
CI is associated with decreased quality of life and increased functional impairment, caregiver distress, and placement in nursing homes.3,4 Early detection of CI will permit appropriate planning for financial and medical contingencies while cognitive abilities are relatively preserved, reduce caregiver stress, and potentially delay placement in nursing homes.5 In recognition of the importance of CI in PD, the Movement Disorder Society published recommendations for the diagnosis of dementia in PD in 2007,1, 6 in which the Task Force on Dementia in Parkinson’s Disease proposed using the Mini-Mental State Examination (MMSE)7 as a primary clinical tool in screening for PDD during an office visit.6 Importantly, under the Task Force’s original guidelines, MMSE ≥ 26 precluded further assessment for PDD.
The utility of the MMSE in PD has been questioned, however, in part because the MMSE emphasizes language and orientation, which are relatively preserved in PDD.1 Recent validation studies that examined the Task Force PDD criteria found the checklist had low sensitivity, in large part due to the cutoff requirement on the MMSE.8, 9 Others have demonstrated the MMSE’s poor sensitivity for detecting CI (including MCI) in PD, by comparing it to the Montreal Cognitive Assessment (MoCA)10-12 or to a larger battery of neuropsychological tests,13, 14 and the MDS Task Force recommendations for the assessment of PD-MCI omitted the MMSE completely.15 Thus, the use of this measure as a screen for CI may lead to a large number of missed cases in PD patients. Indeed, the wide variation reported in the prevalence of CI in PD may be due in part to differences in cognitive measures employed, with less sensitive tasks such as the MMSE yielding lower prevalence rates.
The current study differs from prior studies in its scope, scale, and primary objective. This multi-center, cross-sectional, observational study examined a substantially larger cohort of PD subjects than previous studies and used an extensive battery of neuropsychological instruments to examine cognitive performance. Subjects were selected a priori to have MMSE scores ≥ 26. We hypothesized that this cohort would have a large variation in cognitive performance, further questioning the use of the MMSE in screening for CI in patients with PD.
METHODS
Subjects
Neuropsychological data were collected as part of a collaboration between three Morris K. Udall Centers of Excellence for Parkinson’s Disease Research, the Pacific Northwest Udall Center, located jointly at the University of Washington (Seattle, WA) and the Oregon Health and Science University (Portland, OR); the University of Pennsylvania (Philadelphia, PA); and the University of California-Los Angeles; as well as with Emory University (Atlanta, GA). Subjects were recruited from the participating academic centers, affiliated Veterans Affairs hospitals, community-based neurology clinics, PD support groups, and public service announcements.
Patients were diagnosed with PD according to the UK PD Society Brain Bank criteria16, 17 at all sites, except for those recruited at UCLA (N=189), where diagnosis was based on criteria described in detail elsewhere.18 From this group, all subjects with an MMSE score ≥ 26 were included in the primary analysis. Subjects completed a large battery of neuropsychological tests, although there were some differences in test batteries performed at each site. Where a discrepancy in test version or differences in technique of administration existed, only data from the version used most commonly across sites were included in analysis. Thus, not all subjects completed all neuropsychological tests. Results from a subset of this data (obtained at the University of Pennsylvania) have been previously published.10, 13
Standard protocol approvals, registrations, and patient consents
The institutional review boards at all institutions approved the study, and all subjects (or their legal surrogates) provided written informed consent.
Neuropsychological Examinations
A neuropsychological test battery was administered by trained research staff. Global cognitive screening measures included the Mini Mental State Examination (MMSE), a widely used instrument that emphasizes orientation, language, and attention, but also tests registration, recall, and visuospatial skills,7 the Montreal Cognitive Assessment (MoCA),19 which briefly assesses orientation, attention, memory, language, abstract verbal reasoning, and visuospatial skills, and the Mattis Dementia Rating Scale (DRS),20 which assesses attention, perseveration, construction, memory and conceptualization. More sensitive neuropsychological measures were incorporated to measure a number of cognitive domains, including attention, working memory, processing speed, learning and recall, visuospatial abilities, verbal fluency, and language. These tests included the Trail-Making Test (Parts A and B, as well as Part B minus Part A to account for possible disease related slowing/motor impairment)21 Digit-Symbol Coding and Digit Span (Forward and Backward) from the Wechsler Adult Intelligence Scale-Revised,22 Letter-Number Sequencing from the Wechsler Adult Intelligence Scale-III,23 the Hopkins Verbal Learning Test-Revised (Immediate and Delayed),24 Logical Memory (Immediate and Delayed) from the Wechlser Memory Scale-Revised,25 Judgment of Line Orientation,26 Boston Naming Test,27 and Semantic and Phonemic verbal fluency.28 Normative data for Digit Span, Digit Symbol Coding, Trailmaking, Logical Memory, Boston Naming Test, and semantic verbal fluency were derived from the National Alzheimer’s Coordinating Center Uniform Data set,29, 30 while normative data for the HVLT-R, Letter-Number Sequencing, and Judgment of Line Orientation were obtained from published test manuals (above). Normative data provided by Tombaugh et al.28 were used for phonemic fluency, and normative data provided by Drane et al.31 were used for Trailmaking A minus B.
Consensus Cognitive Diagnosis
In order to conduct secondary sensitivity and specificity analyses to determine whether the MMSE can accurately predict assignment of clinical cognitive diagnoses, subjects who underwent clinical consensus diagnosis procedures at the Pacific Northwest Udall Center were evaluated separately, regardless of MMSE score (n=342). These subjects received a cognitive diagnosis of No CI (NCI), PD-MCI, or PDD as described previously.32 Briefly, this cognitive diagnosis was made on the basis of the subjects’ scores on neuropsychological tests and any functional limitation caused by their cognitive impairment. All of these cases were discussed in conference with, at minimum, one movement disorders neurologist, one behavioral neurologist, and one neuropsychologist. This conference was held within two months of the testing, although the diagnosis was based on testing done on the same date as the MMSE. A diagnosis of dementia was made according to the Level II evaluation proposed by the MDS Task Force on PD Dementia, which excludes the MMSE in favor of more detailed neuropsychological testing.1, 6 Diagnosis of PD-MCI was based on a performance on two neuropsychological tests of one or more cognitive domains that were at least 1 standard deviation below the normative mean. Information concerning activities of daily living was gathered via clinical interview of the patient and caregiver by the clinician assessing the patient, and a diagnosis of PD-MCI was assigned if the cognitive deficits were not significant enough to substantially interfere with activities of daily living. This is consistent with recently published guidelines for PD with mild cognitive impairment, PD-MCI,15 although these guidelines were not available at the time of case review. When PD motor symptoms substantially impaired performance on neuropsychological tests that were motor-dependent, these tests were considered invalid and not used to form a diagnostic opinion. Cognitive diagnoses were based on neuropsychological scores that were corrected for pre-morbid intelligence using the Shipley Institute of Living Scale-2.33
Analysis
Because the goal of this study was to examine the breadth of cognitive ability among those with MMSE scores ≥ 26, the primary outcome measure was the Z-score for each neuropsychological test. Z-scores are a measurement of an individual’s performance relative to a reference population. A Z-score of -1.0, for example, indicates that the value is 1 standard deviation below the normative mean for the reference population. Here, Z-scores were computed from normative clinical reference data, and hence, the reference population is healthy, age-matched controls. We refer to normative means throughout to remind readers that these are not Z-distributions based on our sample data. In addition, for each neuropsychological test, the mean, standard deviation, and 95% confidence intervals were calculated. In secondary analyses, sensitivity and specificity were calculated for participants who underwent clinical diagnostic consensus procedures; all participants who completed the MMSE were included in these analyses, regardless of MMSE score. All data were sent to the Pacific Northwest Udall Center Data Core and were merged into a single dataset for analysis. Statistical analysis was carried out using commercially available software (SPSS 16.0, IBM, Armonk, NY; and Excel 2007, Microsoft, Redmond, WA). Missing responses were excluded from the analysis.
RESULTS
1,015 subjects qualified for a diagnosis of PD based on criteria described above. Of those, 928 had completed the MMSE. Subjects were selected who had MMSE scores of ≥ 26. This produced a total sample of 788 subjects. Of these, 59 subjects (7%) had a score of 26, 135 (17%) scored a 27, 141 (18%) scored a 28, 219 (28%) scored a 29, and 234 (30%) scored a perfect 30. 140 subjects meeting PD criteria scored below 26 on the MMSE. Table 1 shows demographic characteristics of the included subjects, as well as a breakdown of neuropsychological test z score means by MMSE score.
Table 1. Demographic characteristics and neuropsychological test z-scores, by MMSE score.
| MMSE ≥ 26 | MMSE <26 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean (SD) | Range | N | Mean (SD) | Range | |
| Age at Assessment | 786 | 68.4 (9.0) | 38 - 91 | 140 | 74.4 (7.4) | 52 -94 |
| Age at Onset | 601 | 58.8 (10.3) | 23 - 86 | 125 | 64.4 (9.9) | 39 -84 |
| Hoehn and Yahr Stage (median score) | 754 | 2 | 1 - 5 | 123 | 2.5 | 1 - 5 |
| Years of Education | 788 | 15.8 (2.8) | 7 - 29 | 139 | 15.1 (2.8) | 8 - 20 |
| % Male | 788 | 63% | - | 140 | 76% | - |
| MMSE | 788 | 28.55 (1.28) | 26 - 30 | 140 | 22.72 (3.05) | 6 - 25 |
| MoCA | 585 | 24.97 (3.28) | 11 - 30 | 97 | 19.31 (4.72) | 6 - 30 |
| Mattis DRS (z-score) | 380 | .11 (0.90) | -2.33 - 2 | 99 | -1.20 (1.03) | -2.67 - 1.67 |
| Trails A (z-score) | 595 | -1.10 (1.88) | -10.82 – 1.42 | 71 | -2.96 (2.76) | -11.2 – 0.79 |
| Trails B (z-score) | 584 | -1.25 (1.80) | -6.8 – 1.34 | 64 | -3.06 (1.9) | -6.80 – 0.65 |
| Digit Symbol Coding (z-score) | 424 | -0.99 (1.07) | -4.69 – 1.97 | 61 | -1.75 (1.05) | -4.11 – 1.01 |
| Digit Span, Forward (z-score) | 230 | 0.07 (0.93) | -3.23 – 1.7 | 26 | -0.29 (1.00) | -2.3 – 1.7 |
| Digit Span, Backward (z-score) | 230 | -0.31 (0.99) | -2.36 – 2.66 | 26 | -0.97 (0.65) | -2.36 – 0.04 |
| Letter-Number Sequencing (z-score) | 586 | 0.11 (1.04) | -3.0 – 3.0 | 98 | -0.98 (1.00) | -3.0 – 1.0 |
| HVLT-R, Immediate (z-score) | 734 | -0.60 (1.19) | -4.85 – 2.25 | 123 | -1.98 (1.20) | -4.85 – 1.10 |
| HVLT-R, Delayed (z-score) | 731 | -0.77 (1.50) | -5.45 – 1.54 | 121 | -2.04 (1.43) | -5.45 – 1.30 |
| Logical Memory I (z-score) | 451 | -0.52 (1.10) | -3.47 – 2.2 | 42 | -1.53 (1.19) | -4.08 – 1.5 |
| Logical Memory II (z-score) | 451 | -0.53 (1.10) | -3.14 – 2.28 | 42 | -1.48 (0.96) | -3.14 – 0.75 |
| Judgment of Line Orientation (z-score) | 597 | 1.13 (2.16) | -2.45 – 3.99 | 102 | -0.26 (1.95) | -2.45 – 3.99 |
| Boston Naming Test (z-score) | 403 | -0.06 (0.86) | -4.21 – 1.05 | 34 | -0.26 (1.95) | -2.45 – 3.99 |
| Fluency, Semantic (z-score) | 783 | -0.58 (1.04) | -3.43 – 2.83 | 125 | -1.42 (0.93) | -3.61 – 2.12 |
| Fluency, Phonemic (z-score) | 768 | 0.05 (1.08) | -2.44 – 3.83 | 114 | -0.71 (0.95) | -2.81 – 1.37 |
Abbreviations: Std Dev = standard deviation; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; DRS = Dementia Rating Scale; HVLT-R = Hopkins Verbal Learning Test – Revised.
Figure 1 shows the broad distribution of MoCA scores for those with MMSE scores ≥ 26. Over half (51.9%) of those with MMSE scores ≥ 26 scored 25 or below on the MoCA. For the MoCA, both mean and median were 25. Z-scores were not calculated for the MoCA because only limited normative data are available for this test. A MoCA score of 26 is the proposed lower limit of normal (http://www.mocatest.org/normative_data.asp), meaning that just over half of the subjects who had “normal” MMSE scores showed evidence of CI on the MoCA.
Figure 1. Distribution of MoCA scores for all subjects with MMSE ≥ 26.
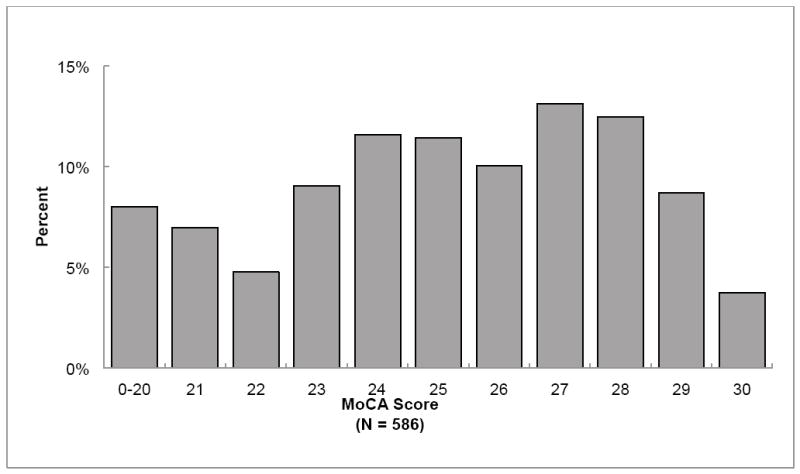
Abbreviations: MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Results of specific neuropsychological tests show that for Digit Symbol Coding, 47.3% of subjects scored at least 1 standard deviation below the normative mean, 31.1% scored at least 1.5 standard deviations below the normative mean, and 16.7% scored at least 2 standard deviations below the normative mean. Similar results were seen for delayed recall on the HVLT-R, with 28.6% scoring at least 1.5 standard deviations below the normative mean, and 16.9% scoring at least 2 standard deviations below the normative mean (Supplemental Table).
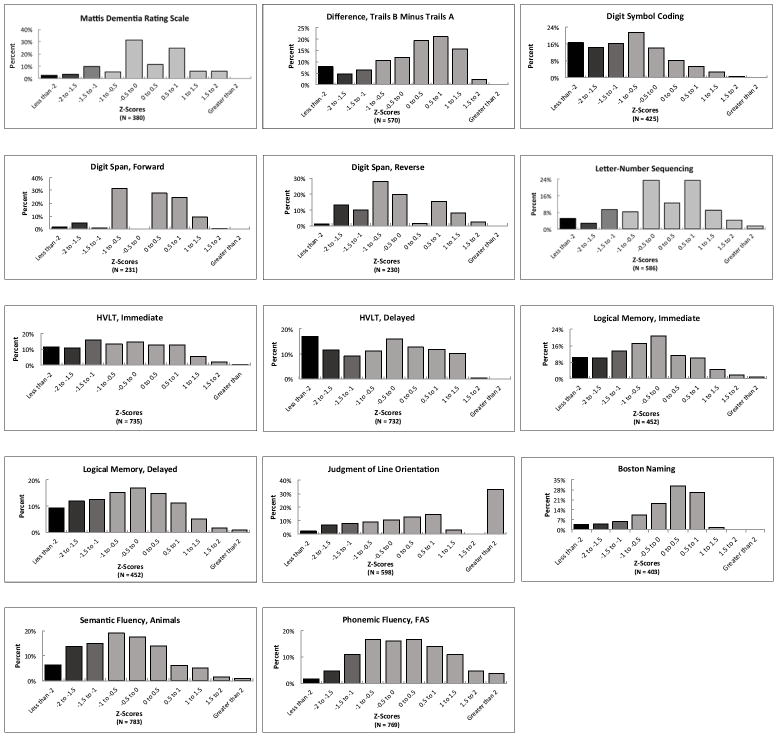
Figure 2 expands on these data by presenting histograms with the full distributions of Z-scores for each cognitive test performed. In every case, there is a broad distribution of performance. On 8 of the cognitive tests administered (Mattis, Digit-Symbol Coding, HVLT Immediate Recall, HVLT Delayed Recall, Logical Memory Immediate Recall, Logical Memory Delayed Recall, Semantic Fluency, and Phonemic Fluency), the median Z-score was below 0, meaning over half of the subjects – all of whom had “normal” MMSE scores – performed below average on these tests. Overall, eight of the 15 neuropsychological tests (excluding the MoCA) had a similar distribution, with more than 20% of subjects scoring at least 1.5 standard deviations below the clinical normative mean. All told, 67% of the 788 subjects with “normal” MMSE scores performed at least 1.5 standard deviations below the normative mean on one or more of the 15 tests and 45% scored at least 1.5 standard deviations below the mean on two or more. Sixty-four percent of these subjects scored at least 1.0 standard deviation below the mean on two or more tests.
Figure 2. Distribution of Z-scores on neuropsychological tests for all subjects with MMSE ≥ 26.
Abbreviations: MoCA = Montreal Cognitive Assessment; HVLT-R = Hopkins Verbal Learning Test-Revised.
Sensitivity and specificity were calculated for the entire subset of subjects who underwent consensus diagnosis procedures at the Pacific Northwest Udall Center, including the full range of MMSE scores (see “Consensus cognitive diagnosis” in the Methods section) (Figure 3). Of 342 total PD subjects at this Center, 290 had a “normal” MMSE score (26 or greater). Of the 290 with “normal” MMSE scores, 42 (15%) were given a consensus diagnosis of dementia and 170 (58.6%) were given a consensus diagnosis of PD-MCI. Thus, for dementia in the subset of subjects that underwent consensus review, the MMSE had a sensitivity of 45%, specificity of 94%, negative predictive value of 86%, and a positive predictive value of 67%.
Figure 3. Cognitive diagnosis by MMSE score, all subjects.
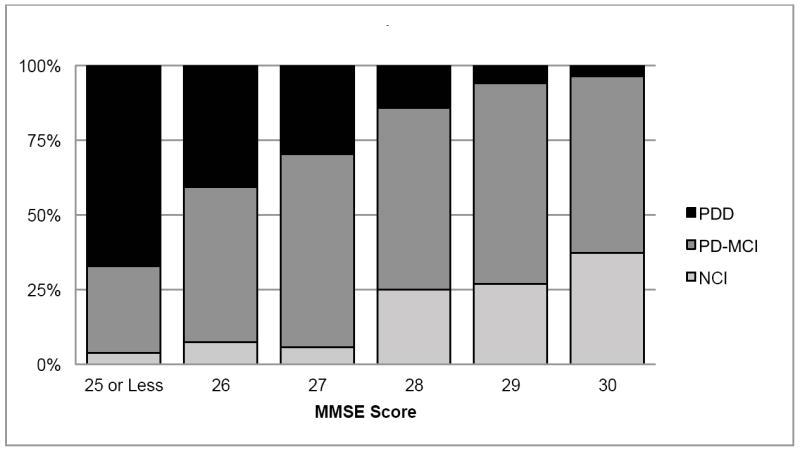
Abbreviations: PDD = Parkinson’s Disease Dementia;PD-MCI = Mild Cognitive Impairment; NCI = No cognitive impairment; MMSE = Mini-Mental State Examination. Percent frequency of cognitive diagnosis grouped by the score on the MMSE, for a subset of subjects who received a clinical cognitive diagnosis by consensus of an expert panel.
Figure 3 also shows the breakdown of cognitive diagnoses for subjects from the Pacific Northwest Udall Center with MMSE scores of 26 or greater. Of those with a perfect MMSE score, 3.5% (3 of 86) received a diagnosis of dementia on the basis of other cognitive testing and functional limitations. Of those with scores of 26 to 28, 25.5% were diagnosed with dementia (35 of 137).
The supplemental figure shows mean scores for each cognitive test by MMSE score, which will be of interest to readers familiar with the scoring for these tests. The figure demonstrates the broad range of scores in this population.
DISCUSSION
As predicted, we observed a remarkably broad range of cognitive performance in PD subjects who scored ≥ 26 on the MMSE. Although some subjects did have normal cognition, many showed evidence of CI, including a number who fulfilled MDS criteria for dementia. If the MMSE were used as the only instrument to detect dementia in PD, as is the case in many clinical settings, 55% of the dementia cases in our study would have been missed. Individual neuropsychological tests perhaps best demonstrated the wide variation in performance among subjects with MMSE scores ≥ 26. Many subjects had significant impairment on at least one test, often performing far below the normative mean, particularly on measures of processing speed and learning/memory, in which 20-30% scored more than 1.5 standard deviations below the age-based mean. For perspective, a randomly distributed normal distribution would have about 7% of scores more than 1.5 standard deviations below the normative mean.
This study provides additional evidence that the MMSE is not sensitive to CI in PD, even in those diagnosed with dementia. Recent studies that examine the validity of the original MDS Task Force screening checklist for PDD have likewise found that the MMSE cutoff ≥ 26 fails to identify a substantial proportion of PD patients with dementia.8, 9 However, these studies included a relatively small number of subjects, and thus our findings in this large cohort help to bolster their conclusions that the MMSE component of the checklist be reconsidered or revised. Other prior studies, also with smaller sample sizes, found that a substantial portion of PD subjects with MMSE ≥ 26 scores had MoCA scores in the impaired range (below 26).11,10, 12 We similarly found a broad range of performance on the MoCA in our sample with a MMSE ≥ 26, but also demonstrated variability in performance on more sensitive cognitive tests. Two additional studies (n=13213 and n=11414 ) compared the ability of the MoCA and MMSE to detect PD subjects categorized as demented, MCI, or non-demented, and found that the MoCA performed superiorly to the MMSE in detecting either category of CI. These previous results have led these investigators to suggest that the MoCA, versus the MMSE, is a superior screening tool for CI in PD. However, the MoCA also has important limitations when assessing CI in PD, including reduced specificity.13 These limitations are inherent given that both the MoCA and MMSE are intended for use as screening measures only, even when more rigorous cut-off scores are used. Thus, more detailed neuropsychological evaluation, as performed in this study, is needed to accurately detect the full spectrum of CI in PD.
A primary limitation of the study is the lack of a non-PD control group. The National Alzheimer’s Coordinating Center Uniform Data Set includes a large sample (n=3258) of cognitively normal older adults. Similar to the current study, this group was found to have significantly negatively skewed MMSE scores, yet a broader range of performance across other neuropsychological measures.30 This study did not focus solely on the upper range of MMSE scores, however, thus future research should endeavor to better delineate any differences between PD and control populations. This study is further limited by its cross-sectional design. Data collection continues and future studies will examine changes in cognition in PD as predicted by the MMSE or other tests. For example, a recent study suggested that the MMSE may better track cognitive change over time than the MoCA.34 Thus, further longitudinal studies may help to clarify the best methods for screening and tracking cognitive decline. The current data may also be limited in their generalizability, since the majority of subjects were white, male, and highly educated (16 median years of education). Differences across sites may also have impacted the results; for example, slight differences in administration technique of the MMSE across sites may have led to minor differences in total MMSE scores. There was also differential contribution from the sites in terms of the neuropsychological assessments conducted; thus not every participant completed each cognitive measure examined. Further, variability in prevalence of CI noted across studies may be related to issues inherent to inconsistencies in the methods used to assess CI; notably, the level of impairment required on testing to assess PD-MCI in our sub-sample may have led to a higher rate of PD-MCI than would have been identified using more stringent cutoffs. This method, however, permits diagnosis of subjects who are experiencing notable cognitive decline but have high premorbid abilities. Finally, the MMSE is sensitive to education,35 which may have elevated MMSE scores relative to tests that are less education-sensitive. Nonetheless, the current study, using a broad battery of neuropsychological tests in the largest sample of PD subjects reported to date, lends further support to the supposition that the MMSE misses much CI, including dementia, in PD. Given these results and those of previous studies, clinicians should be cautious when utilizing the MMSE as a bedside test to detect CI in PD, and should consider referring patients with cognitive concerns for more detailed neuropsychological assessments.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs and grants from the American Parkinson Disease Association, Consolidated Anti-Aging Foundation, National Institutes of Health (P50 NS062684, P50 NS053488, P50 NS038367, R01 NS065070, RO1 ES010544), and Northwest Collaborative Care.
This study received no funding or sponsorship from industry sources.
DOCUMENTATION OF AUTHOR ROLES
Research project (Conception, organization, and execution): Drs Burdick, Watson, Cholerton, Montine, Zabetian, Leverenz
Analysis or interpretation of data: A. Design: Drs Burdick, Watson, Edwards; B. Execution: Drs Burdick, Watson, Edward, Cholerton; C. Review & Critique: Drs. Siderowf, Edwards, Trojanowski, Weintraub, Ritz, Rhodes, Rausch, Factor, Wood-Siverio, Quinn, Chung, Cholerton, Srivatsal, Edwards, Montine, Zabetian, Leverenz
Manuscript preparation: A. Writing of the first draft: Drs Burdick & Watson, B. Review & Critique: Siderowf, Trojanowski, Weintraub, Ritz, Rhodes, Rausch, Factor, Wood-Siverio, Quinn, Chung, Cholerton, Srivatsal, Edwards, Montine, Zabetian, Leverenz
Acquisition of Data: Drs Burdick, Watson, Siderowf, Trojanowski, Weintraub, Ritz, Rhodes, Rausch, Factor, Wood-Siverio, Quinn, Chung, Cholerton, Srivatsal, Montine, Zabetian, Leverenz
Study supervision and coordination: Drs Watson, Montine, Zabetian, Leverenz
Obtaining funding: Drs Trojanowski, Ritz, Factor, Quinn, Edwards, Montine, Zabetian, Leverenz
FINANCIAL DISCLOSURES
Dr. Burdick reports honoraria from Teva Neuroscience and is funded by a grant from the Department of Veterans Affairs.
Dr. Watson reports no disclosures.
Dr. Siderowf reports he is a full time employee of Avid Radiopharmaceuticals
Dr. Trojanowski serves as an Associate Editor of Alzheimer’s & Dementia; may accrue revenue on patents held by the University of Pennsylvania wherein he is inventor. Modified avidin-biotin technique, Method of stabilizing microtubules to treat Alzheimer’s disease, Method of detecting abnormally phosphorylated tau, Method of screening for Alzheimer’s disease or disease associated with the accumulation of paired helical filaments, Compositions and methods for producing and using homogeneous neuronal cell transplants, Rat comprising straight filaments in its brain, Compositions and methods for producing and using homogeneous neuronal cell transplants to treat neurodegenerative disorders and brain and spinal cord injuries, Diagnostic methods for Alzheimer’s disease by detection of multiple MRNAs, Methods and compositions for determining lipid peroxidation levels in oxidant stress syndromes and diseases, Compositions and methods for producing and using homogenous neuronal cell transplants, Method of identifying, diagnosing and treating alpha-synuclein positive neurodegenerative disorders, Mutation-specific functional impairments in distinct tau isoforms of hereditary frontotemporal dementia and parkinsonism linked to chromosome-17: genotype predicts phenotype, Microtubule stabilizing therapies for neurodegenerative disorders, and Treatment of Alzheimer’s and related diseases with an antibody; and he receives research support from the NIH (AG 10124, AG 17586, AG-19724AG 024904, NS053488,AG029213 and the Marian S. Ware Alzheimer Program).
Dr. Weintraub reports consulting or advisory board membership with honoraria from Teva Pharmaceuticals, Eli Lilly and Company, Lundbeck Inc., Biogen, Pfizer, Avanir Pharmaceuticals, and Merck & Co. Honoraria from Michael J. Fox Foundation for Parkinson’s Research, American Psychiatric Publishing, Teva Pharmaceuticals, CHDI Foundation, and Alzheimer’s Disease Cooperative Study. Intellectual property rights from licensing fees from the University of Pennsylvania. Research funding support from National Institutes of Health, Michael J. Fox Foundation, and Novartis Pharmaceuticals.
Dr. Ritz reports no disclosures and is funded by grants from the NIH.
Dr. Rhodes reports no disclosures and is funded by grants from the NIH.
Dr. Rausch reports no disclosures.
Dr. Factor reports consulting fees from Ipsen Biopharmaceuticals and Merz. Grant support from Teva Neurosciences, Ipsen Biopharmaceuticals, EMD Serono, Ceregene, Michael J Fox Foundation, NIH/NHLBI (PO1 eS 016731-01 (PI Gary Miller, PhD), NIH 1 RO1 NS065070-01A1 (PI Cyrus Zabetian, MD, MSc), NINDS U01 NS052592-2CARE (PI Merit Cudkowica, MD), Royalties from UpToDate, Blackwell Futura, Demos.
Ms. Wood-Siverio reports no disclosures.
Dr. Quinn reports consulting fees from Merck and speaking fees from Novartis. Dr. Quinn is also reimbursed by Elan, Baxter, Bristol-Meyers Squibb, and Roche for the conduct of clinical trials. Dr. Quinn is also funded by grants from the NIH and Department of Veterans Affairs.
Dr. Chung reports no disclosures.
Dr. Cholerton reports no disclosures.
Dr. Srivatsal reports no disclosures and is funded by a grant from the Department of Veterans Affairs.
Dr. Edwards reports no disclosures and is funded by grants from the NIH.
Dr. Montine reports honoraria from invited scientific presentations to universities and professional societies not exceeding $5,000 per year and is funded by grants from the NIH.
Dr. Zabetian reports no disclosures and is funded by grants from the American Parkinson Disease Association, Department of Veterans Affairs, NIH, Northwest Collaborative Care, and Parkinson’s Disease Foundation.
Dr. Leverenz reports consulting fees from Bayer Healthcare Pharmaceuticals, Piramal Healthcare, and Navidea Biopharmaceuticals and is funded by grants from the Department of Veterans Affairs, American Parkinson Disease Association, Michael J. Fox Foundation, NIH, and the Parkinson’s Disease Foundation.
References
- 1.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 2.Goldman JG, Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 3.Marras C, McDermott MP, Rochon PA, et al. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Movement disorders : official journal of the Movement Disorder Society. 2008;23(5):653–659. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 4.Martin RC, Okonkwo OC, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson’s disease patients without dementia. Movement disorders : official journal of the Movement Disorder Society. 2008;23(13):1867–1874. doi: 10.1002/mds.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Movement disorders : official journal of the Movement Disorder Society. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Barton B, Grabli D, Bernard B, et al. Clinical validation of Movement Disorder Society-recommended diagnostic criteria for Parkinson’s disease with dementia. Movement disorders : official journal of the Movement Disorder Society. 2012;27(2):248–253. doi: 10.1002/mds.24059. [DOI] [PubMed] [Google Scholar]
- 9.Isella V, Mapelli C, Siri C, et al. Validation and attempts of revision of the MDS-recommended tests for the screening of Parkinson’s disease dementia. Parkinsonism & related disorders. 2014;20(1):32–36. doi: 10.1016/j.parkreldis.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. Journal of the American Geriatrics Society. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 12.Hu MT, Szewczyk-Krolikowski K, Tomlinson P, et al. Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Movement disorders : official journal of the Movement Disorder Society. 2014;29(Suppl 3):351–359. doi: 10.1002/mds.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 15.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement disorders : official journal of the Movement Disorder Society. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang GA, Bronstein JM, Masterman DL, Redelings M, Crum JA, Ritz B. Clinical characteristics in early Parkinson’s disease in a central California population-based study. Movement disorders : official journal of the Movement Disorder Society. 2005;20(9):1133–1142. doi: 10.1002/mds.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 21.Army Individual Test Battery: Manual of directions and scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale-Revised manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 23.Wechsler D. WAiS-III® Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation Harcourt Brace & Company; 1997. [Google Scholar]
- 24.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and inter-rater reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 26.Benton AL, Sivan AB, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment-A Clinical Manual. Lutz, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- 27.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 28.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 29.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer disease and associated disorders. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer disease and associated disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry, neuropsychology, and behavioral neurology. 2002;15(1):39–43. [PubMed] [Google Scholar]
- 32.Cholerton BA, Zabetian CP, Quinn JF, et al. Pacific northwest udall center of excellence clinical consortium: study design and baseline cohort characteristics. Journal of Parkinson’s disease. 2013;3(2):205–214. doi: 10.3233/JPD-130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shipley WC, Gruber CP, Martin TA, Klein AM. Shipley-2 Manual. Los Angeles, CA: Western Psychological Services; 2009. [Google Scholar]
- 34.Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on brief cognitive instruments over time in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2012;27(9):1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- 35.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA : the journal of the American Medical Association. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.