Abstract
Programmed cell death 1 (PD-1) is an inhibitory molecule expressed by activated T cells. Its ligands (PD-L1 and -L2; PD-Ls) are expressed not only by a variety of leukocytes but also by stromal cells. To assess the role of PD-1 in CD8 T cell-mediated diseases, we used PD-1-knockout (KO) OVA-specific T cell-receptor transgenic (Tg) CD8 T cells (OT-I cells) in a murine model of mucocutaneous graft-versus-host disease (GVHD). We found that mice expressing OVA on epidermal keratinocytes (K14-mOVA mice) developed markedly enhanced GVHD-like disease after transfer of PD-1-KO OT-I cells as compared to those mice transferred with wild-type OT-I cells. In addition, K14-mOVA×OT-I double Tg (DTg) mice do not develop GVHD-like disease after adoptive transfer of OT-I cells, while transfer of PD-1-KO OT-I cells caused GVHD-like disease in a Fas/Fas-L independent manner. These results suggest that PD-1/PD-Ls-interactions have stronger inhibitory effects on pathogenic CD8 T cells than does Fas/Fas-L-interactions. Keratinocytes from K14-mOVA mice with GVHD-like skin lesions express PD-L1, while those from mice without the disease do not. These findings reflect the fact that primary keratinocytes express PD-L1 when stimulated by interferon-γ in vitro. When co-cultured with K14-mOVA keratinocytes for 2 days, PD-1-KO OT-I cells exhibited enhanced proliferation and activation compared to wild-type OT-I cells. In addition, knockdown of 50% PD-L1 expression on the keratinocytes with transfection of PD-L1-siRNA enhanced OT-I cell proliferation. In aggregate, our data strongly suggest that PD-L1, expressed on activated target keratinocytes presenting autoantigens, regulates autoaggressive CD8 T cells, and inhibits the development of mucocutaneous autoimmune diseases.
Keywords: programmed cell death 1, programmed cell death ligand 1, Fas, keratinocyte, CD8 T cell, graft-versus-host-disease
1. Introduction
Programmed cell death 1 (PD-1) expressed on T cells is an immunoreceptor that negatively regulates antigen receptor signaling upon interacting with either of two ligands, PD-L1 and PD-L2 (PD-Ls) [1]. While PD-L1 has a wide distribution on leukocytes and stromal cells including tumor cells [2], PD-L2 is predominantly expressed on dendritic cells [3]. Upon engagement with PD-L1 or PD-L2, PD-1 on T cells inhibits TCR/CD3 signaling and CD28 signaling via phosphatase SHP2 (and possibly SHP1 as well) that dephosphorylates phosphatidylinositol-3-kinase and ZAP70/CD3ζ [4-6]. Clinically, blockade of PD-1-PD-L1 has recently been founded to be an effective treatment for cancer by enhancing cancer immunity [7, 8]. On the other hand, PD-1 deficiency leads to lupus-like autoimmune disease with glomerulonephritis and arthritis or fatal dilated cardiomyopathy in mice [9, 10]. It has been also shown that rodent models of autoimmune disease are modulated via the PD-1-PD-Ls system. Blockade of PD-1-PD-Ls interaction with anti-PD-1/PD-L1 blocking antibodies or using PD-1/PD-L1-deficient rodents accelerates disease in prediabetic non-obese diabetic (NOD) mice, a murine model of type I diabetes [11, 12], experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis [13], MRL/MpJ-Fas (lpr) mice and (New Zealand Black × New Zealand White)F1 mice, murine models of lupus [14, 15], and collagen-induced arthritis, a murine model of rheumatoid arthritis [16]. In aggregate, PD-1/PD-Ls system seems to regulate autoaggressive T cells during the breakdown of peripheral tolerance and development of autoimmunity.
To dissect the specific roles of autoaggressive CD8 T cells against epidermal keratinocytes in mucocutaneous diseases, we generated a transgenic mouse that expresses chicken ovalbumin (OVA) in skin and mucosal epithelia under the control of the keratin 14 promoter (K14-mOVA mouse). After adoptive transfer of transgenic T cells, OT-I cells, that express a TCR for the CD8-epitope of OVA (SIINFEKL), recipient K14-mOVA mice develop erosive skin and mucosal lesions as well as weight loss [17, 18]. Their bimodal weight loss curve may be caused by difficulty in chewing and swallowing because of mucosal lesions. In the late phase of this disease, the mice are partially saved by ingesting a gel meal. Mucocutaneous lesions in these mice show histological features characterized by liquefaction degeneration of the basal epidermal layer. This inflammatory reaction is reminiscent of human diseases including acute graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation, drug eruptions such as erythema multiforme, lupus erythematosus, dermatomyositis and lichen planus, and is called a lichenoid tissue reaction/interface dermatitis [19]. In addition to the K14-mOVA mice described above, we also generated double transgenic (DTg) mice, which are K14-mOVA mice crossed with OT-I mice. DTg mice are consistently resistant to developing GVHD-like disease after adoptive transfer of OT-I cells [20]. In a previous report, we assumed that one of the mechanisms that peripherally inhibits transferred OT-I cells might be Fas-FasL killing of transferred syngeneic OT-I cells by double negative (DN) OT-I cells (CD3+B220−CD4−CD8−Vα2+Vβ5+cells) that are increased in the DTg mice.
Here, we report that PD-1 expression on autoaggeressive CD8 T cells is critical in inhibiting the development of mucocutaneous GVHD-like disease. In addition, PD-L1 expressed on target keratinocytes regulates the antigen-specific CD8 T cells that are involved in the inflammation.
2. Results
2.1. Adoptive transfer of PD-1-knockout (KO) OT-I cells causes markedly enhanced GVHD-like disease in K14-mOVA mice and causes de novo GVHD-like disease in K14-mOVA/OT-I DTg mice when compared to mice adoptively transferred with wild-type OT-I cells or Fas-KO OT-I cells
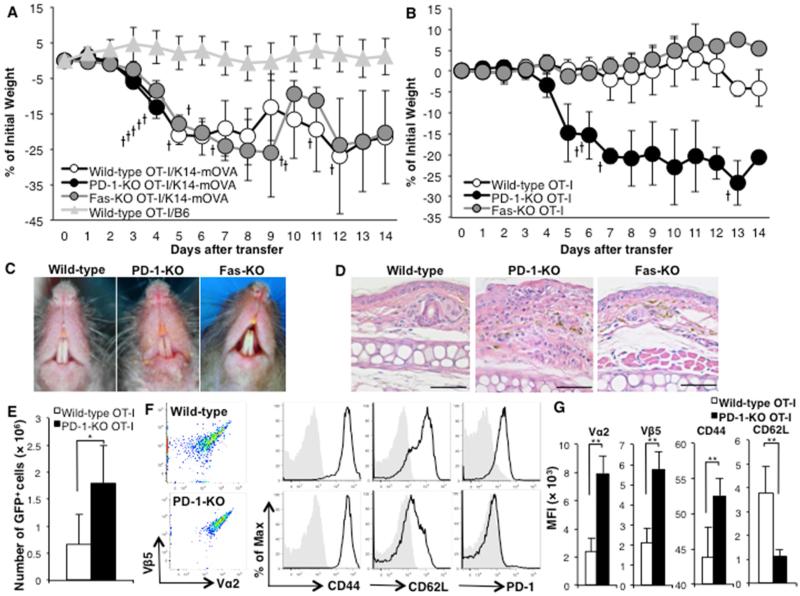
K14-mOVA mice develop GVHD-like disease, weight loss and erosive skin and mucosal lesions characterized by interface dermatitis, when adoptively transferred with more than 5 × 105 OT-I cells, and 10 – 20% of them die within 2 weeks with severe weight loss. To determine whether PD-1 and Fas expressed on effector CD8 T cells have inhibitory roles in the disease, we transferred 1 × 106 wild-type OT-I cells, PD-1-KO or Fas-KO OT-I cells into K14-mOVA mice. The mice transferred with PD-1-KO OT-I cells rapidly lost weight, shivered severely and suddenly died within 4 days after the transfer without any clinical skin or mucosal lesions or pathology in internal organs (brain, heart, lung, liver and kidney), while those transferred with Fas-KO OT-I cells followed the same GVHD-like disease course as those transferred with wild-type OT-I cells (Fig. 1A). Control B6 mice do not develop GVHD-like disease after the transfer of wild-type OT-I cells. As shown in Table 1, serum levels of proinflammatory cytokines in the mice that were transferred with PD-1-KO OT-I cells were markedly elevated 3 days after transfer (just before sudden death) compared to cytokines in mice transferred with wild-type or Fas-KO OT-I cells.
Figure 1. Adoptive transfer of PD-1-KO OT-I cells, but not wild-type or Fas-KO OT-I cells, induces severe GVHD-like disease in K14-mOVA mice.
(A) GVHD-like weight loss curves of K14-mOVA mice with transfer of 1 × 106 of wild-type (open circles), Fas-KO (grey circles) or PD-1-KO (black circles) OT-I cells. Triangles represent the weight curve of B6 mice after transfer of 1 × 106 wild-type OT-I cells. (B) Weight loss curves of K14-mOVA mice following transfer of reduced numbers (5 × 104) of wild-type (open circles), Fas-KO (grey circles) or PD-1-KO (black circles) OT-I cells. The cross marks represent death of recipient mice. The bars represent SD. (C) Clinical and (D) histologic photographs of K14-mOVA mice 2 weeks after the transfer of 5×104 of wild-type, PD-1-KO or Fas-KO OT-I cells. (E) Cell numbers ± SD and (F) expression of activation markers and PD-1 of transferred GFP+OT-I cells in skin-draining lymph nodes (SDLNs) 7 days after transfer of 5 × 104 wild-type (upper) or PD-1-KO (lower) OT-I cells. (G) The graphs show median fluorescence intensities (MFIs) ± SD of the markers. 3 mice per group. *P<0.05, **P<0.01, t test. Data is representative of 3 separate experiments.
Table 1.
Transfer of 1 million of PD-1-KO OT-I cells markedly increases serum levels of pro-inflammatory cytokines in K14-mOVA mice. Concentrations of cytokines in sera collected from K14-mOVA or B6 mice 4 days after adoptive transfer of 1 × 106 wild-type, PD-1-KO or Fas-KO OT-I cells were measured by ELISA. Averages of 4 mice ± SD.
| Recipient | OT-I cell | IFNγ | TFNα | IL-6 | IL-1b |
|---|---|---|---|---|---|
| K14-mOVA | Wild-type | 574.0 ± 229.4 | 60.1 ± 4.8 | 75.8 ± 12.9 | 42.3 ± 15.1 |
| K14-mOVA | PD-1-KO | 1527.9 ± 599.6* | 102.7 ± 28.7* | 204.2 ± 86.4* | 29.3 ± 6.4 |
| K14-mOVA | Fas-KO | 176.5 ± 17.4** | 52.0 ± 2.1* | 52.1 ± 2.5** | 46.5 ± 19.0 |
| B6 | Wild-type | 21.2 ± 0.3** | 54.3 ± 1.1* | 31.0 ± 4.2** | 62.3 ± 12.6 |
P<0.05
P<0.01 compared to the concentrations in sera from K14-mOVA mice transferred with wild-type OT-I cells, t-test.
We next titrated the number of transferred OT-I cells to 5 × 104, which is far less than is required to cause GVHD-like disease in K14-mOVA mice. Only mice transferred with reduced numbers of PD-1-KO OT-I cells lost weight, and 4 of 5 mice died (Fig. 1B). The mouse that survived 14 days after the transfer of 5 × 104 PD-1-KO OT-I cells developed severe skin and mucosal lesions with erosions and crusts, characterized histologically by liquefaction degeneration of the basal epidermal cell layer, while all mice transferred with 5 × 104 wild-type or Fas-KO OT-I cells exhibited no skin or mucosal lesions (Fig. 1C and 1D).
To determine whether transferred PD-1-KO OT-I cells are activated to a greater extent than wild-type OT-I cells in K14-mOVA mice, skin-draining lymph node (SDLN) cells were analyzed by flow cytometry 7 days after the adoptive transfer of 5 × 104 wild-type or PD-1-KO OT-I cells, both expressing green florescence protein (GFP). There were greater numbers of PD-1-KO OT-I cells in SDLNs compared with wild-type cells (Fig. 1E). Both groups adoptively transferred with OT-I cells expressed the specific TCR (Vα2 and Vβ5), CD44 and CD25, and down-regulated expression of CD62L on their surface, and wild-type OT-I cells also expressed PD-1 (Fig. 1F). Expression of Vα2, Vβ5 and CD44 was higher and of CD62L was lower on GFP+OT-I cells in SDLNs of mice transferred with PD-1-KO OT-I cells compared to those transferred with wild-type OT-I cells (Fig. 1G). Both types of naïve OT-I cells express high Vα2, Vβ5, CD62L, and low CD44, CD25 and CD69 before transfer (Suppl. Fig. 1). These results demonstrate that PD-1KO OT-I cells were more numerous and activated to a greater extent than wild-type OT-I cells in SDLNs of K14-mOVA mice.
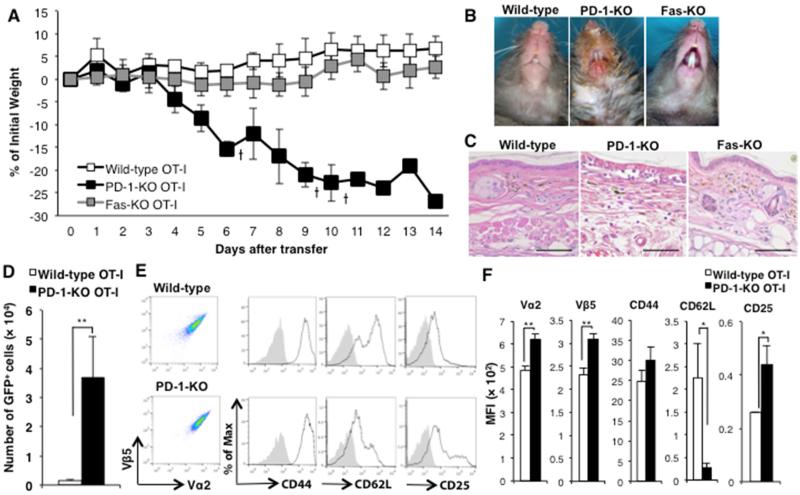
Consistent with our prior studies [20], when DTg mice were adoptively transferred with 1 × 106 OT-I cells they did not develop GVHD-like disease. On the other hand, DTg mice that were adoptively transferred with 1 × 106 PD-1-KO OT-I cells developed severe disease with marked weight loss and skin/mucosal lesions and many died (Fig. 2A, B, C). Although we showed that double negative T cells (CD3+CD4−CD8−Vα2+Vβ5+; DN T cells) found in increased numbers in LNs and spleens of DTg mice might have inhibitory effects on transferred OT-I cells via the Fas-FasL system, DTg mice that received 1 × 106 Fas-KO OT-I cells did not develop GVHD-like disease clinically (Fig. 2A, B, C). When SDLN cells were analyzed by flow cytometry 7 days after the adoptive transfer of 1 × 106 wild-type or PD-1-KO GFP+OT-I cells, we found that total cell numbers of PD-1-KO OT-I cells occupied larger populations of SDLNs than wild-type OT-I cells (Fig. 2E), and that PD-1KO OT-I cells transferred into DTg mice were more activated than wild-type OT-I cells (Fig 2D, E).
Figure 2. Transfer of PD-1KO-OT-I cells causes GVHD-like disease in K14-mOVA/OT-I double transgenic (DTg) mice, while the DTg mice are completely resistant to transfer of wild-type or Fas-KO OT-I cells.
(A) GVHD-like weight loss curves of DTg mice with transfer of 1 × 106 of wild-type (open squares), Fas-KO (grey squares) or PD-1-KO (black squares) OT-I cells. The cross marks represent death of recipient mice. The bars represent SD. (B) Clinical and (C) histology photographs of DTg mice 2 weeks after the transfer of 1 × 106 of wild-type, PD-1-KO or Fas-KO OT-I cells. (D) Cell numbers ± SD and (E) expression of activation markers of transferred GFP+OT-I cells in SDLNs 7 days after transfer of 1 × 106 wild-type (upper) or PD-1-KO (lower) OT-I cells. (F) MFIs ± SD of the activation markers. 3 mice per group. **P<0.01, t test. Data is representative of 3 separate experiments.
In aggregate, our data strongly suggest that the expression of PD-1 on effector CD8 T cells inhibits the development and progression of the CD8 T cell-mediated mucocutaneous autoimmune disease.
2.2. SDLN cells and epidermal cells from K14-mOVA mice with GVHD-like disease express the ligands of PD-1
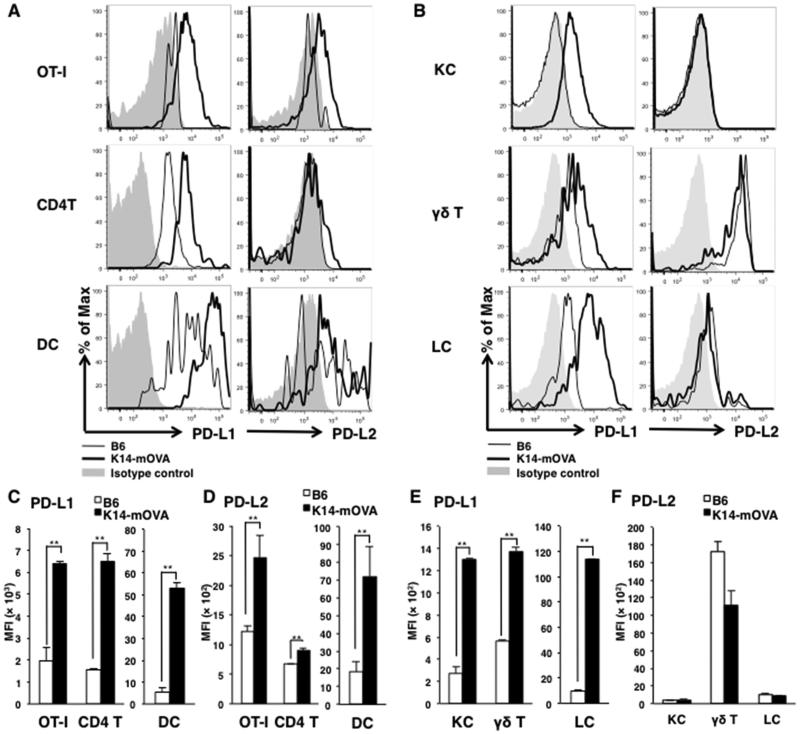
To determine which cells express the ligands of PD-1 (PD-L1 and PD-L2; PD-Ls), SDLN cells and ear epidermal cells were collected from B6 mice and K14-mOVA mice after transfer of 1 × 106 OT-I cells and analyzed by flow cytometry. PD-L1 was expressed on CD4 T cells and dendritic cells (DCs) in SDLNs not only from K14-mOVA mice but also from B6 mice 5 days after transfer of OT-I cells (Fig 3A). The median fluorescence intensities (MFIs) of PD-L1 were significantly higher in SDLN cells from K14-mOVA mice than those from B6 mice (Fig 3C). While PD-L2 were expressed only on DCs but not on OT-I cells and CD4 T cells in SDLNs from B6 mice after OT-I transfer, these three cell types expressed PD-L2 in SDLNs from K14-mOVA mice (Fig 3A, D). Thus, PD-Ls were expressed on a wide-range immune cells.
Figure 3. Immune cells and keratinocytes of mice with GVHD-like disease expresse PD-Ls.
(A) Expression of PD-L1 and PD-L2 on indicated cell populations in SDLN cells from K14-mOVA mice (bold lines) and B6 mice (thin lines) 5 days after OT-I cell transfer were analyzed in each population. The cells were gated into OT-I cells (GFP+), CD4 T cells (GFP−CD3+CD4+), and dendritic cells (DCs, GFP−CD3−CD11c+I-A/I-Ehigh). (B) Expression of PD-Ls on ear skin epidermal cells from mice 10 days after OT-I cell transfer were analyzed in each population, KCs (CD45−), γδ T cells (CD45+TCR γδ+) or Langerhans cells (LCs, CD45+CD11c+I-A/I-Ehigh). (C - F) MFIs ± SD of PD-L1 (C – SDLN, E - ear) or –L2 (D - SDLN, F - ear) on each cell type. Open bars are cells from B6 mice and closed bars are from K14-mOVA mice. *P<0.05, **P<0.01, t test. Data is representative of 3 separate experiments.
We also analyzed epidermal cells by flow cytometry. PD-L1 were expressed on γδ T cells and Langerhans cells (LCs) in the epidermis not only from K14-mOVA mice but also from B6 mice 5 days after transfer of OT-I cells (Fig 3B) whereas the MFIs were significantly higher in epidermal cells from K14-mOVA mice with GVHD-like skin lesions than those from B6 mice (Fig 3E). Interestingly, keratinocytes (KCs) of K14-mOVA mice expressed PD-L1, while those of B6 mice did not (Fig 3B, E). KCs of naïve B6 and K14-mOVA mice never express PD-L1 or PD-L2 (Suppl. Fig. 2). These results show that target KCs express PD-L1 only when activated CD8 T cells infiltrate to skin.
In addition, γδ T cells and, to a lesser extent, LCs also expressed PD-L2 not only in K14-mOVA mice with skin lesions but also in B6 mice (Fig 3B, F). All γδ T cells expressed PD-L2, while small populations of LC expressed PD-L2 in K14-mOVA mice transferred with OT-I cells (Fig 3B). These results indicate that γδ T cells may inhibit autoaggressive CD8 OT-I cells expressing PD-1.
2.3. Keratinocytes expressing OVA modulate the proliferation and the activation of OT-I cells
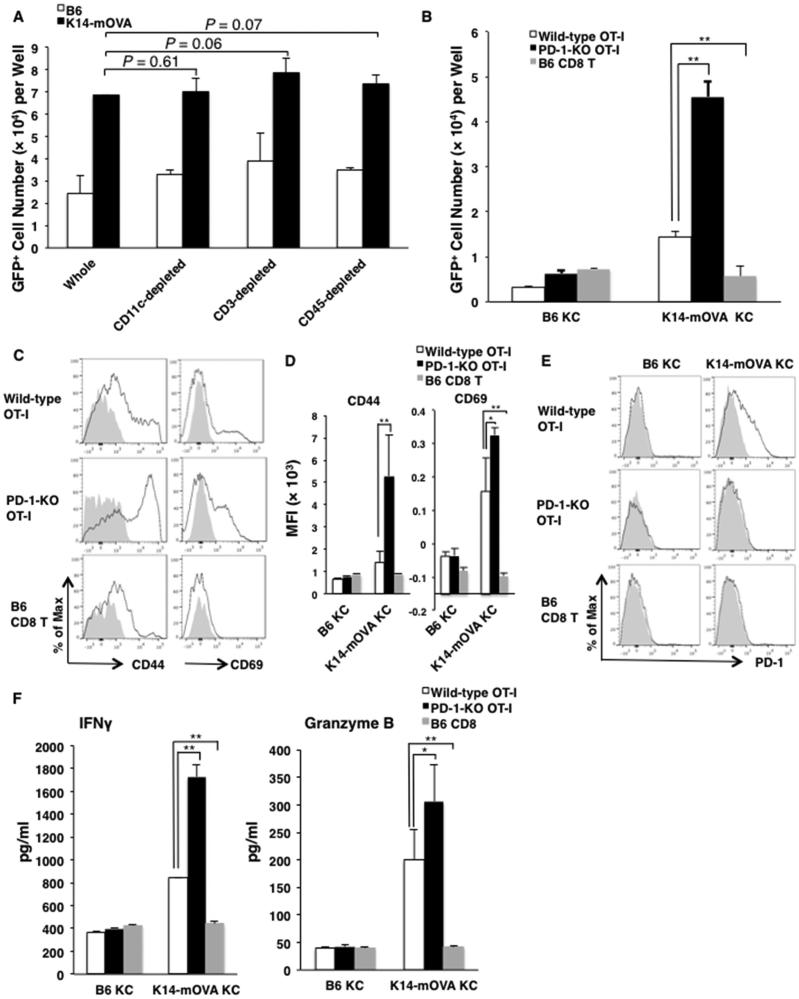
In our previous studies [21], epidermal cells devoid of LCs from K14-mOVA mice could induce naïve OT-I cells to proliferate. Although LCs and γδ T cells of K14-mOVA mice with GVHD-like disease upregulated PD-Ls (Fig 3B, F), LC (CD11c+ cell)-depleted, γδ T cell (CD3+cell)-depleted or all hematopoietic cell (CD45+cell)-depleted of epidermal cells from K14-mOVA mice had no significant effect on GFP+OT-I cell proliferation in vitro (Fig. 4A). We suggest that the effects of LCs and γδ T cells on OT-I cell proliferation in vitro are minor, at best. When PD-1-KO OT-I cells were assessed in co culture experiments with K14-mOVA KCs for 3 days, they proliferated and were activated to a greater extent than wild-type OT-I cells under the stimulation of K14-mOVA KCs (Fig. 4B, C, D). Wild-type OT-I cells expressed PD-1 only when co-cultured with K14-mOVA KCs (Fig. 4E). We also observed that, in culture, PD-1-KO OT-I cells produced greater amounts of the cytotoxic molecules, IFN-γ and granzyme B, than do wild-type OT-I cells (Fig. 4E). These results indicate that K14-mOVA KCs stimulate not only TCRs but also PD-1 expressed on OT-I cells, and that PD-1 on activated OT-I cells modulates proliferation and activation of OT-I cells.
Figure 4. PD-1KO OT-I cells exhibit enhanced proliferation and activation compared to wild-type OT-I cells when co-cultured with OVA-expressing K14-mOVA keratinocytes (KC).
(A) Whole epidermal cells (ECs), CD11c+cell-depleted ECs, CD3+cell-depleted ECs and CD45+cell-depleted ECs (keratinocytes; KCs) from K14-mOVA mice or B6 mice were co-cultured with GFP+ wild-type OT-I cells. (B) CD45+cell-depleted ECs (keratinocytes; KCs) from K14-mOVA mice or B6 mice were co-cultured with GFP+ wild-type or PD-1-KO OT-I cells, or GFP+ CD8 T cells. Cell numbers of the GFP+ cells per well were counted by flow cytometry 2 days later. The bars present means ± SD of 3 wells. (B) Expression of CD44 and CD69 on the cultured GFP+ cells 3 days later. (C) MFIs ± SD of the markers. (D) Expression of PD-1 on the GFP+ cells 3 days later. (E) Concentrations ± SD of interferon (IFN)-γ and granzyme B in culture supernatants from wells of the GFP+ cells co-cultured with KCs for 3 days. *P<0.05, **P<0.01, t test. Data is representative of 4 separate experiments.
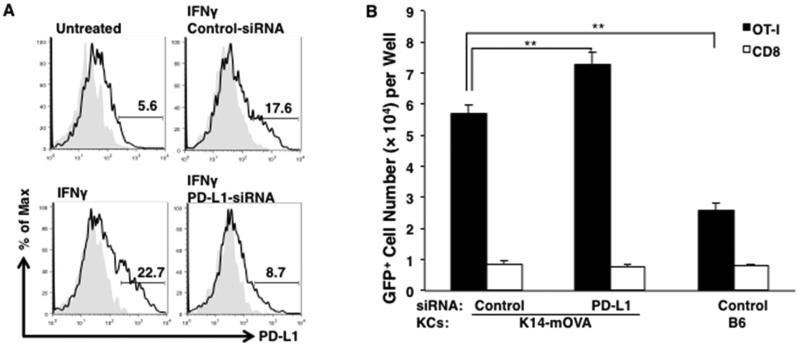
KCs upregulated PD-L1 expression when stimulated with interferon (IFN)-γ, a cytokine produced by activated OT-I cells (Fig. 5A). The viability of these KCs, when transfected with 160 μM control- or PD-L1-specific-siRNA was reduced by 50% when compared to untreated KCs. Transfection of PD-L1-siRNA inhibited 50 % of expression of PD-L1 on keratinocytes compared with that of control-siRNA (Fig. 5A). To determine whether PD-L1 expressed on KCs inhibits the proliferation of OT-I cells, we attempted to knock down PD-L1 expressed on K14-mOVA KCs that were co-cultured with GFP+OT-I cells. OT-I cells co-cultured with PD-L1-siRNA-transfected K14-mOVA KCs exhibited greater proliferation than those transfected with control-siRNA-transfected KCs (Fig. 5B). In repeated experiments with PD-L1-siRNA and experiments using another PD-L1-siRNA with a different sequence, we found that transfection PD-L1-siRNA enhanced proliferation of OT-I cells by 10 – 28 %.
Figure 5. Knockdown of PD-L1 on K14-mOVA KCs enhances the proliferation of OT-I cells.
(A) Expression of PD-L1 that KCs upregulates after stimulation of interferon (IFN)-γ was reduced by 50 percent (from 17.6 % PD-L1-positive cells to 8.7 % positive) with the transfection of PD-L1-siRNA. (B) GFP+ OT-I cells or CD8 T cells were co-cultured with KCs from K14-mOVA mice or B6 mice that were transfected with control- or PD-L1-siRNA. Numbers of the GFP+ cells per well were counted by flow cytometry 2 days later. The bars represent means ± SD of 3 wells. *P<0.05, **P<0.01, t test. Data is representative of 2 sequences and 3 separate experiments.
In aggregate, these in vitro results demonstrated that PD-L1 expressed on KCs presenting autoantigens inhibit the proliferation of the autoantigen-specific CD8 T cells.
3.Discussion
PD-1 expressed on pathogenic CD8 T cells plays an important role in regulating GVHD-like mucocutaneous lesions in our murine models of autoaggressive CD8 T cell-mediated disease. In the present study we show that K14-mOVA mice expressing membrane-bound OVA on epidermal keratinocytes developed GVHD-like disease after adoptive transfer of small numbers of PD-1-KO OT-I cells. It has previously been demonstrated that transgenic mice expressing membrane-bound OVA exclusively on cardiac myocytes (cMy-mOVA mice,) transferred with PD-1-KO OT-I cells develop more severe myocarditis by immunization with OVA in Freund's Complete Adjuvant than those transferred with wild-type OT-I cells [22]. Similar to what has been previously reported in cMy-mOVA mice, PD-1-KO OT-I cells proliferated to a greater extent than wild-type OT-I cells in K14-mOVA mice. The mice quickly died after transfer of 1 × 106 PD-1-KO OT-I cells, and had either a high mortality (80%) or severe disease with ulcerative skin and mucosal lesions even after transfer with as few as 5 × 104 PD-1-KO OT-I cells. This finding may rely not only on the vigorous proliferation of PD-1-KO OT-I cells but also on their ability to kill SIINFEKL-presenting cells [22].
We also have observed that a few mice in groups of wild-type OT-I cell-transferred K14-mOVA mice, not only PD-1KO OT-I cell-transferred mice, suddenly died with severe shivering early (4-6 days) after cell transfer. This sudden death is probably due to a cytokine storm. We have never detected severe skin or mucosal lesions or inflammatory pathology in internal organs. As shown in Table 1, serum levels of some proinflammatory cytokines in K14-mOVA mice transferred with PD-1-KO OT-I cells were elevated compared to mice transferred with wild-type or Fas-KO OT-I cells. Because PD-1-KO OT-I cells were increased in SDLNs of K14-mOVA mice to a greater extent than were wild-type or Fas-KO OT-I cells, the increased OT-I cells in LNs are probably the source of the cytokines. We also observed that OT-I mice develop the same condition (sudden death following severe shivering) within 24 hours after an intravenous injection of SIINFEKL peptides, and that IFNγ-, TNFα- or IL-6-deficiency blocked the death (unpublished data). When transferred with lower numbers of PD-1KO OT-I cells, the K14-mOVA mice survived through an early phase of weight loss and developed severe skin and mucosal lesions in the later phase (7 – 14 days after the transfer) of GVHD-like disease.
In our mucocutaneous GVHD model, leukocytes in SDLNs upregulated both PD-L1 and PD-L2 as expected. Interestingly, KCs from K14-mOVA mice upregulated expression of PD-L1 but not PD-L2 after transfer of OT-I cells. It has been shown that KCs upregulated mRNA of PD-L1 after IFN-γ-stimulation [2]. Likewise, we have shown here that PD-L1 appears on the membrane of K14-mOVA KCs when stimulated with IFNγ. Although we have already reported that OT-I cells can proliferate when co-cultured with K14-mOVA KCs devoid of any co-stimulating molecules like CD80/86 [21], PD-1-KO OT-I cells proliferated and were activated to a greater extent than wild-type OT-I cells. This finding indicates that the proliferation and activation of antigen-specific CD8 T cells may be inhibited via PD-L1 on antigen-presenting KCs. To exclude inhibitory effects of PD-Ls expressed on OT-I cells themselves, transfection of siRNA for PD-L1 was used to knockdown expression of PD-L1 on KCs. OT-I cells proliferated to a greater extent when co-cultured with PD-L1-knockdown K14-mOVA KCs than with control KCs. The enhancement of OT-I proliferation induced by transfection of PD-L1-siRNA on antigen-presenting KCs was, however, not dramatic (10 – 28%), and this may be because only 50 % of PD-L1 was knocked down. In aggregate, PD-L1, expressed on activated target keratinocytes presenting autoantigens, regulates autoaggressive CD8 T cells, and inhibits the development of autoimmune mucocutaneous disease with interface dermatitis. Interestingly, in human mucosal samples from patients with lichen planus, a mucocutaneous disease with interface dermatitis, infiltrating inflammatory cells expressed PD-1 and PD-Ls, and KCs expressed PD-L1 [23]. In that article, it was shown that human allogeneic T-cell proliferative responses and IFN-γ production induced by IFN-γ-treated KCs were augmented preferentially by anti-PD-L1 blocking antibodies. K14-PD-L1-Tg mice were also shown to be resistant to developing contact hypersensitivity [24].
In addition to cutaneous diseases, PD-L1 expression on parenchymal cells is critical for suppression of acute GVHD in irradiated B6 mice after transfer of bone marrow (BM) cells from BALB/c mice, another murine model of GVHD. Chimeras that lack PD-L1 on parenchymal cells but not on hematopoietic cells developed GVHD to a greater extent than other chimeras lacking PD-L1 only on hematopoietic cells after transfer of BM cells from BALB/c mice [25]. While NOD severe combined immunodeficiency (SCID) mice developed diabetes after adoptive transfer of splenocytes from NOD mice, early onset of diabetes was observed in PD-L1/L2-KO NOD SCID mice [26]. Another group also showed that PD-L1-deficiency in rat-insulin promotor (RIP)-B7.1 Tg mice accelerated to the development diabetes after immunization with preproinsulin-encoding vectors [27]. In a murine model of autoimmune myocarditis that develops in cMy-mOVA mice, PD-L1/L2-KO cMy-OVA Tg mice transferred with OT-I cells developed more severe myocarditis after immunization with OVA than do wild-type cMy-OVA Tg mice [28]. In patients with malignant tumors, it have been suggested that PD-L1 expressed on tumor cells correlated with the number of infiltrating inflammatory cell around the tumors [29-32]. These reports indicate that PD-Ls expressed on stromal cells might be critical in inhibiting effector T cells moreso than those on hematopoietic cells. Our results also suggest that target cells expressing PD-L1 protect themselves from autoantigen-specific CD8 T cells in autoimmune mucocutaneous disease.
When we utilized K14-mOVA×OT-I double Tg (DTg) mice that never develop GVHD-like disease after adoptive transfer of OT-I cells, transfer of PD-1-KO OT-I cells caused GVHD-like disease. Although DN OT-I cells that were found in increased numbers in DTg mice kill syngeneic OT-I cells via Fas-FasL interactions in vitro [20], transfer of Fas-KO OT-I cells could not cause the disease in DTg mice as well as wild-type OT-I cells. These results suggest that PD-1/PD-Ls-interactions have stronger inhibitory effects on pathogenic CD8 T cells than do the Fas/Fas-L-interactions in vivo. Although activated DN OT-I cells expressed PD-L1, PD-1KO-OT-I cells were killed by DN OT-I cells after SIINFEKL-stimulation to the same extent as wild-type OT-I cells in vitro (Suppl. Fig. 3). In addition, treatment with PD-L1-IgG-Fc protein that stimulates PD-1 (kindly provided by Dr. G. Freeman, Harvard School of Medicine, Boston, Massachusetts) [33] did not inhibit GVHD-like disease in our model (Suppl. Fig. 4). All of these findings thus strongly suggest that expression of PD-L1 on antigen-presenting cells may be more important than that on surrounding leukocytes.
Though the pathway of PD-1-mediated programmed cell death (PCD) is still unknown, previous reports in cancer or infection showed that PD-1-expression on T cells induced PCD of the T cells [34-37]. Because it has been reported that CD3/CD8-mediated signaling induces PCD on OT-I cells more so than does TCR-mediated signaling [38], we stimulated wild-type, PD-1KO or Fas-KO OT-I cells with anti-CD3/CD8 antibodies to address whether PD-1- or Fas-deficiency inhibits PCD of OT-I cells. Stimulation by anti-CD3/CD8 antibodies accelerated PCD and reduced live cells of all types of OT-I cells (Suppl. Fig. 5). PD-1- and Fas-deficiency do not seem to be related in PCD induced by CD3/CD8-stimulation.
In conclusion, target KCs that express PD-L1 only at the time of inflammation protect themselves from destruction by antigen-specific CD8 T cells. Thus, upregulation of PD-L1 in skin and/or PD-1 on CD8 T cells provide novel approaches to the treatment of autoimmune mucocutaneous diseases.
4. Materials and Methods
4.1. Mice
K14-mOVA mice have been generated [17]. Rag-1-deficient OT-I mice (Taconic) were crossed with human ubiqutin C promoter-GFP transgenic mice (The Jackson Laboratory) to generate Rag-1−GFP+OT-I mice. PD-1-KO mice [9] were provided by T. Honjo (Kyoto University, Kyoto, Japan) via W. Kastenmuller (NIAID, Bethesda, MD). PD-1-KO mice and Fas-KO mice (The Jackson Laboratory) were crossed with GFP+OT-I mice to generate PD-1-KO and Fas-KO Rag-1−GFP+OT-I mice. All strains were on a C57BL/6 background. All animal studies were conducted with prior approval by the Animal Care and Use Committee of the NCI.
4.2. Cells
GFP+OT-I cells were collected from LNs of GFP+OT-I mice. For epidermal cells, ear and/or whole body skin were incubated for 30 min at 37°C in 0.5% trypsin (United States Biochemical) and separated into epidermis and dermis. Single epidermal cells were dissociated from the epidermal sheets in 0.05 % DNase (Sigma) by pipetting and filtering. LC-depleted epidermal cells, γδ T cell-depleted epidermal cells, and KCs were negatively selected from the epidermal cells using CD11c−, CD3− and CD45− MicroBeads (Miltenyi Biotec), respectively.
4.3. Adoptive transfer
Mice were injected intravenously with OT-I cells in 200 μl RPMI1640 medium with 5 % fetal bovine serum (FBS). The mice were weighed and clinically observed daily after the adoptive transfer.
4.4. Flow cytometry
SDLN cells and epidermal cells from mice and cultured OT-I cells were stained with PE-conjugated anti-CD44 (clone: IM7) and PD-L1 (MIH5), PE-CF594-conjugated anti-PD-1 (J43), CD4 (RM4-5) and CD45 (30-F11), Per-CP5.5-conjugated anti-CD62L (MEL-14), PE-Cy7-conjugated anti-Fas (Jo2) and CD3e (145-2C11), BV450-conjugated anti-TCR γδ (GL3), V500-conjugated anti-I-A/I-E (M5/114.15.2), allophycocyanin (APC)-conjugated anti-Vβ5 (MR9-4) and PD-L2 (TY25), mouse CD25 (PC61), AlexaFlour 700-conjugated anti-Vα2 (B20.1) and CD11c (N418), APC-Cy7-conjugated anti-CD25 (PC61) monoclonal antibodies (BD Pharmingen). LIVE/DEAD® fixable dead cell staining kit (violet and Near IR, Life technologies) were used to detect dead cells. Isotype matched antibodies were used as controls. Stained cells were analyzed on a LSR-II flow cytometer.
4.5. OT-I cell proliferation assay with epidermal cells
OT-I cells (1 × 105) were co-cultured with epidermal cells (1 × 105) from whole skin of naïve B6 mice or K14-mOVA mice for 2 days in RPMI 1640 with 10% FBS in flat bottom wells of 96-well plates at 37°C in a CO2 incubator. The cells were suspended in 400 μl of PBS. GFP+ cells per 50 μl of the cell suspension were counted by a flow cytometer, then GFP+cell numbers per well were calculated.
4.6. Knockdown of PD-L1 in KCs
KCs (1 × 105) from whole skin of naïve B6 mice or K14-mOVA mice were cultured for an hour in RPMI 1640 with 10% fetal bovine serum in 96-well flat bottom wells at 37°C in a CO2 incubator, then added to a mixture of 160 μM PD-L1-siRNA (4 types of sequences) or control-siRNA (QIAGEN) with GenMute® siRNA Transfection Reagent for Primary Keratinocytes (SignaGen Laboratories). Two hours later, 20 ng/ml IFN-γ (PeproTech) or OT-I cells were added into the plates.
4.7. Measurement of cytokines
OT-I cells (4 × 105) were co-cultured with KCs (4 × 105) from whole skin of either naïve B6 mice or K14-mOVA mice for 3 days in RPMI 1640 with 10% fetal bovine serum in 24-well plates at 37°C in a CO2 incubator. Concentrations of IFNγ and granzyme B in the culture supernatants were measured by enzyme-linked immunosorbent assay using the DuoSet® Kit (R&D Systems) according to the manufacturer’s instructions.
Supplementary Material
Highlights.
In a CD8 T cell-mediated mucocutaneous GVHD model, target keratinocytes upregulate PD-L1.
PD-1-KO autoaggressive CD8 T cells cause severe autoimmune mucocutaneous GVHD-like disease.
PD-1-KO autoaggressive CD8 T cells are activated to a great extent when they interact with target keratinocytes.
Target keratinocyes protect themselves from destruction by antigen-specific CD8 T cells via the expression of PD-L1.
Acknowledgments
We thank Jay T. Linton (NCI, NIH) for his outstanding technical support, Drs. Gaku Tsuji, Hong Zhang and Vadim A. Villarroel (NCI, NIH) for advice, Prof.Tasuku Honjo (Kyoto University, Kyoto, Japan) and Dr. Wolfgang Kastenmuller (NIAID, NIH) for sharing mice, and Dr. Gordon Freeman (Dana-Farber Cancer Institute, MA) for sharing reagents.
Abbreviations
- K14-mOVA mouse
keratin 14 promoter-membrane ovalbumin-transgenic mouse
- GVHD
graft-versus-host disease
- DTg mouse
K14-mOVA/OT-I double transgenic mouse
- DN T cell
double negative T cell (CD3+B220−CD4−CD8−cell)
- SDLN
skin-draining lymph node
- DC
dendritic cell
- LC
Langerhans cell
- KC
keratinocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no financial conflict of interest.
References
- [1].Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- [2].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Current opinion in immunology. 2002;14:384–90. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- [4].Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. Journal of immunology. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- [5].Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS letters. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- [6].Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. The Journal of experimental medicine. 2012;209:1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- [10].Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- [11].Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–9. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823–8. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–8. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181:2513–21. doi: 10.4049/jimmunol.181.4.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aad G, Abbott B, Abdallah J, Abdelalim AA, Abdesselam A, Abdinov O, et al. Determination of the strange-quark density of the proton from ATLAS measurements of the W-->lnu and Z-->ll cross sections. Phys Rev Lett. 2012;109:012001. doi: 10.1103/PhysRevLett.109.012001. [DOI] [PubMed] [Google Scholar]
- [16].Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum. 62:1870–80. doi: 10.1002/art.27500. [DOI] [PubMed] [Google Scholar]
- [17].Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–15. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- [18].Miyagawa F, Gutermuth J, Zhang H, Katz SI. The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J Autoimmun. 2010;35:192–8. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. 2009;129:1088–99. doi: 10.1038/sj.jid.2009.42. [DOI] [PubMed] [Google Scholar]
- [20].Miyagawa F, Okiyama N, Villarroel V, Katz SI. Identification of CD3+CD4−CD8− T cells as potential regulatory cells in an experimental murine model of graft-versus-host skin disease (GVHD) J Invest Dermatol. 2013;133:2538–45. doi: 10.1038/jid.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim BS, Miyagawa F, Cho YH, Bennett CL, Clausen BE, Katz SI. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J Invest Dermatol. 2009;129:2805–17. doi: 10.1038/jid.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. Journal of immunology. 2012;188:4876–84. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Youngnak-Piboonratanakit P, Tsushima F, Otsuki N, Igarashi H, Machida U, Iwai H, et al. The expression of B7-H1 on keratinocytes in chronic inflammatory mucocutaneous disease and its regulatory role. Immunology letters. 2004;94:215–22. doi: 10.1016/j.imlet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [24].Ritprajak P, Hashiguchi M, Tsushima F, Chalermsarp N, Azuma M. Keratinocyte-associated B7-H1 directly regulates cutaneous effector CD8+ T cell responses. Journal of immunology. 2010;184:4918–25. doi: 10.4049/jimmunol.0902478. [DOI] [PubMed] [Google Scholar]
- [25].Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–73. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rajasalu T, Brosi H, Schuster C, Spyrantis A, Boehm BO, Chen L, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes. 2010;59:1966–73. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–71. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- [29].Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- [30].Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. International journal of cancer Journal international du cancer. 2007;121:751–8. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- [31].Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6341–7. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- [32].Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Medical oncology. 2011;28:682–8. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- [33].Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis and rheumatism. 2010;62:1870–80. doi: 10.1002/art.27500. [DOI] [PubMed] [Google Scholar]
- [34].Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- [35].Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. International journal of cancer Journal international du cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- [36].Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Critical care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon gamma-producing T cells from apoptosis in patients with pulmonary tuberculosis. The Journal of infectious diseases. 2013;208:603–15. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- [38].Wang X, Simeoni L, Lindquist JA, Saez-Rodriguez J, Ambach A, Gilles ED, et al. Dynamics of proximal signaling events after TCR/CD8-mediated induction of proliferation or apoptosis in mature CD8+ T cells. Journal of immunology. 2008;180:6703–12. doi: 10.4049/jimmunol.180.10.6703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.