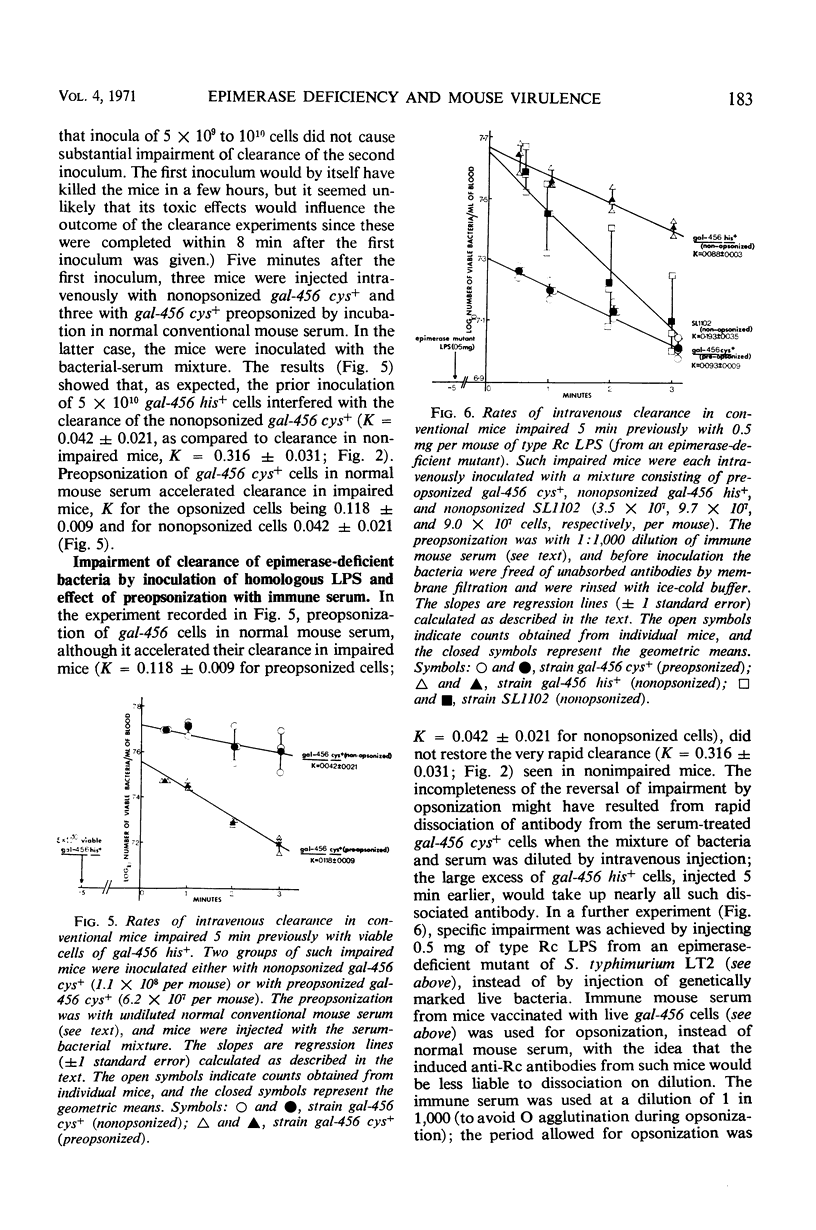
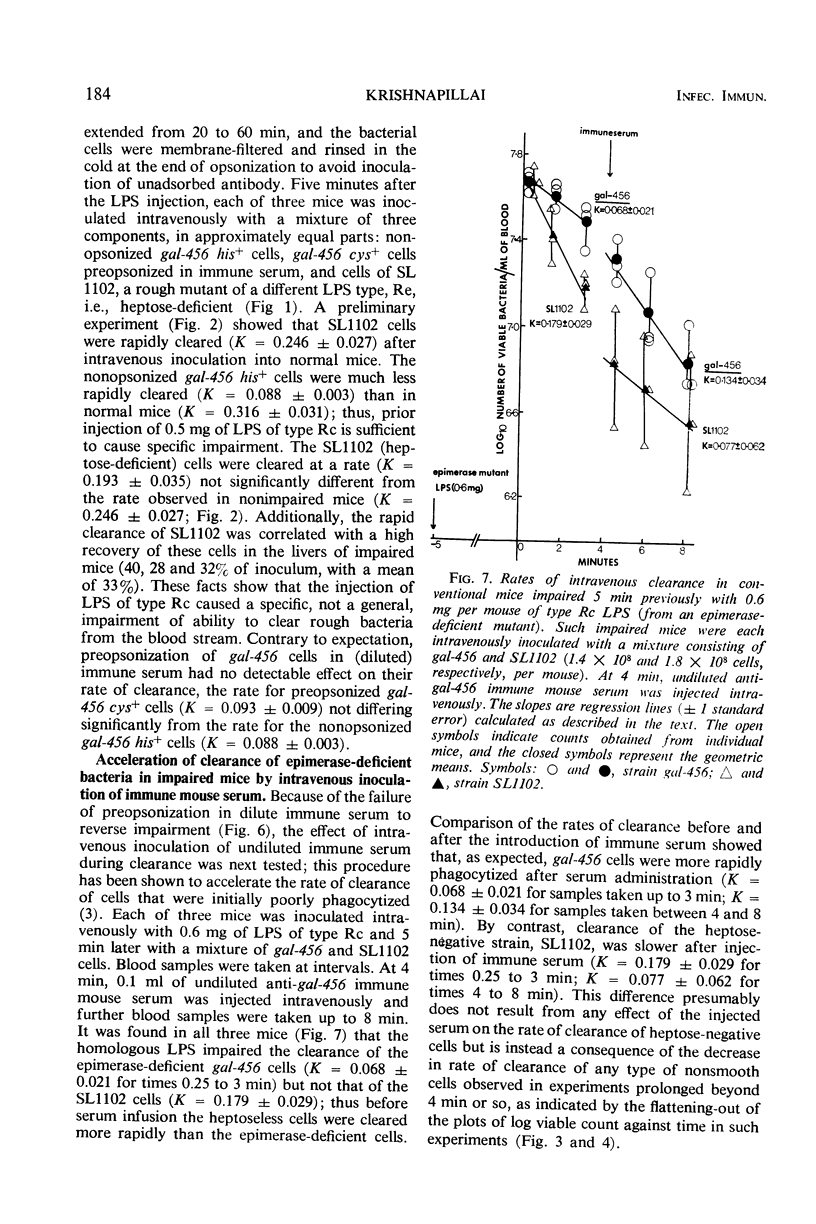
Abstract
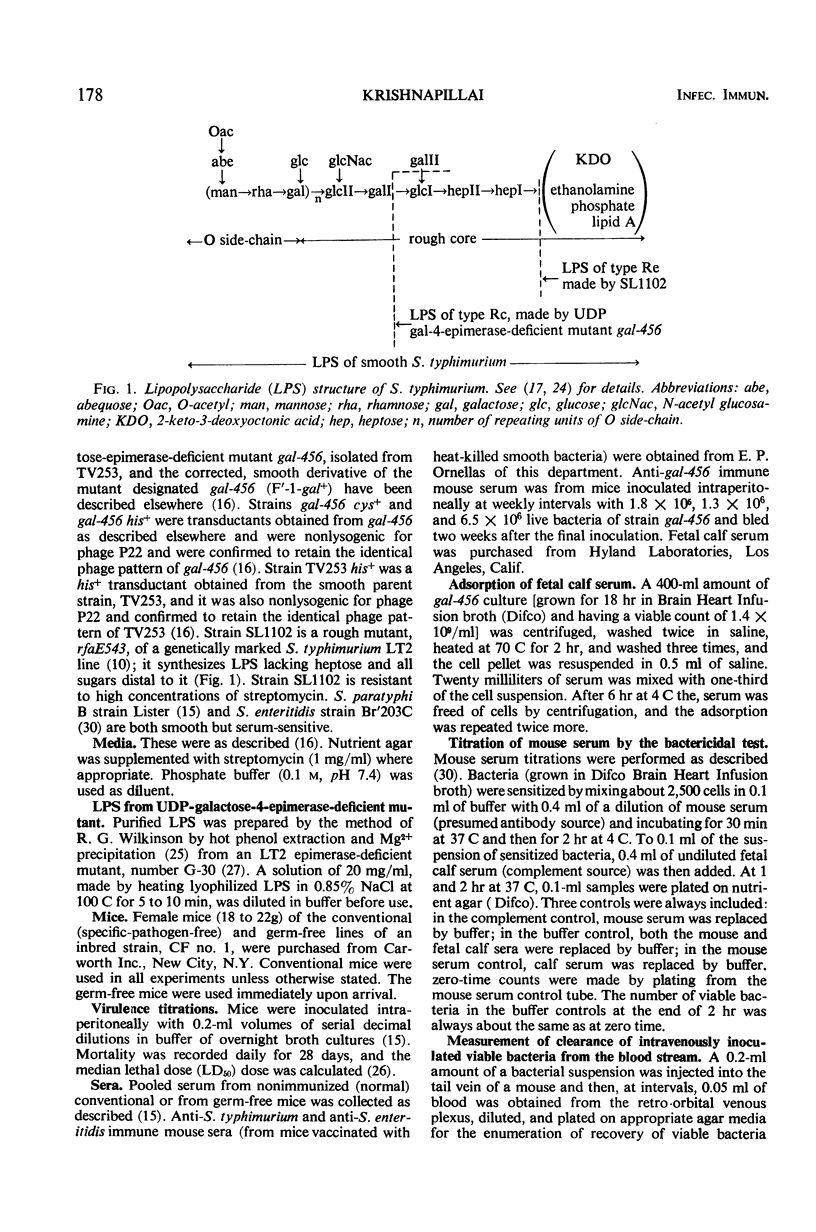
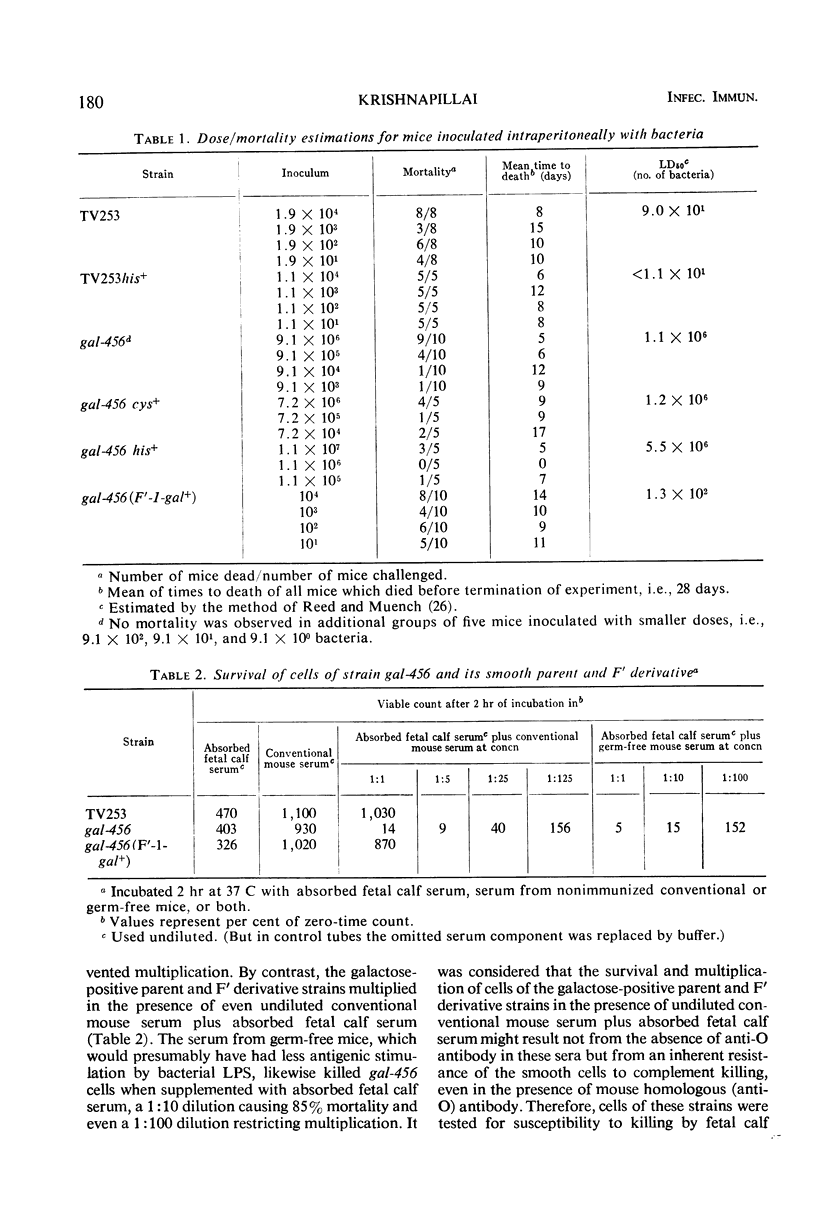
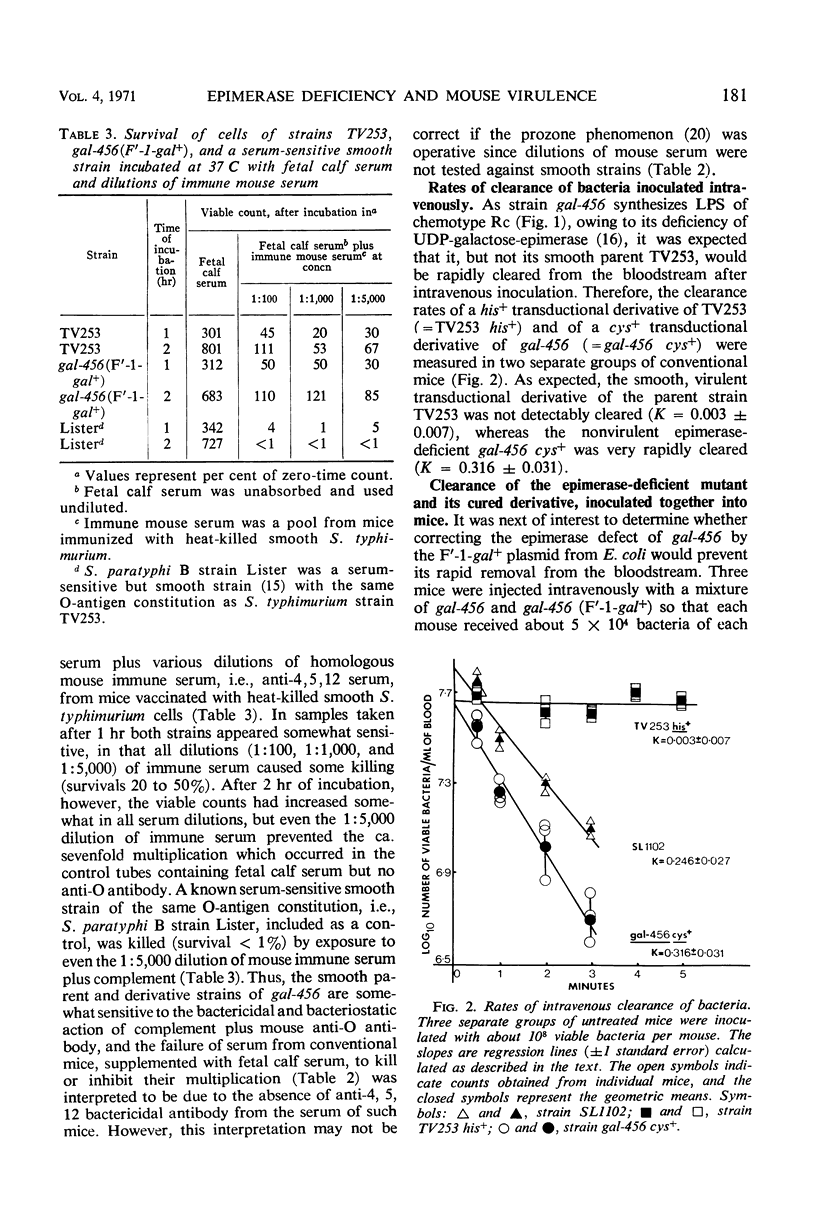
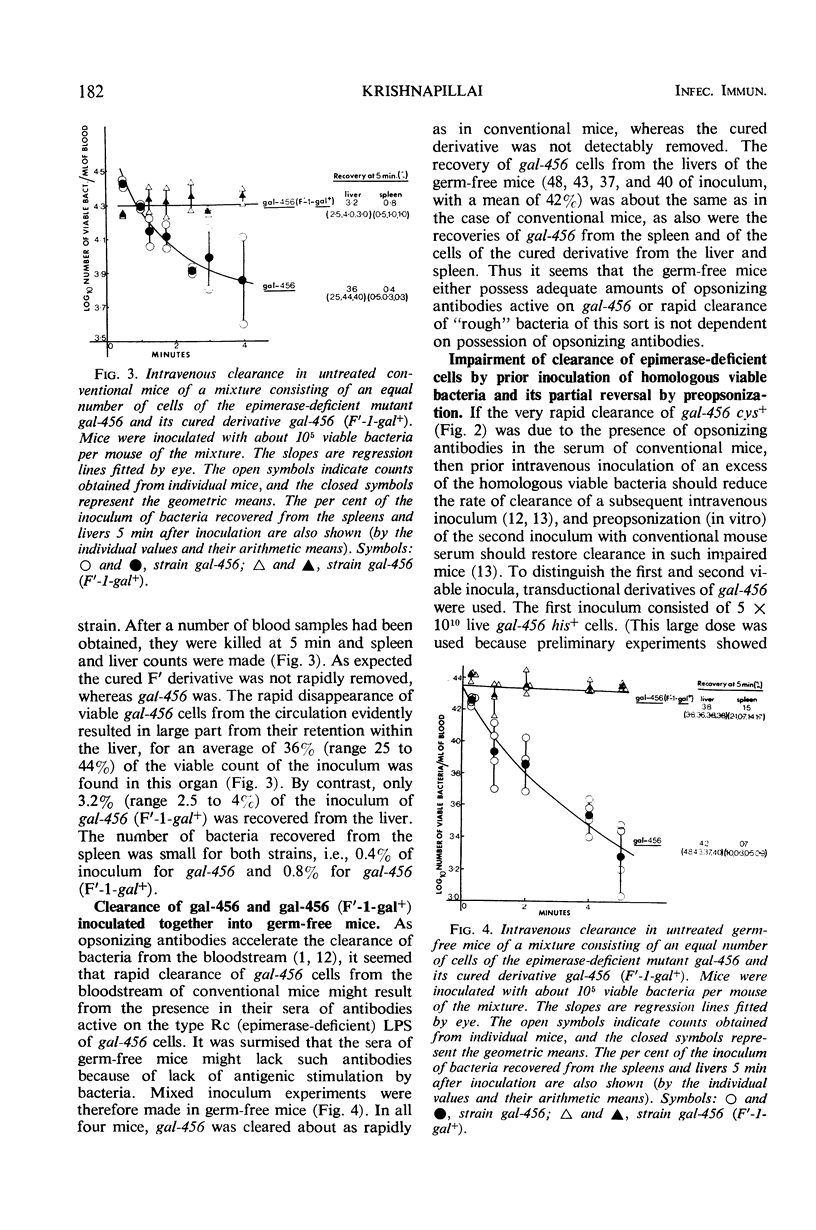
The synthesis of smooth lipopolysaccharide (LPS) in relation to mouse virulence and resistance to serum bactericidal activity in vitro and to rapid intravenous clearance in vivo was studied in Salmonella typhimurium by using a virulent [median lethal dose (LD50) = 102], smooth, and genetically marked strain, a uridinediphosphogalactose epimerase-deficient mutant of it which was, therefore, rough, and a derivative of the mutant made smooth again by acquisition of the galactose-positive genes of Escherichia coli. The mutant was of reduced virulence (LD50 = 106) but the smooth derivative regained the virulence character typical of the parent. The non-smooth phenotype also made the mutant, but not the smooth relatives (parent and derivative), susceptible to serum bactericidal activity and also to rapid intravenous clearance by phagocytosis by the liver. The mutant was similarly treated by germ-free mice (expected to be relatively free of opsonizing antibodies). The clearance of the mutant could be impaired by prior intravenous inoculation of homologous bacteria or their LPS but was reversible by preopsonization of the second inoculum with nonimmune mouse serum, suggesting that the initial inoculum preempted the opsonizing antibodies. Independent evidence of clearance specificity was also provided in mixed inoculum experiments on impaired mice by the rapid clearance of an antigenically unrelated heptose-deficient mutant while maintaining the decelerated clearance of the epimerase mutant. The latter, however, was converted to accelerated clearance by the intravenous inoculation during the impaired state of anti-epimerase mutant immune mouse serum.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., SEBESTYEN M. M., SCHLOSSMAN S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959 Jul 1;110(1):27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., HOWARD J. G., HALPERN B. N., STIFFEL C., MOUTON D. The kinetics of blood clearance o isotopically labelled Salmonella entertidis by the reticulo-endothelial system in mice. Immunology. 1960 Jan;3:74–89. [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. Interference immunity produced by pertussis vaccine to pertussis infection in mice. Br J Exp Pathol. 1954 Dec;35(6):603–608. [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. The ability of pertussis vaccine to produce in mice specific immunity of a type not associated with antibody production. Br J Exp Pathol. 1954 Aug;35(4):322–330. [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. The production of both interference and antibody immunity by pertussis vaccine to pertussis infection in mice. Br J Exp Pathol. 1955 Aug;36(4):391–401. [PMC free article] [PubMed] [Google Scholar]
- Edebo L., Normann B. Virulence and immunogenicity of mutant strains of Salmonella typhimurium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):75–84. doi: 10.1111/j.1699-0463.1970.tb04271.x. [DOI] [PubMed] [Google Scholar]
- FISHER S. Multiplication of H. pertussis in the mouse lung following intranasal infection. Aust J Exp Biol Med Sci. 1955 Dec;33(6):609–628. doi: 10.1038/icb.1955.61. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R. An antigenic basis for virulence in strains of Salmonella typhimurium. J Exp Med. 1962 Apr 1;115:731–743. doi: 10.1084/jem.115.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNAPILLAI V., BARON L. S. ALTERATIONS IN THE MOUSE VIRULENCE OF SALMONELLA TYPHIMURIUM BY GENETIC RECOMBINATION. J Bacteriol. 1964 Mar;87:598–605. doi: 10.1128/jb.87.3.598-605.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V., Karthigasu K. Salmonella abony-Salmonella typhimurium recombinant nonvirulent for the mouse. J Bacteriol. 1969 Mar;97(3):1343–1351. doi: 10.1128/jb.97.3.1343-1351.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V., MacPhee D. G., Stocker B. A. Properties of a Salmonella typhimurium mutant with an incomplete deficiency of uridinediphosphogalactose-4-epimerase. J Bacteriol. 1971 Jul;107(1):155–161. doi: 10.1128/jb.107.1.155-161.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T., Hellerqvist C. G., Svensson S. Bacteriophage receptor development and synthesis of O-specific side chains after addition of D-galactose to the uridine diphosphate-galactose-4-epimeraseless mutant Salmonella typhimurium LT2-M1. J Bacteriol. 1970 May;102(2):540–547. doi: 10.1128/jb.102.2.540-547.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth A., Duguid J. P. Demonstration of different mutational sites controlling rhamnose fermentation in FIRN and non-FIRN rha-strains of Salmonella typhimurium: an essay in bacterial archaeology. Genet Res. 1968 Apr;11(2):151–169. doi: 10.1017/s0016672300011320. [DOI] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Nakano M., Saito K. The chemical compositions in the cell wall of Salmonella typhimurium affecting the clearance-rate in mouse. Jpn J Microbiol. 1968 Dec;12(4):471–478. doi: 10.1111/j.1348-0421.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. I. Linkage between o side chains and R core. J Biol Chem. 1969 Jun 10;244(11):2835–2845. [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- SAITO K. On the variation of mutabile type (Murase) originated from rough phase of Salmonella. I. Mode of variation and characteristics of each variant. Jpn J Med. 1951 Dec;4(6):347–359. doi: 10.7883/yoken1948.4.347. [DOI] [PubMed] [Google Scholar]
- Steward J. P., Collis L. R., Roantree R. J. Effects of immunization of guinea pigs lacking bactericidal antibody against Salmonella enteritidis. J Immunol. 1966 Aug;97(2):224–230. [PubMed] [Google Scholar]



