Abstract
Objectives
Both patients with pediatric bipolar disorder (BD) and unaffected youth at familial risk (AR) for the illness show impairments in face emotion labeling. Few studies, however, have examined brain regions engaged in AR youth when processing emotional faces. Moreover, studies have yet to explore neural responsiveness to subtle changes in face emotion in AR youth.
Methods
Sixty-four unrelated youth, including 20 patients with BD, 15 unaffected AR youth, and 29 healthy comparisons (HC) completed functional magnetic resonance imaging. Neutral faces were morphed with angry or happy faces in 25% intervals. In specific phases of the task, youth alternatively made explicit (hostility) or implicit (nose width) ratings of the faces. The slope of blood oxygenated level-dependent activity was calculated across neutral to angry and neutral to happy face stimuli.
Results
Behaviorally, both subjects with BD (p ≤ 0.001) and AR youth (p ≤ 0.05) rated faces as less hostile relative to HC. Consistent with this, in response to increasing anger on the face, subjects with BD and AR youth showed decreased modulation in the amygdala and inferior frontal gyrus [(IFG); BA 46] compared to HC (all p ≤ 0.05). Amygdala dysfunction was present across both implicit and explicit rating conditions, but IFG modulation deficits were specific to the explicit condition. With increasing happiness, AR youth showed aberrant modulation in the IFG, which was also sensitive to task demands (all p ≤ 0.05).
Conclusions
Decreased amygdala and IFG modulation in subjects with BD and AR youth may be pathophysiological risk markers for BD, and may underlie the social cognition and face emotion labeling deficits observed in BD and AR youth.
Keywords: at risk, bipolar disorder, face emotion, fMRI, parametric modulation
The identification of endophenotypes for complex illnesses, such as bipolar disorder (BD), could assist in the search for risk-related genes, early intervention, and ultimately the development of prevention initiatives (1). Studying unaffected youth at risk (AR) for BD provides an opportunity to examine intermediate pathophysiological markers for the illness without the common potential confounding effects of mood state, psychotropic medications, and prior mood episodes. Face emotion processing deficits have been proposed as a candidate BD endophenotype; face labeling impairments are present in patients with BD during euthymia (2, 3), and in AR youth (2, 4). The neural correlates of this behavioral deficit have been investigated in both pediatric and adult patients with BD. Meta-analyses have established limbic hyperactivation and prefrontal cortex (PFC) hypoactivation during face emotion processing (5-10) in BD. However, few studies have determined whether a similar pattern of neural dysfunction is present in AR youth (11-14).
Most studies examining the neural circuitry mediating face emotion processing have focused on full, prototypical emotional expressions. Both positive and negative face emotions, such as happy (15-31) and angry (19, 26, 28, 30-32) faces, elicit aberrant neural activity in patients with BD. Few studies, however, have explored the neural correlates of more ecologically valid, subtle changes in emotional expressions. Moreover, systematically varying face emotion intensity levels may defend against amygdala habituation (33). Indeed, only one study has examined the neural correlates of subtle changes in emotional expressions by statistically modeling neural activation as a function of increasing face emotion (30). In this study, relative to healthy comparison (HC) children, patients with BD failed to modulate the amygdala and frontal cortex in response to increasing anger on the face (30). Failure to modulate in BD indicates that these regions are not as neurally responsive to subtle changes in anger intensity, compared to HC youth. To date, research has yet to examine amygdala and PFC responsiveness to subtle changes in face emotion in AR youth. Aberrant modulation of these areas may contribute to the face emotion deficits observed in AR youth for BD, and may be a potential pathophysiological endophenotypic marker for the illness.
Here, we examine neural activation in youth AR using a parametric face emotion processing task, and compared their activation to a previously published overlapping sample of BD and HC youth (30). This prior study indicated that youth with BD showed aberrant patterns of activation in the amygdala and PFC (30). Given this prior work (5-10, 30), we conducted an amygdala region-of-interest (ROI) analysis, as well as an exploratory wholebrain analysis. We hypothesized that AR youth would exhibit abnormal linear trends in the amygdala and PFC, similar to deficits previously reported in pediatric BD (30).
Materials and methods
Participants
Sixty-four unrelated youth (8–19-years-old), including 20 patients with BD, 15 AR youth, and 29 HC were enrolled in an Institutional Review Board-approved study at the National Institute of Mental Health. Parental/child informed consent/assent was obtained. Data from 16 subjects with BD and 22 HC, but no AR youth, have been published previously (30).
Children were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (34). Youth with BD met narrow phenotype criteria (35). We also completed depression and hypo/mania ratings to determine mood state at time of scanning in youth with BD. The Children’s Depression Rating Scale (36) and Young Mania Rating Scale (37) were completed within 48 hours of scanning. Interviewers were master’s and doctoral-level clinicians, with excellent inter-rater reliability (κ > 0.9 for identifying hypo/manic episodes). Diagnoses were based on best-estimate procedures generated in a consensus conference led by a psychiatrist.
AR youth had a first-degree relative (parent: 60%; sibling: 33.3%; both: 6.7%) whose BD diagnosis was confirmed via semi-structured interview (34, 38, 39). AR youth with attention-deficit hyperactivity disorder (ADHD) or anxiety disorders were included; history of major depressive or other mood disorders was exclusionary.
Exclusion criteria for all participants were: IQ < 70, history of head trauma, neurological disorder, pervasive developmental disorder, chronic medical illness, or substance abuse within two months.
Of the 108 youth scanned, data were excluded from 29 subjects [BD (n = 15), AR (n = 3), HC (n = 11)] due to poor task performance; six subjects [BD (n = 3), AR (n = 1), HC (n = 2)] for a sibling in the study; seven subjects [BD (n = 2), AR (n = 2), HC (n = 3)] for movement; and two HC for equipment failure.
Parametric faces paradigm
Task details have been described elsewhere (30). Faces displaying neutral, angry, and happy expressions (40) were used to create two stimulus sets of morphs between an angry or a happy face and a neutral face. There were five morph intensities: 100% neutral; 25% emotion and 75% neutral; 50% emotion and 50% neutral; 75% emotion and 25% neutral; and 100% emotion. During face presentation, there were two rating conditions: implicit (how wide is the nose?) or explicit (how hostile is the face?). Ratings were made using a five-button response device: 1 = least wide/hostile to 5 = most wide/hostile. Faces appeared for 3000 msec per trial; a blank screen was presented for 750–1250 msec (average = 1000 mses). Different morph intensities were presented randomly. Each of four blocks was 6.3 min (total = 25 min).
Functional magnetic resonance imaging (fMRI) acquisition
Neuroimaging data were acquired on two General Electric 3T scanners: a Signa VH/i (16 BD, 9 AR, 22 HC) and a Signa HDx (4 BD, 6 AR, 7 HC), with the same eight-channel GE head coil. Identical scanning parameters were used: a high-resolution structural scan (T1-weighted axial acquisition, 124 1.2-mm slices, 15° flip angle, 256 ×256 matrix, 24-cm field-of-view) and gradient echo-planar imaging images (38 contiguous 2-mm3 slices, repetition time = 2300 msec, echo time = 25 msec, flip angle = 90°, 96 ×96 mm). Although the number of subjects in each group did not differ across scanners (p = 0.38), Scanner was included as a covariate in the fMRI analyses.
Data analyses
Demographic analyses
Analysis of variance (ANOVA) was used to compare age and IQ, and chi-square test was used to compare sex distribution.
Behavioral data analyses
Ratings and reaction time (RT) for angry and happy faces were compared in separate Group (BD, AR, HC) ×Condition (implicit, explicit) ×Face intensity repeated measures ANOVAs. Post-hoc t-tests decomposed interactions.
Imaging data analyses
Analysis of Functional Neuroimages (41) was used, including standard preprocessing methods (30). Linear-trend analyses were used to model the degree to which blood oxygenated level-dependent signal was related to face emotion intensity. Separate trend analyses were performed for each condition (implicit, explicit) and each face emotion (angry, happy). Positive linear trends (positive slope value) indicate that activation increased with increasing emotion intensity; negative linear trends (negative slope value) indicate that activation decreased with increasing emotion intensity. We performed two types of analyses on the imaging data: an anatomical amygdala ROI and a wholebrain analysis.
For the anatomical amygdala ROI analysis, we performed a Group (BD, AR, HC) ×Condition (implicit, explicit) analysis of covariance (ANCOVA), with Scanner as a covariate. Scanner was not a significant (all p > 0.80) covariate in these models. We conducted separate ANCOVAs for linear trends between neutral to angry, and neutral to happy faces. ROI data were extracted using the BOLD signal averaged across the entire anatomical ROI. There was one trend value per hemisphere, and separate linear trends for each rating condition. Analyses and post-hoc t-tests were performed in SPSS. This analysis was repeated with Age included as an additional covariate; significant main effects of Group remained.
At the wholebrain level, we examined linear trends using a Group ×Condition ANCOVA, with scanner as a covariate. Scanner was not a significant covariate in the analyses for neutral to angry (all p > 0.15); however, as noted below, it was significant in the neural to happy analysis. To balance type I and II errors, we used criteria suggested by Lieberman and Cunningham (42) (p ≤ 0.005; k ≥10); separate analyses were conducted for angry and happy faces. Post-hoc analyses were performed in SPSS to decompose interactions. This analysis was repeated with Age included as an additional covariate; significant Group ×Condition interactions remained. Post-hoc analyses tested the effects of potentially confounding variables on our results in AR youth. We examined proband status [parent (n = 9) versus sibling (n = 5); one ‘both sibling and parent probands’ was excluded from this post-hoc analysis]. In addition, post-hoc analyses examined the presence of ADHD or an anxiety disorder on activation.
Results
Demographics
Groups did not differ on age, IQ, or sex distribution (Table 1).
Table 1.
Demographic and clinical characteristics of subjects with bipolar disorder, at-risk youth, and healthy comparisons
| Characteristics | Bipolar disorder (n = 20) | At risk (n = 15) | Healthy comparisons (n = 29) |
|---|---|---|---|
| Age, years, mean (SD) | 15.6 (2.3) | 14.5 (2.2) | 14.9 (1.9) |
| WASI IQ, mean (SD) | 104.6 (16.3) | 108.2 (13.6) | 110.0 (12.7) |
| YMRS score, mean (SD) | 6.5 (5.1) | – | – |
| CDRS score, mean (SD) | 28.5 (8.1) | – | – |
| CGAS score, mean (SD)a | 46.4 (19.6) | 79.9 (24.9) | – |
| No. of medications, mean (SD) | 3.1 (1.5) | 0 | 0 |
| Sex, male, n (%) | 7 (35) | 9 (60) | 16 (55.2) |
| Diagnoses, n (%) | |||
| BD-I | 15 (75) | 0 (0) | 0 (0) |
| BD-II | 5 (25) | 0 (0) | 0 (0) |
| Anxiety disorderb | 9 (45) | 1 (6.7) | 0 (0) |
| ADHDc | 13 (65) | 1 (6.7) | 0 (0) |
| ODDd | 5 (25) | 0 | 0 (0) |
| Mood state, n (%) | |||
| Euthymic | 17 (85) | – | – |
| Depressed | 1 (5) | – | – |
| Hypo/manic | 1 (5) | – | – |
| Mixed | 1 (5) | – | – |
SD = standard deviation; WASI IQ = Wechsler Abbreviated Scale of Intelligence two-scale IQ; YMRS = Young Mania Rating Scale; CDRS = Children’s Depression Rating Scale; CGAS = Clinical Global Assessment Scale; BD-I = bipolar I disorder; BD-II = bipolar II disorder; ADHD = attention-deficit hyperactivity disorder; ODD = oppositional defiant disorder; BD = bipolar disorder; AR = at risk.
Missing data for three subjects with BD and one AR youth. t(29) = -4.20, p ≤ 0.001.
Includes generalized anxiety disorder, separation anxiety disorder, social phobia, panic disorder, posttraumatic stress disorder, and obsessive compulsive disorder. BD versus AR (χ2 = 5.69, p ≤ 0.05).
BD versus AR (χ2 = 11.38, p ≤ 0.01).
BD versus AR (χ2 = 4.10, p ≤ 0.05).
Behavioral results
Angry faces
There was a Group ×Condition interaction [F(2,244) = 3.54, p ≤ 0.05], with BD (mean = 2.84 ±0.52, p ≤ 0.001) and AR (mean = 3.00 ±0.44, p ≤ 0.05) rating angry faces as less hostile than HC (mean = 3.27 ±0.37).
Happy faces
There was a Group ×Condition ×Face intensity interaction [F(8,244) = 3.17, p ≤ 0.01], with BD (mean = 2.25 ±0.50, p ≤ 0.01; mean = 1.87 ±0.47, p ≤ 0.01) and AR (mean = 2.28 ±0.52, p ≤ 0.05; mean = 1.94 ±0.38, p ≤ 0.05) rating neutral and 100% happy faces as less hostile than HC (mean = 2.62 ±0.42; mean = 2.28 ±0.48).
There were no group differences in reaction time.
Anatomical amygdala ROI results
Angry faces
Linear-trend analyses in left and right amygdala revealed a main effect of Group: left [F(2,60) = 6.85, p ≤ 0.01]; right [F(2,60) = 5.29, p ≤ 0.01)]. Compared to HC, both BD (left p ≤ 0.01; right p ≤ 0.05) and AR (left p ≤ 0.001; right p ≤ 0.01) showed less amygdale modulation with increasing anger on the face (Fig. 1, Table 2).
Fig. 1.
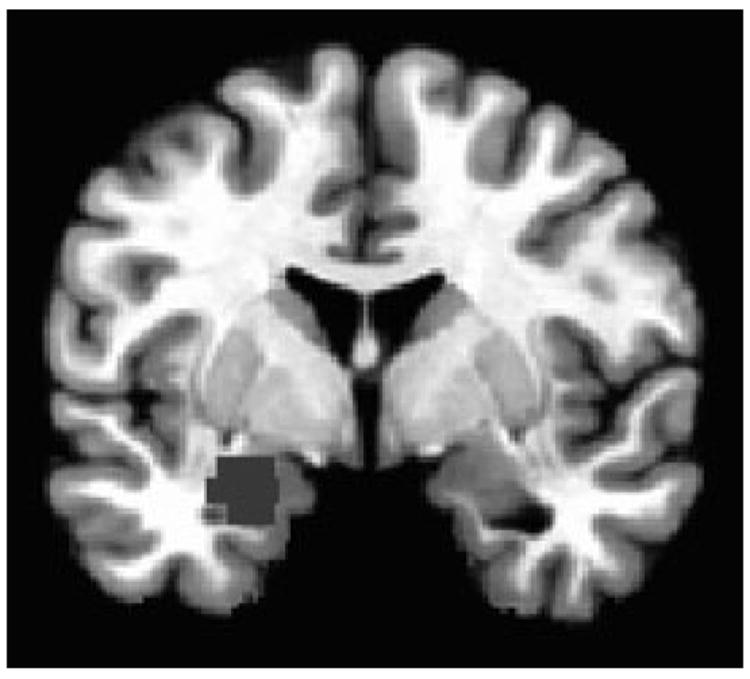
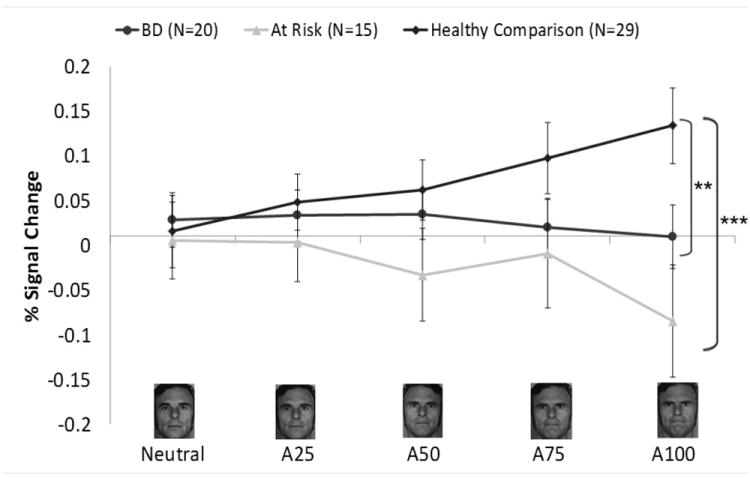
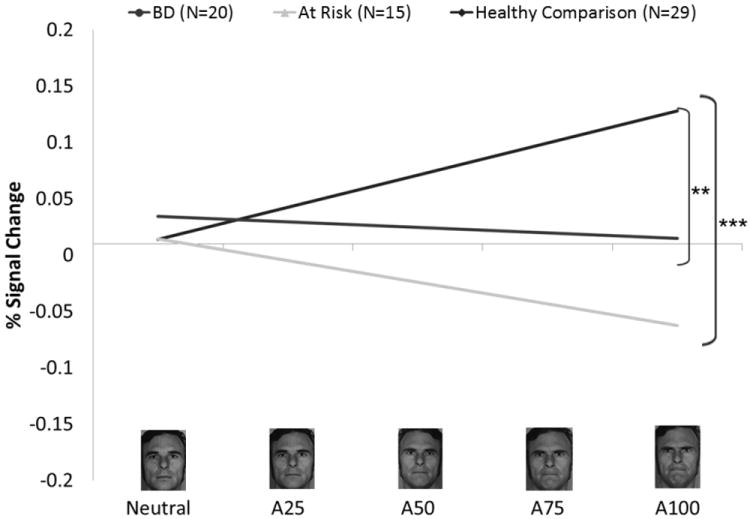
Left amygdala region-of-interest (ROI) from the angry face analysis. Panel A shows the left anatomical ROI used in the analysis. Panel B shows the blood oxygenated level-dependent signal at each intensity level for each group. Panel C shows the linear-trend values for each group. A25 = 25% Angry and 75% Neutral; A50 = 50% Angry and 50% Neutral; A75 = 75% Angry and 25% Neutral; A100 = 100% Angry; BD = bipolar disorder. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Table 2.
Amygdala anatomical region-of-interest and wholebrain results
| Region-of-interest | Hemisphere | Post-hoc analyses | |||||
|---|---|---|---|---|---|---|---|
| Neutral to angry | |||||||
| Amygdala | L | HC versus BDb, ARc | |||||
| Amygdala | R | HC versus BDa, ARb | |||||
|
| |||||||
| Wholebrain analysis | BA | Hemisphere | k | RAI Coordinates | Post-hoc analyses | ||
|
| |||||||
| x | y | z | |||||
| Neutral to angry | |||||||
| IFG | 46 | L | 18 | 49 | -31 | 10 | HC versus BDa, ARb |
| ACC | 25 | R | 36 | -3 | -13 | -10 | BD versus HCa, ARb |
| Neutral to happy | |||||||
| IFG | 46 | L | 214 | 39 | -37 | 4 | AR versus BDa, HCa |
BA = Brodmann area; L = left; R = right; HC = healthy comparison; BD = bipolar disorder; AR = at risk; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex; RAI = right, left; anterior, posterior; inferior, superior.
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001.
Happy faces
There were no Group or Group ×Condition effects.
Wholebrain results
Angry faces
Two clusters showed a Group ×Condition interaction: left inferior frontal gyrus (IFG) [Brodmann area (BA) 46, k = 18; x = 49, y = -31, z = 10] and right anterior cingulate (ACC) (BA 25, k = 36; x = -3, y = -13, z = -10). In the IFG (BA 46), both BD (p ≤ 0.05) and AR (p ≤ 0.01) youth showed decreased modulation relative to HC during hostility ratings [F(2,60) = 7.70, p ≤ 0.001] (Fig. 2, Table 2). In the ACC (BA 25), increased modulation was observed in BD during hostility ratings relative to both AR (p ≤ 0.01) and HC (p ≤ 0.05), while decreased modulation in BD was seen during nose width ratings relative to HC (p ≤ 0.01) [F(2,60) = 10.65, p ≤ 0.001].
Fig. 2.
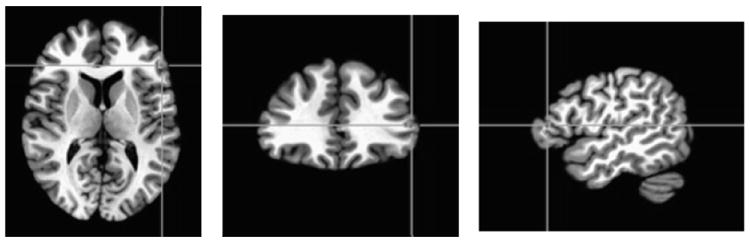
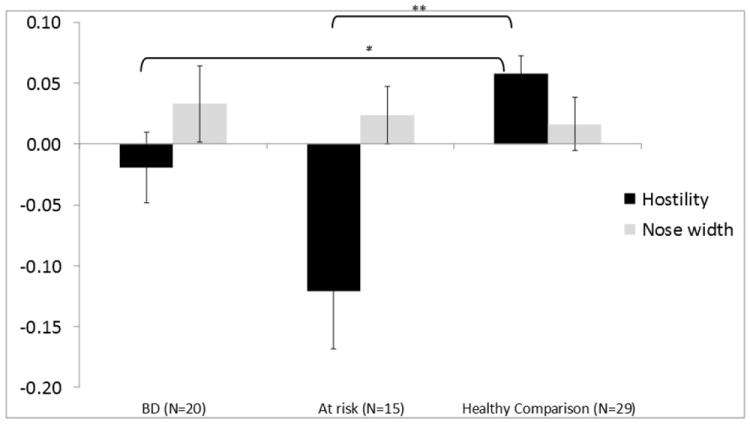
Inferior frontal gyrus (IFG) cluster identified by a Group ×Condition interaction from the wholebrain angry face analysis. Panel A shows the IFG cluster identified from the wholebrain analysis (p ≤ 0.005; k ≥ 10; x = 49, y = -31, z = 10). Panel B depicts the linear-trend value collapsed across all face intensities. Positive linear trend indicates that activation in a region increased with increasing face emotion intensity (i.e., positive slope value); negative linear trend indicates that activation in a region decreased with increasing face emotion intensity (i.e., negative slope value). BD = bipolar disorder. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Happy faces
There was a Group ×Condition interaction in the left IFG (BA 46, k = 214; x = 39, y = -37, z = 4). During hostility ratings, AR (all p ≤ 0.05) showed decreased modulation, but increased modulation during nose width ratings relative to HC [F(2,60) = 11.27, p ≤ 0.001] (Table 2). Scanner was a significant covariate in this model [F(1,60) = 5.80, p = 0.02]. Post-hoc analyses in AR: proband status and Axis I diagnosis
AR with an affected parent differed from those with an affected sibling only in the ACC, where AR with an affected parent showed decreased modulation during nose width ratings (p = 0.008). When the n = 2 AR with an Axis I diagnosis were excluded, the pattern of AR versus HC findings remained the same.
Discussion
Few studies have mapped neural circuitry engaged by face emotion displays in youth at familial risk for BD (11-14). Here, we used a parametric design to demonstrate that the amygdala and PFC dysfunction found previously in BD (5-10) also occurs in AR youth. Specifically, as in the only prior study using the same paradigm in patients with BD (30), we also found deficient amygdala modulation when faces subtly morphed from neutral to angry expressions in AR youth AR. In addition, AR youth, like pediatric patients with BD, showed reduced modulation of the IFG as faces morphed from neutral to angry. AR youth also demonstrated modulation impairments in the IFG when neutral faces morphed towards happy. Together, these findings extend prior work suggesting that fronto-amygdala dysfunction may be an endophenotypic marker for BD (12, 14, 24).
Reduced amygdala modulation was present as faces morphed towards angry during both implicit and explicit rating conditions. In contrast, BD and AR IFG dysfunction in both angry and happy face conditions was sensitive to task demands. For both angry and happy faces, when explicitly rating the level of hostility of the face, AR demonstrated decreased modulation of the IFG; in BD, aberrant modulation of the IFG was only present during the angry face condition. Consistent with this and prior work indicating that patients with BD and AR youth require more intense emotional information to identify face emotions (4), both youth with BD and AR youth rated faces as less hostile relative to HC, suggesting downstream behavioral effects from deficient IFG modulation. Together, the amygdala and IFG are crucial in the integration of emotional information (43, 44) and emotion regulation (45). Dysfunctional modulation of these areas may contribute to the social and emotion labeling deficits observed in youth with BD and AR youth (2, 4). AR youth by virtue of a parent versus a sibling with BD did not differ, with the exception of those with an affected parent showed decreased modulation during implicit ratings in the ACC. However, these findings are subject to type II error due to the small sample sizes in each group. It is also important to note that modulation of the ACC did not differ between the entire AR and HC groups, suggesting that this finding should be interpreted with caution. Our findings should be considered in light of additional limitations. First, our BD and AR sample sizes were small. Studies with larger samples are needed to replicate the current findings. Second, most patients with BD were medicated; and, while the majority (85%) of patients with BD were euthymic at the time of scanning, some were not. However, AR children showed a similar pattern of dysfunction. AR youth were all unaffected by a mood disorder and medication naïve, suggesting that the deficits observed may be a risk marker, as opposed to a consequence of mood state or medication exposure. Consistent with this, studies indicate the neural dysfunction may normalize with treatment; medications are not likely to cause between-group differences in activation (46, 47). Third, fMRI data were combined across two scanners and a scanner effect may be present. Of note, Scanner was included as a covariate in the analyses. However, we have not tested inter-scanner reliability of the two scanners used in this study.
Our design, which included different gradations of emotional expressions, may be particularly useful in research on amygdala dysfunction, as the amygdala habituates rapidly to repeated presentations of similar stimuli (33). Thus, parametric designs, which model linear changes along a continuum, may be more sensitive to subtle between–group differences in activation. Future studies should include larger samples and a longitudinal design to determine whether the neural deficits associated with face processing predict the onset of BD in AR youth. With further study, risk stratification and preventive interventions could be used to potentially mitigate the development and prevalence of BD.
Acknowledgments
Funding for this study was provided by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health. We would like to thank the staff of the Emotion and Development Branch at NIMH and the patients and their parents for their participation.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 3.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 4.Brotman MA, Skup M, Rich BA, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry. 2008;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond BN, Fredericks CA, Blumberg HP. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord. 2012;14:340–355. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 7.Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacology. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Houenou J, Frommberger J, Carde S, et al. Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. J Affect Disord. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res. 2011;193:71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Strakowski SM, Adler CM, Almeida J, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajek T, Cullis J, Novak T, et al. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol Psychiatry. 2013;73:144–152. doi: 10.1016/j.biopsych.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladouceur CD, Diwadkar VA, White R, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neuros. 2013;5:185–196. doi: 10.1016/j.dcn.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts G, Green MJ, Breakspear M, et al. Reduced inferior frontal gyrus activation during response inhibition to emotional stimuli in youth at high risk of bipolar disorder. Biol Psychiatry. 2013;74:55–61. doi: 10.1016/j.biopsych.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Olsavsky AK, Brotman MA, Rutenberg JG, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:294–303. doi: 10.1016/j.jaac.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Hassel S, Almeida JR, Frank E, et al. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. J Affect Disord. 2009;118:19–27. doi: 10.1016/j.jad.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassel S, Almeida JR, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida JR, Mechelli A, Hassel S, Versace A, Kupfer DJ, Phillips ML. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry Res. 2009;174:195–201. doi: 10.1016/j.pscychresns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah MP, Wang F, Kalmar JH, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surguladze SA, Marshall N, Schulze K, et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 25.Versace A, Thompson WK, Zhou D, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology. 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrett AS, Reiss AL, Howe ME, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:821–831. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keener MT, Fournier JC, Mullin BC, et al. Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychological Med. 2012;42:1913–1924. doi: 10.1017/S0033291711002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourão-Miranda J, Oliveira L, Ladouceur CD, et al. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PloS One. 2012;7:e29482. doi: 10.1371/journal.pone.0029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas LA, Brotman MA, Muhrer EJ, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Archiv Gen Psychiatry. 2012;69:1257–1266. doi: 10.1001/archgenpsychiatry.2012.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas LA, Kim P, Bones BL, et al. Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder. Neuroimage Clin. 2013;2:637–645. doi: 10.1016/j.nicl.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlman SB, Almeida JR, Kronhaus DM, et al. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disord. 2012;14:162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 36.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Nurnberger JI, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archiv Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 40.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- 41.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cog Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 44.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 45.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 47.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]


