Abstract
Monocyte development is a tightly regulated and multi-staged process, occurring through several defined progenitor cell intermediates. The key transcription factors, including PU.1, IRF8 and KLF4, growth factors, such as M-CSF and IL-34 and cytokines that drive monocyte development from hematopoietic progenitor cells are well defined. However, the molecular controls that direct differentiation into the Ly6Chi inflammatory and Ly6Clo monocyte subsets are yet to be completely elucidated. This review will provide a summary of the transcriptional regulation of monocyte development. We will also discuss how these molecular controls are also critical for microglial development despite their distinct haematopoetic origins. Furthermore, we will examine recent breakthroughs in defining mechanisms that promote differentiation of specific monocyte subpopulations.
Keywords: Ly6Chi monocytes, Ly6Clo monocytes, microglia, monocyte development, transcription factors, IRF8, PU.1, KLF4, NR4A1
1. Introduction
Monocytes are a heterogeneous population of circulating phagocytes that give rise to tissue macrophage and dendritic cell (DC) populations under homeostatic and inflammatory conditions [1, 2]. In the mouse, monocytes can be divided into two primary subsets based on phenotype and function. Inflammatory monocytes express high levels of CCR2 and Ly6C and are rapidly recruited to sites of infection or injury, where they give rise to proinflammatory macrophages and DC [3–5]. Inflammatory monocytes are critical for the control of a number of pathogens, including bacteria such as Listeria and Mycobacterium [6–8], fungi including Cryptococcus and Candida [9–11], parasites such as Toxoplasma [12, 13] and viruses including Herpes simplex and Murine hepatitis virus [14–16]. However, these cells also significantly contribute to immunopathology and autoimmunity in models of atherosclerosis and cardiac infarction [3, 17–19], rheumatoid arthritis [20], multiple sclerosis [21], inflammatory bowel disease [22], stroke [23] and encephalitis [5, 24–28]. Ly6Clo monocytes, identified by high expression of the chemokine receptor CX3CR1 and low expression of CCR2, patrol blood vessels and mediate early responses against insult [29, 30]. These cells have also been shown to promote wound healing and angiogenesis in models of atherosclerosis and cardiac infarction [31, 32].
The developmental relationship between the Ly6Chi and Ly6Clo subsets is yet to be completely elucidated, however a large body of evidence suggests that circulating Ly6Clo monocytes derive from Ly6Chi monocytes [33–36]. Following clodronate liposome depletion, Ly6Chi monocytes are the first subset to repopulate the blood, followed by Ly6Clo monocytes, suggesting that the latter derive from Ly6Chi cells [33, 37]. Supporting this, experiments tracing circulating Ly6Chi monocytes with fluorescent beads or Dil-labeled liposomes after clodronate depletion showed that these cells convert into a Ly6Clo phenotype after two days in the circulation [33, 37]. Furthermore, adoptive transfer studies have shown that Ly6Chi monocytes can shuttle between the blood and bone marrow and downregulate Ly6C expression [34, 36]. However, there is some evidence to suggest that circulating Ly6Clo monocytes may derive directly from hematopoietic precursors in the absence of their Ly6Chi counterparts, highlighting the potential plasticity of monocyte differentiation [1].
Although significant efforts have been made to define the developmental relationship between Ly6Chi and Ly6Clo monocytes, there is distinct lack of data examining the molecular controls i.e. transcription factors, growth factors and cytokine/chemokine signaling that directs differentiation into these two phenotypically and functionally distinct subsets. This review will provide a current overview of the development of Ly6Chi and Ly6Clo monocyte subsets and the transcriptional regulation of this process. A better understanding of the molecular signals that mediate the differentiation of these subsets will be beneficial in understanding the development and function of these cells under homeostatic conditions and during disease.
2. The monocyte development pathway
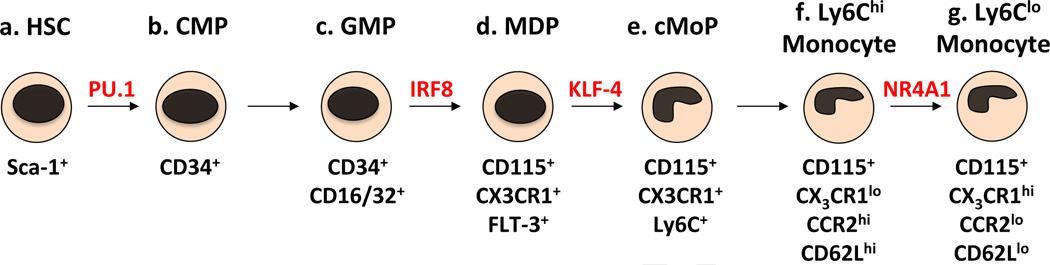
The development of monocytes from hematopoietic stem cells (HSC) in the bone marrow and spleen occurs via several myeloid-committed progenitors (Figure 1.). The earliest precursors derived from HSC are the common myeloid precursors (CMP), which express the surface glycoprotein CD34, but not stem cell antigen-1 (SCA-1) [38–41]. These cells in turn give rise to a multipotent pool of precursors known as the granulocyte/macrophage precursors (GMP), which are identified by expression of the Fcγ receptors CD16 and CD32 [38]. Included within this subset are the more recently defined macrophage/DC precursors (MDP), which express the cytokine receptors CD115 (CSF-1R/M-CSFR), CX3CR1 and Flt-3 (CD135) [42–47]. These cells have no significant granulocytic potential, but give rise to monocytes and tissue macrophage subsets. MDP also differentiate into some conventional DC (cDC) and plasmacytoid DC (pDC) subsets directly, without a monocytic intermediate, whereas monocytes themselves give rise to some DC subsets including inflammatory DC and mucosal DC [1, 36, 47]. Recently, a DC-restricted progenitor, known as the common DC precursor (CDP), was described [47–49]. The CDP shares the phenotypic markers of MDP and has been shown to give rise to cDC and pDC, but not monocytes. There is some debate in regards to whether CDP represent a distinct GMP precursor population, a population overlapping with MDP, or a DC-restricted population derived from MDP [47–49].
Figure 1.
The monocyte development pathway. Monocytes are derived from HSC in the bone marrow and spleen via several myeloid-restricted progenitors. Sca-1+ HSC (a) give rise to CD34+ CMP (b). These cells in turn give rise to CD34+CD16/32+ GMP (c). A population of these precursors also expresses CD115, CX3CR1 and Flt-3, known as the MDP (d). A recently identified monocyte precursor downstream of the MDP loses expression of Flt-3 and upregulates Ly6C, known as the cMoP (e). This cell gives rise to Ly6Chi monocytes (f), which have also been shown to differentiate into Ly6Clo monocytes (g).
Recently, a monocyte and macrophage-restricted clonogenic progenitor downstream of the MDP was identified (Figure 1.). The common monocyte progenitor (cMoP) loses expression of Flt-3, gains expression of Ly6C and maintains expression of CD115 and CX3CR1 [50]. These cells have been shown to give rise to monocytes and macrophages in vitro and in vivo, but not DC. Further supporting the hypothesis that Ly6Clo monocytes are derived from Ly6Chi monocytes, cMoP were shown to first give rise to Ly6Chi monocytes in vivo 1–2 days post transfer, which then differentiated into Ly6Clo monocytes 3–4 days post transfer. Similar but delayed developmental kinetics were also shown following the adoptive transfer of MDP in this study [50]. Furthermore, genomic analysis comparing the expression of transcription factors by cMOP, Ly6Chi and Ly6Clo monocytes indicated a closer relationship between cMoP and Ly6Chi monocytes compared to cMoP and Ly6Clo monocytes. This data further supports the hypothesis that Ly6Chi monocytes give rise to Ly6Clo monocytes [50].
3. Molecular control of the monocyte development pathway
Transcription Factors
Monopoeisis is controlled by the expression and suppression of specific transcription factors at defined timepoints in the developmental pathway. Targeted gene knock-out and knock-in technologies have provided significant insight into the temporal roles of specific transcription factors, as well as cytokines, growth factors and other molecules which play a critical role in this developmental process [49, 51]. The transcription factor PU.1 plays a prominent role in monocyte differentiation at various stages of commitment. As homozygous PU.1-deficient mice are embryonic lethal or die within a few days after birth, fetal liver stem cells or conditional knockouts have been used to investigate the role of this transcription factor in myeloid cell development [52]. High expression of PU.1 antagonizes key regulators of other developmental pathways, such as GATA-1, GATA-2 and C/EBPα [53–55] and activates myeloid-specific factors such as Interferon regulatory factor-8 (IRF8), Kruppel-like Factor 4 (KLF-4) and Erg1 [56–59]. Conversely, low expression of PU.1 favors the development of granulocyte lineage cells. In the absence of PU.1, HSC fail to give rise to myeloid progenitors, resulting in the loss of monocytes and most DC subsets. PU.1 deficiency also favors the development of the granulocyte lineage, resulting in excess proliferation of neutrophils [52, 60–62].
IRF8, also known as interferon consensus sequence-binding protein (ICSBP), is an IFN-γ-regulated transcription factor that is also critical for monocyte differentiation. Interestingly, although PU.1-and KLF4-deficient mice are homozygous lethal, IRF8-deficient mice are viable. HSC express low levels of IRF8, which is upregulated as these cells differentiate into CMP and GMP [63]. In conjunction with PU.1, IRF8 promotes the differentiation of monocytes from GMP and inhibits the differentiation of granulocytes [64]. IRF8-deficient animals show accumulation of GMP, significant expansion of neutrophils and defective monocyte development [65–68]. Differentiation into macrophage and DC subsets is also defective [67, 69–72]. Recent studies indicate that IRF8 is critical for both the differentiation of Ly6Chi and Ly6Clo monocytes. Mice lacking IRF8 have significantly reduced numbers of Ly6Chi and Ly6Clo monocytes in the blood, bone marrow and spleen [71, 73]. We have also shown that Ly6Chi monocytes fail to express high levels of CCR2 in IRF8-deficient mice and as a consequence these cells show defective migration to sites of inflammation (Terry et al, unpublished data).
The PU.1 and IRF8-activated transcription factor KLF4 also plays an important role in monocyte development [73]. As homozygous KLF4-deficient mice die within a few days after birth, fetal liver stem cells have been used to investigate the role of this transcription factor in vitro and in vivo [74]. KLF4-deficient hematopoietic cells fail to differentiate into Ly6Chi monocytes and have very few Ly6Clo monocytes in the bone marrow, blood and spleen. These cells also showed increased apoptosis and failed to express key trafficking molecules such as CD62L [74]. Increased numbers of neutrophils are also produced by KLF4-deficient HSC [56]. Overexpression of KLF4 in HSC or CMP restricts these progenitors to the monocytic lineage [56]. Furthermore, reintroduction of KLF4 into PU.1 or IRF8-deficient progenitor cells was sufficient to rescue monocyte development [56, 73]. These data indicate that KLF4 is a major downstream target of IRF8 and PU.1 that is critical for development of the monocyte lineage.
Growth Factors
PU.1 is critical for the expression of the receptor tyrosine kinase CD115 (CSF-1R/M-CSFR) in early myeloid progenitors and the upregulation of this receptor during the later stages of differentiation [42–45]. Expression of this growth factor receptor is indispensable for monocyte development as it is a key regulator of survival, proliferation and differentiation [75]. Mice that lack functional expression of CD115 show deficiencies in most monocyte and macrophage populations, as well as some DC subsets. The two known ligands of CD115, M-CSF (CSF-1) and IL-34, are both important for development of monocytes from their hematopoietic progenitors, as M-CSF- and IL-34-deficient mice exhibit a milder phenotype than animals lacking CD115 expression [76–79].
Cytokine signaling
The proinflammatory cytokine Interferon-γ (IFN-γ) plays an import role in driving monocyte development under inflammatory conditions. During infection, the expression of PU.1 and IRF8 are upregulated in GMP derived from wild-type (WT) but not IFN-γ-deficient mice. Furthermore, direct stimulation of GMP with IFN-γ results in the upregulation of these transcription factors critical for monocyte development [80]. In vivo, WT mice showed significant expansion of monocytes at the expense of neutrophils in response to lymphocytic choriomeningitis virus (LCMV) infection, resulting in increased numbers of these cells in the blood and bone marrow. In comparison, IFN-γ-deficient mice showed significantly increased numbers of neutrophils, highlighting the role of IFN-γ in directing monocyte development during inflammation [80].
Type I interferon (IFN-I) signaling also plays an important role in monocyte development under inflammatory conditions. Mice lacking the IFN-I receptor show increased migration of neutrophils and decreased recruitment of monocytes to the influenza-infected lung, and as a result show increased mortality [81]. Interestingly, IFN-I receptor-deficient HSC fail to give rise to Ly6Chi monocytes. A significant Ly6Cint monocyte population was instead observed in the bone marrow and infected lung, which shows altered morphology and chemokine production compared to WT Ly6Chi monocytes [81]. Culture of WT and IFN-I receptor-deficient bone marrow cells with bronchiolar lavage fluid or influenza directly, revealed that WT bone marrow cells were able to differentiate into Ly6Chi monocytes, IFN-I receptor-deficient cells were not. The role of the IFN-I receptor was confirmed by culturing infected WT bone marrow cells in the presence or absence of IFN-I receptor-blocking antibodies, in which IFN-I receptor blockade inhibited differentiation into Ly6Chi monocytes. These findings confirm the role of type I IFN signaling in promoting inflammatory monocyte development during infection [81].
4. Transcriptional control of monocyte subset differentiation
The molecular controls that promote the differentiation of specific monocyte subsets remain for the most part unknown. However, a significant breakthrough has been made in recent years in identifying the role of transcription factor nuclear receptor subfamily 4, group A, member 1 (NR4A1) in Ly6Clo monocyte development. Hanna and colleagues (2011) showed that NR4A1 plays a critical role in mediating the differentiation and survival of Ly6Clo but not Ly6Chi monocytes [82]. MDP in NR4A1-deficent mice fail to give rise to a significant Ly6Clo population and these animals do not have mature Ly6Clo monocytes circulating in the blood, spleen, or patrolling the endothelium. The authors show that the few Ly6Clo cells that remain in the bone marrow are unable to differentiate into mature monocytes and rapidly undergo apoptosis [82]. This is a particularly significant finding as there is a distinct lack of knowledge in regards to the molecular controls determining the differentiation of Ly6Chi or Ly6Clo monocytes. A recent study has also confirmed that NR4A1 is not expressed by MDP, cMoP or Ly6Chi monocytes, but is exclusively expressed by Ly6Clo monocytes, further supporting the hypothesis that this transcription factor plays a critical role in directing the differentiation of Ly6Clo monocytes from Ly6Chi precursors [50].
5. Monocytes and microglia arise via distinct haematopoetic pathways but are dependent on the same molecular controls
Microglia, the resident macrophages of the brain, play an important role in protecting the central nervous system (CNS) from infection. However, these cells also contribute to the pathogenesis of some inflammatory diseases [83, 84]. Recent studies have confirmed that microglia arise from myeloid progenitors in the yolk sac during embryogenesis, through a developmental pathway distinct to that of bone marrow monocytes [85–88]. However, a major question that is still under contention is whether circulating monocytes can replenish microglial populations in the adult. While it is clear that in some models of infection and injury, Ly6Chi inflammatory monocytes migrate to the CNS and exhibit a microglial phenotype, there is little evidence to suggest that this process occurs under homeostatic conditions in unperturbed animals [27, 73, 87, 89, 90].
Despite their distinct haematopoetic origins, the development of both monocytes and microglia is critically dependent on the transcription factor PU.1. Mouse embryos lacking PU.1 expression are completely devoid of microglia in the CNS [88]. The PU.1-regulated chemokine receptor CD115 is also critical for normal microgliogenesis. Mice deficient in CD115 or its ligand IL-34 lack microglia in the CNS [79, 86, 88]. It is also clear that the transcription factor IRF8 plays an important role in microglial development, however there is some dispute in regards to its precise function. While a recent study has shown that numbers of microglia are significantly reduced in the brain of IRF8−/− embryonic mice compared to the WT, in the adult CNS, normal or even slightly increased microglial cell numbers were reported in several other studies [73, 91, 92]. Furthermore, IRF8 deficiency was also shown to have a role in the downstream morphology and function of microglia [73, 91, 92]. The discrepancies between these studies may be primarily due to changes in microglial populations that occur with the aging process, or the result of different experimental techniques used to enumerate microglial populations. Nevertheless, these data indicate that further investigation of the role of IRF8 in the embryonic vs. adult brain is warranted.
6. Summary
The molecular control of monocyte differentiation, specifically transcription factors, growth factors and cytokine/chemokine signaling critical for the development of these cells, have been well described in recent years [93–95]. However, the factors that promote the differentiation of specific monocyte subsets remain for the most part unknown. Recent studies have identified some key molecular signals that are critical for driving subset differentiation, of which the transcription factor NR4A1 represents a significant breakthrough. NR4A1 is not only the first transcription factor to be identified that is primarily critical for the development of Ly6Clo monocytes, but furthermore supports the current model of monocyte development in which Ly6Clo precursors arise via a Ly6Chi monocyte precursor. Future studies which identify other transcription factors, growth factors and cytokine/chemokine signaling pathways critical for Ly6Chi and/or Ly6Clo monocyte differentiation will be undoubtedly be key in broadening our understanding of myeloid cell development and the role that these cells play in infections, cancer and autoimmune diseases.
Supplementary Material
Highlights.
Monocyte development is a tightly regulated molecular process
Transcription factors PU.1, IRF8 and KLF4 are critical for monocyte development
NR4A1 is critical for Ly6Clo monocyte development from Ly6Chi progenitors
Abbreviations
- cDC
conventional dendritic cells
- CDP
common dendritic cell precursors
- cMoP
common monocyte progenitor
- CMP
common myeloid precursors
- CNS
central nervous system
- DC
dendritic cells
- GMP
granulocyte-macrophage precursors
- HSC
hematopoietic stem cells
- IRF8
interferon regulatory factor-8
- KLF
Kruppel-like factor
- MDP
macrophage/dendritic cell precursors
- pDC
plasmacytoid dendritic cells
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 2.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 3.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. Journal of neuroinflammation. 2012;9:270. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 7.Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. eLife. 2013;2 doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst JD, Charo IF. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. Journal of immunology. 2004;172:7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 9.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. Journal of immunology. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. Journal of immunology. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 11.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory Monocytes Mediate Early and Organ-Specific Innate Defense During Systemic Candidiasis. The Journal of infectious diseases. 2013 doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infection and immunity. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunay IR, Sibley LD. Monocytes mediate mucosal immunity to Toxoplasma gondii. Current opinion in immunology. 2010;22:461–466. doi: 10.1016/j.coi.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojtasiak M, Pickett D, Tate M, Londrigan S, Bedoui S, Brooks A, Reading P. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol. 2010;91:2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen BP, Kuziel WA, Lane TE. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. Journal of immunology. 2001;167:4585–4592. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- 17.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 18.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-Directed RNAi Targeting CCR2 Improves Infarct Healing in Atherosclerosis-Prone Mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Presumey J, Courties G, Louis-Plence P, Escriou V, Scherman D, Pers YM, Yssel H, Pene J, Kyburz D, Gay S, Jorgensen C, Apparailly F. Nicotinamide phosphoribosyltransferase/visfatin expression by inflammatory monocytes mediates arthritis pathogenesis. Annals of the rheumatic diseases. 2013;72:1717–1724. doi: 10.1136/annrheumdis-2012-202403. [DOI] [PubMed] [Google Scholar]
- 21.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. Journal of neuroinflammation. 2010;7:92. doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christophi GP, Massa PT. Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1. Viral Immunol. 2009;22:371–387. doi: 10.1089/vim.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophi GP, Hudson CA, Panos M, Gruber RC, Massa PT. Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease. J Virol. 2009;83:522–539. doi: 10.1128/JVI.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett JL, Elhofy A, Charo I, Miller SD, Dal Canto MC, Karpus WJ. CCR2 regulates development of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Viral Immunol. 2007;20:19–33. doi: 10.1089/vim.2006.0068. [DOI] [PubMed] [Google Scholar]
- 27.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Deffrasnes C, Ashhurst TM, Radford J, Hofer M, Thomas S, Campbell IL, King NJ. Targeted blockade in lethal West Nile virus encephalitis indicates a crucial role for very late antigen (VLA)-4-dependent recruitment of nitric oxide-producing macrophages. Journal of neuroinflammation. 2012;9:246. doi: 10.1186/1742-2094-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 31.Nahrendorf M, Swirski F, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J, Libby P, Weissleder R, Pittet M. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski F, Weissleder R, Pittet M. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. Journal of immunology. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 34.Davison AM, King NJ. Accelerated dendritic cell differentiation from migrating Ly6C(lo) bone marrow monocytes in early dermal West Nile virus infection. Journal of immunology. 2011;186:2382–2396. doi: 10.4049/jimmunol.1002682. [DOI] [PubMed] [Google Scholar]
- 35.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Varol C, Landsman L, Fogg D, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tacke F, Randolph G. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 39.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 40.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 41.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 42.Reddy MA, Yang BS, Yue X, Barnett CJ, Ross IL, Sweet MJ, Hume DA, Ostrowski MC. Opposing actions of c-ets/PU.1 and c-myb protooncogene products in regulating the macrophage-specific promoters of the human and mouse colony-stimulating factor-1 receptor (c-fms) genes. J Exp Med. 1994;180:2309–2319. doi: 10.1084/jem.180.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krysinska H, Hoogenkamp M, Ingram R, Wilson N, Tagoh H, Laslo P, Singh H, Bonifer C. A two-step, PU.1-dependent mechanism for developmentally regulated chromatin remodeling and transcription of the c-fms gene. Mol Cell Biol. 2007;27:878–887. doi: 10.1128/MCB.01915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeKoter RP, Walsh JC, Singh H. PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 1998;17:4456–4468. doi: 10.1093/emboj/17.15.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tagoh H, Himes R, Clarke D, Leenen PJ, Riggs AD, Hume D, Bonifer C. Transcription factor complex formation and chromatin fine structure alterations at the murine c-fms (CSF-1 receptor) locus during maturation of myeloid precursor cells. Genes Dev. 2002;16:1721–1737. doi: 10.1101/gad.222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 47.Auffray C, Fogg D, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume D, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nature immunology. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 49.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nature immunology. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 51.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nature reviews. Immunology. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 52.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 53.Rekhtman N, Radparvar F, Evans T, Skoultchi AI. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev. 1999;13:1398–1411. doi: 10.1101/gad.13.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Radomska HS, Auron PE, Tenen DG, Sun Z. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 58.Krishnaraju K, Nguyen HQ, Liebermann DA, Hoffman B. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Mol Cell Biol. 1995;15:5499–5507. doi: 10.1128/mcb.15.10.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schonheit J, Kuhl C, Gebhardt ML, Klett FF, Riemke P, Scheller M, Huang G, Naumann R, Leutz A, Stocking C, Priller J, Andrade-Navarro MA, Rosenbauer F. PU.1 level-directed chromatin structure remodeling at the Irf8 gene drives dendritic cell commitment. Cell reports. 2013;3:1617–1628. doi: 10.1016/j.celrep.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201:1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Lee CH, Qi C, Tailor P, Feng J, Abbasi S, Atsumi T, Morse HC., 3rd IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood. 2008;112:4028–4038. doi: 10.1182/blood-2008-01-129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 65.Koenigsmann J, Rudolph C, Sander S, Kershaw O, Gruber AD, Bullinger L, Schlegelberger B, Carstanjen D. Nf1 haploinsufficiency and Icsbp deficiency synergize in the development of leukemias. Blood. 2009;113:4690–4701. doi: 10.1182/blood-2008-05-158485. [DOI] [PubMed] [Google Scholar]
- 66.Scheller M, Foerster J, Heyworth CM, Waring JF, Lohler J, Gilmore GL, Shadduck RK, Dexter TM, Horak I. Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood. 1999;94:3764–3771. [PubMed] [Google Scholar]
- 67.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC, 3rd, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 68.Tsujimura H, Nagamura-Inoue T, Tamura T, Ozato K. IFN consensus sequence binding protein/IFN regulatory factor-8 guides bone marrow progenitor cells toward the macrophage lineage. Journal of immunology. 2002;169:1261–1269. doi: 10.4049/jimmunol.169.3.1261. [DOI] [PubMed] [Google Scholar]
- 69.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. Journal of immunology. 2003;170:1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 71.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, Beutler B, Theofilopoulos AN. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2940–2945. doi: 10.1073/pnas.1222798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121:1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. Journal of immunology. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Droin N, Solary E. Editorial: CSF1R, CSF-1, and IL-34, a "menage a trios" conserved across vertebrates. Journal of leukocyte biology. 2010;87:745–747. doi: 10.1189/jlb.1209780. [DOI] [PubMed] [Google Scholar]
- 76.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 77.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 78.Wiktor-Jedrzejczak W, Gordon S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev. 1996;76:927–947. doi: 10.1152/physrev.1996.76.4.927. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nature immunology. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–1554. doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- 81.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C(−) monocytes. Nature immunology. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Town T, Nikolic V, Tan J. The microglial "activation" continuum: from innate to adaptive responses. Journal of neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. Journal of leukocyte biology. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 85.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 86.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59:177–187. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- 88.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature neuroscience. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 89.Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 90.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch U-K, Mack M, Heikenwalder M, Brück W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nature neuroscience. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 91.Horiuchi M, Wakayama K, Itoh A, Kawai K, Pleasure D, Ozato K, Itoh T. Interferon regulatory factor 8/interferon consensus sequence binding protein is a critical transcription factor for the physiological phenotype of microglia. Journal of neuroinflammation. 2012;9:227. doi: 10.1186/1742-2094-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minten C, Terry R, Deffrasnes C, King NJ, Campbell IL. IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS. PloS one. 2012;7:e49851. doi: 10.1371/journal.pone.0049851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mak KS, Funnell AP, Pearson RC, Crossley M. PU.1 and Haematopoietic Cell Fate: Dosage Matters. Int J Cell Biol. 2011;2011:808524. doi: 10.1155/2011/808524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 95.Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377–3390. doi: 10.1038/sj.onc.1205324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.