Abstract
Recent findings suggest the existence of a frontoparietal control system consisting of ‘flexible hubs’ that regulate distributed systems (e.g., visual, limbic, motor) according to current task goals. A growing number of studies are reporting alterations of this control system across a striking range of mental diseases. We suggest this may reflect a critical role for the control system in promoting and maintaining mental health. Specifically, we propose that this system implements feedback control to regulate symptoms as they arise (e.g., excessive anxiety reduced via regulation of amygdala), such that an intact control system is protective against a variety of mental illnesses. Consistent with this possibility, recent results indicate that several major mental illnesses involve altered brain-wide connectivity of the control system, likely altering its ability to regulate symptoms. These results suggest that this ‘immune system of the mind’ may be an especially important target for future basic and clinical research.
Keywords: prefrontal cortex, frontoparietal system, psychiatric disease, cognitive control, executive functions
A fundamental mystery of clinical neuroscience is why some patients have much better outcomes despite similar neural or experiential disturbances (e.g., a virtually identical trauma). One major factor may be individual differences in cognitive control abilities – a set of cognitive processes that coordinate goal pursuit (Miller and Cohen 2001; Schneider and Chein 2003). We suggest that those individuals who are better able to pursue their goal of recovery are likely to experience better outcomes. Extensive evidence suggests the existence of a cortical ‘control’ system that implements cognitive control (Cole and Schneider 2007; Duncan 2010). This suggests the control system’s capacity may be an important factor in the maintenance and improvement of mental health. Here we review the properties of the control system and introduce an integrative theory that postulates this system may play a central role in mental health.
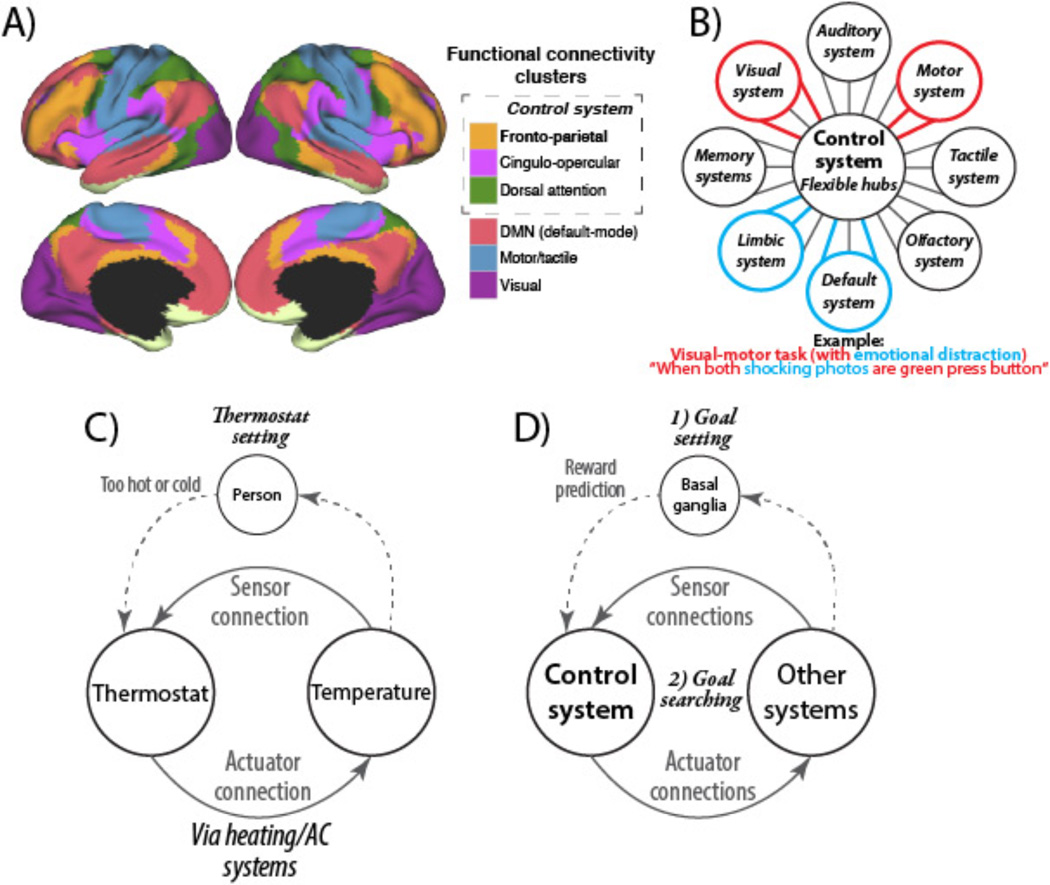
Recent studies have begun to identify the basic properties of the control system. Such decomposition of the control system is critical for avoiding the ‘homunculus’ (i.e., ‘little man’) fallacy, wherein goal-directedness of a person is explained away by an undifferentiated internal entity that is nearly equivalent to another person. First, the control systems consists of distinct cortical regions that are highly interconnected (Cole and Schneider 2007; Vincent and others 2008; Thomas Yeo and others 2011) – consistent with the notion that this set of regions forms an internally differentiated yet unified system (Cole and Schneider 2007). Second, the control system is thought to consist of several sub-systems (Figure 1A) – defined as having especially high within-sub-system connectivity – that have related but not identical functions (Power and others 2011). For instance, the ‘frontoparietal’ portion is thought to be especially involved in highly adaptive control processes, the ‘cingulo-opercular’ portion is thought to be especially involved in time-extended control processes, and the ‘dorsal attention’ portion is thought to coordinate attention to external stimuli (Corbetta and Shulman 2002; Dosenbach and others 2007). Third, the control system – especially the frontoparietal sub-system – has especially extensive brain-wide connectivity (Cole and others 2010; Power and others 2011) (i.e., its regions are hubs), suggesting it can communicate with a variety of systems throughout the brain. The ability for these hubs to communicate with many systems may be what allows them to be domain-general (Chein and Schneider 2005; Fedorenko and others 2013) (e.g., involved regardless of sensory or motor modality). Fourth, the pattern of functional connectivity between the control system and a variety of other systems is updated depending on current task demands (Sakai 2008; Cole, Reynolds, and others 2013). This suggests the control system contains flexible hubs – brain regions that implement control via task-dependent biases of their connections throughout the brain (Cole, Laurent, and others 2013; Cole, Reynolds, and others 2013) (Figure 1B).
Figure 1. The control system and flexible hubs.
A) Clustering applied to resting-state functional connectivity MRI identified large-scale neural systems (Thomas Yeo and others 2011). Components of the control system are co-active in a wide variety of task domains (i.e., the system is domain-general) (Duncan 2010; Fedorenko and others 2013), are sensitive to a variety of cognitive control demands (Niendam and others 2012), and this system is split here into three sub-systems. The frontoparietal sub-system is labeled in bold due to its centrality to adaptive task control (Dosenbach and others 2007; Cole, Reynolds, and others 2013), though all sub-systems are highly inter-connected and functionally related (Cole and Schneider 2007; Vincent and others 2008). B) Recent evidence suggests the core control system has highly global functional connectivity (Cole and others 2010; Power and others 2011) that updates systematically across tasks (Cole, Reynolds, and others 2013). Further, the control system inhibits the default-mode system when it is irrelevant to task performance (Shulman and others 1997; A.C. Chen and others 2013), and control system inhibition of the default-mode system is impaired in mental illness (Anticevic, Cole, and others 2012). C) A schematic of how temperature is regulated by a thermostat (a controller in a feedback control loop). D) Several biologically realistic computational models suggest a two-step process of cognitive control (Braver and Cohen 1999; O'Reilly and Frank 2006). The first is reward prediction by the basal ganglia selecting a goal representation via the control system. The second step involves goal maintenance with searching for sub-goals to accomplish the goal (matching the current state to the maintained goal state representation). This is similar to feedback control in other contexts (e.g., controlling temperature with a thermostat).
It is currently unknown exactly how the control system’s flexible hubs utilize changes in functional connectivity to implement control of distal systems. One parsimonious possibility is that flexible hubs implement control via feedback loops with a variety of brain systems, similar to how feedback control is implemented in many engineered and other biological systems (Figure 1C & 1D). For instance, setting the temperature with a thermostat may be akin to (though simpler than) setting a goal in the control system, with the feedback between a temperature sensor (sensor connectivity) and the heating and cooling actuators (actuator connectivity) being used to search for – and eventually achieve – the goal. Similar mechanisms have also been postulated for the motor system’s control of muscles (Diedrichsen and others 2010). Unlike a thermostat’s univariate linear search (pushing the temperature up or down to match a single variable), however, the control system must perform multivariate non-linear search (trying sequences of functional connectivity patterns) until a goal is achieved. Recent results suggest that previously learned functional connectivity patterns can be recalled and re-used in new contexts and in novel combinations to facilitate this search process (Cole, Reynolds, and others 2013).
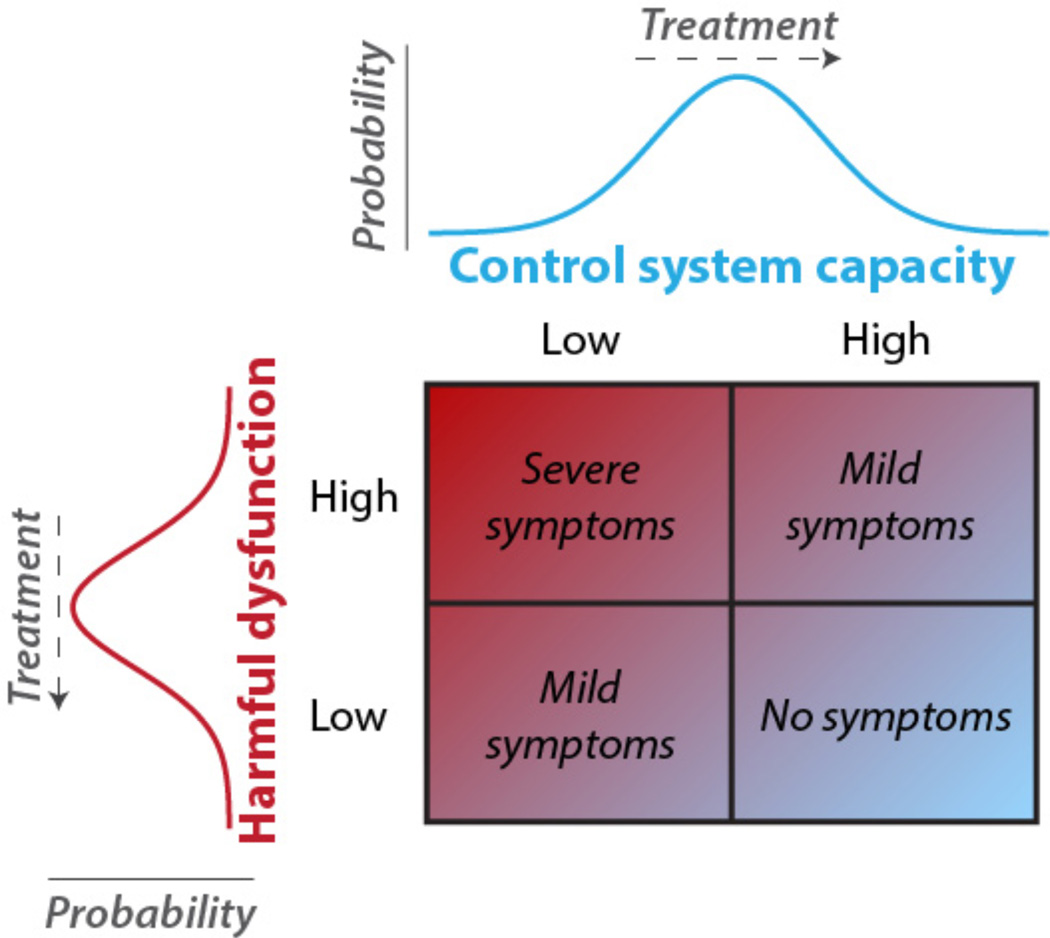
We suggest these mechanisms may provide the control system with important computational properties for promoting and maintaining homeostatic balance across distributed neural systems, increasing optimal behavioral outcomes (i.e. mental health). In other words, the control system’s ability to regulate other systems in a goal-directed manner (Cole, Reynolds, and others 2013) may lead it to reduce goal-disrupting processes as they arise and are manifest overtly as symptoms of mental disease (Figure 2). This suggestion is based on two basic assumptions: First, that complex mental illnesses result primarily from dysregulated brain systems and, second, that these diseases are harmful/undesirable to the individual. This definition of mental disease is consistent with some of the most well established and influential theoretical arguments in the field (Henriques 2002; Wakefield 2007), including the ‘harmful dysfunction’ hypothesis. If the first component of this definition is true (system dysfunction) then it is likely that the control system would be able to correct such dysfunction in many cases via the feedback control mechanism described above. However, what signals could the control system use to detect dysfunction of a distant system? This is where the second component of the definition becomes important (that it is harmful/undesirable to the individual) – the control system is specialized for implementing goal pursuit, such that any undesirable dysfunction would, by definition, interfere with goal pursuit and become the target of regulation by the control system.
Figure 2. Control system capacity interacts with dysfunctions to regulate symptoms.
Like the body’s immune system is protective against symptoms of bodily disease, the control system is postulated to be protective against symptoms of mental disease – likely via the flexible hub mechanisms described above. Theoretical probability distributions are shown to indicate any given individual’s likelihood of control system capacity (top) and the severity of a harmful dysfunction in any given mental process (left). The likely levels of experienced symptoms are indicated at different combinations of control system capacity and dysfunctionality. Treatment for each mental disease is postulated to be specific to that disease when harmful dysfunctions are reduced (left), but may be common across diseases when control system capacity is enhanced (top) due to the domain generality of the control system (Chein and Schneider 2005; Duncan 2010).
In summary, ongoing research into the basic properties of the control system is revealing neural mechanisms by which a variety of mental diseases may be regulated. In particular, it appears that the control system consists of flexible hubs that use feedback control (via dynamic global connectivity) to regulate processes in a variety of brain systems. Many goal-incompatible (i.e., undesirable/harmful) processes contribute to the symptoms of mental diseases, such that the control system likely utilizes these flexible hub mechanisms to regulate symptoms and so promote mental health. Below we articulate the implications of this flexible hub theory, recent evidence relevant to the theory, along with testable predictions stemming from this framework. Note that in the pages that follow all references to the control system and flexible hubs are meant to map directly onto the mechanisms described in this section and illustrated in Figure 1.
The importance of an intact control system across neuropsychiatric conditions
The above theoretical argument suggests that an effective control system would be protective against a variety of mental diseases. Consistent with this, a wide variety of mental diseases involve altered control system functionality. Perhaps the most well-established evidence for control system alterations have been observed in schizophrenia (Van Snellenberg and others 2006; Cole and others 2011; Anticevic, Repovs, and others 2012; Barch and Ceaser 2012). Similar evidence has been identified in bipolar disorder (Anticevic, Cole, and others 2013), obsessive-compulsive disorder (Anticevic, Hu, and others 2013), anxiety disorders (Sylvester and others 2012), eating disorders (Friederich and others 2013), autism (Poljac and Bekkering 2012), attention deficit hyperactivity disorder (Makris and others 2008), post-traumatic stress disorder (Blair and others 2012), and major depression (Zhang and others 2011; Lee and others 2012), through a combination of neuroimaging approaches. Each of these neuropsychiatric conditions involve complex and somewhat distinct mechanisms underlying their pathophysiology, which cause dysfunctions across a distinct set of neural systems (e.g., amygdala in anxiety disorders, orbitofrontal cortex and basal ganglia in obsessive-compulsive disorder) and in turn separable behavioral abnormalities. However, recent efforts – such as the Research Domain Criteria (RDoC) project (Cuthbert and Insel 2013) – have begun to characterize common features across mental diseases in addition to these ‘categorical’ distinctions (Adam 2013) (see Box). Consistent with the RDoC framework, it appears that control system dysfunction may be a common factor cutting across a broad range of mental diseases.
Box: Mental illness as a spectrum of dysregulated systems.
Every day millions of individuals are affected by mental disease worldwide. Progress has been made in identifying and characterizing mental disorders, but inadequacies in current classification schemes are becoming more apparent as prognosis and biomarkers fail to honor diagnostic boundaries. To address this problem, a major effort at the National Institute of Mental Health (NIMH) is underway to redefine diagnostic criteria in terms of brain circuits – the Research Domain Criteria (RDoC) (Insel and others 2010). This paradigm shift – mirrored by several other proposals (Adam 2013) – suggests a re-conceptualization of the field’s rigid categorical diagnostic systems towards a more dimensional framework that is capable of combining levels of inquiry. The NIMH’s RDoC initiative suggests that complex mental disorders are fundamentally brain-based disorders arising due to dysregulation across neural systems, possibly due to shared mechanisms (Cuthbert and Insel 2013). This initiative is designed such that identifying the biological mechanisms indicative of a given complex behavioral disturbance will better link individuals with the proper treatment and improve outcomes, like other areas of medicine. Consistent with the RDoC initiative's emphasis on cross-disease traits, deficits in cognitive control – the ability to influence thoughts and emotions in a goal-directed manner – have been identified in a variety of mental illnesses (Poljac and Bekkering 2012; Sylvester and others 2012). More directly relevant to RDoC, these cognitive control deficits have been linked to alterations in distributed neural system connectivity, providing a possible target for biomarker refinement (Figure 3C). Here we propose a neurocognitive hypothesis that crosses traditional diagnostic boundaries to help explain how a wide variety of symptoms may be exacerbated by disruptions in the functioning of a cognitive control brain system. Conversely, we hypothesize how effective functioning of the control system may be protective against mental illness generally. This aspect of the theory is congruent with the RDoC framework because it proposes a shared neural dysfunction across psychiatric conditions with distinct behavioral profiles. We propose that future work should focus on delineating the underlying computational mechanisms that give rise to control system disruptions that are shared versus distinct across neuropsychiatric conditions. For instance, recent studies implicate glutamatergic dysfunction (possibly at the NMDA receptor) as relevant for coordination of large-scale neural systems involved in higher cognition (Anticevic, Gancsos, and others 2012). Future studies are needed to link our system-level predictions with specific cortical circuit mechanisms operating in particular psychiatric conditions.
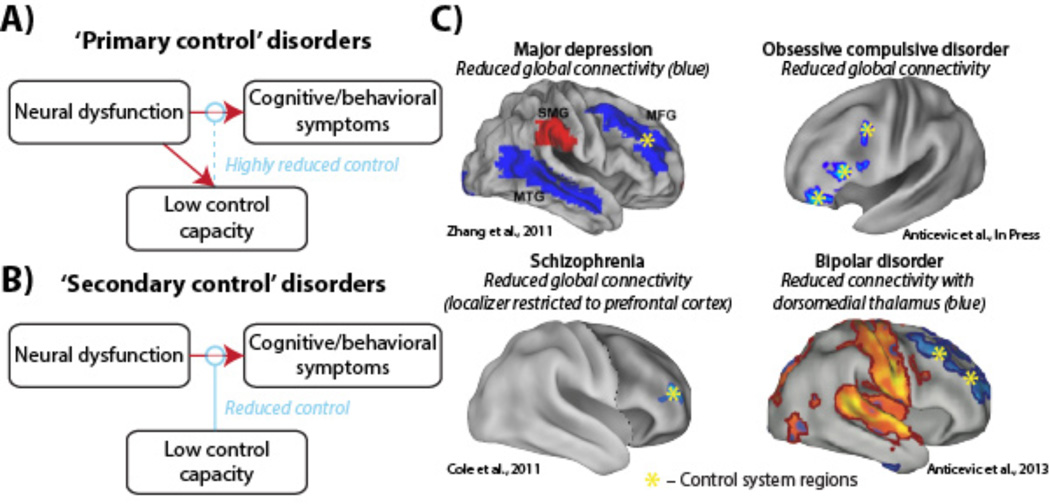
Rather than considering all mental diseases as equal, however, we suggest there may be two broad ways control system capacity interacts with the harmful dysfunctions underlying mental disorders. We hypothesize that in certain neuropsychiatric conditions control system disruption is itself one of the primary dysfunctions of the disease. Such ‘primary control’ disorders (such as schizophrenia and bipolar disorder) likely involve larger reductions of control system capacity on average than other disorders (Barch and Ceaser 2012) (Figure 3A). This account predicts that – like immunodeficiency diseases of the body – primary control disorders are especially difficult to treat (Goff and others 2011) due to the diseases’ disruption of natural health-promoting regulatory processes. Further, primary control disorders may result in dysfunctional control signals that actually exacerbate symptoms, rather than simply failing to reduce them (Cole and others 2011). Consistent with this primary control pathology, typical cognitive therapeutic approaches are usually ineffective for schizophrenia without concurrent pharmacological intervention (Lynch and others 2009).
Figure 3. The control system is disrupted across mental diseases.
A) ‘Primary control’ disorders are defined as involving neural dysfunction of the control system itself, such that control system capacity is more likely to be compromised in all individuals with such disorders. This predicts that primary control disorders are more difficult to treat due to the diseases’ disruption of natural health-promoting processes. B) In contrast, ‘secondary control’ disorders are characterized as those that are exacerbated by low control system capacity (potentially by chance), but whose root neural dysfunction does not directly affect control system capacity. It may be possible, however, that if/when symptoms arise the control system capacity is in turn compromised (as a secondary downstream effect). C) Resting-state functional connectivity (inter-region temporal correlations during rest) disruptions have been found with a key control system region – lateral prefrontal cortex (LPFC) – across a variety of mental diseases. LPFC's global connectivity (temporal correlations across all other regions) was altered in major depression (Zhang and others 2011), obsessive-compulsive disorder (Anticevic, Hu, and others 2013), and schizophrenia (Cole and others 2011). These alterations, as well as altered connectivity with a subcortical hub (mediodorsal thalamus) in bipolar disorder (Anticevic, Cole, and others 2013), are consistent with the flexible hub theory.
In contrast, ‘secondary control’ disorders are defined as mental diseases influenced by the natural variation in control system capacity across individuals (i.e., the general population distribution), without a given disease’s underlying pathological processes causing direct reduction of control system capacity (Figure 3B). Some examples of such secondary control disorders may include major depression, anxiety disorder, and obsessive-compulsive disorder. Note, however, that there may be indirect disruption of the control system (e.g., major depressive episodes are associated with cognitive control deficits (Lee and others 2012)). In particular, it may be that the need to constantly regulate symptoms reduces the capacity of the control system for other cognitive demands (Anticevic, Cole, and others 2012). We develop this possibility further in a later section. Alternatively, it may be that individuals with especially effective control systems do not develop symptoms (despite harmful dysfunctions) due to effective symptom regulation (see Figure 2), such that studies of mental disorders (which depend on diagnosis based on overt symptoms) are biased to include predominantly those with lower-than-average control system capacity.
Consistent with a primary control disorder, there is strong evidence that the control system as a whole is affected in schizophrenia, though most evidence points to alterations of a key region in the control system – lateral prefrontal cortex (LPFC). Evidence for this comes from converging multi-disciplinary work, such as postmortem studies of patients showing altered neurotransmitter and local microcircuit anatomy (Lewis and others 2005) as well as large neuroimaging investigations using both task-based (Repovs and Barch 2012) and resting-state (Fornito and others 2011) approaches. Consistent with these neural alterations affecting behavior, functional MRI (fMRI) studies have shown abnormal control system activation during cognitive control tasks in schizophrenia (Barch 2005). Further, structural MRI studies have shown altered control system anatomy on a large scale as well (Zalesky and others 2011). Most relevant to the flexible hub theory, however, is the observation that LPFC’s global connectivity is altered in schizophrenia patients relative to healthy comparison subjects (Lynall and others 2010; Van Den Heuvel and others 2010; Cole and others 2011) (Figure 3C).
Again consistent with a primary control disorder, there is extensive evidence of control system disruption at multiple levels in bipolar disorder. For instance, postmortem studies have identified altered cellular composition within LPFC and dorsal anterior cingulate in bipolar patients, and have associated this with emotional dysregulation (M.J. Green and others 2007). Further, a recent study identified reduced functional connectivity between LPFC and a subcortical hub (mediodorsal thalamus) in bipolar patients (Anticevic, Cole, and others 2013) (Figure 3C). Finally, several control system regions were associated with an inability to modulate amygdala activity during emotional regulation among manic individuals (Foland and others 2008), consistent with reduced connectivity between LPFC and the amygdala in bipolar illness (Anticevic, Brumbaugh, and others 2012). Further, consistent with the primary vs. secondary control distinction, there are common aspects to the genetics (Van Snellenberg and de Candia 2009), neurobiology (Anticevic, Cole, and others 2013), and cognitive control impairments (M.F. Green 2006) of bipolar disorder and schizophrenia.
A recent study examining obsessive-compulsive disorder found support for LPFC global connectivity disruption (Figure 3C), in addition to disruption of connectivity in orbitofrontal cortex and basal ganglia (Anticevic, Hu, and others 2013). One possibility is that the core functional dysfunction causing this disorder involves orbitofrontal cortex and basal ganglia circuits, but that control system regions (such as LPFC) would have been able to correct that dysfunction had they been as well connected and well functioning as in healthy individuals. Alternatively, it could be that the difficulty of constantly regulating altered systems causes impairment (via cognitive loading) of the control system. We will explore these possibilities in a later section.
We have focused primarily on psychiatric disorders – how does the flexible hub theory relate to neurological conditions? A recent study demonstrated that control system integrity is important for recovering speech after aphasic brain lesions (Brownsett and others 2014). This is consistent with the control system using feedback control of remaining functionally intact language regions to facilitate recovery of speech in a goal-directed manner. This hypothesis makes the prediction that individuals with a robust control system may have improved outcomes from lesions of a variety of brain regions with a variety of possible functional deficits, regardless of specific modality (e.g., visual vs. language lesions). Further, the flexible hub theory suggests that lesions of control system regions may be particularly debilitating to daily life (relative to other association cortex lesions) (Shallice and Burgess 1991), given the control system’s domain-general role in goal-directed cognition.
Possible feedback control mechanisms relevant to mental health
Above we suggested that the control system uses feedback control, similar to many engineered systems (e.g., aircraft autopilot, car cruise control) and also similar to self-organizing and homeostatic feedback control in other biological systems (e.g., body temperature control, the body’s immune system, ant colony organization) (Brun and others 2009). The key mechanism here involves the control system maintaining a goal state representation using ‘sensor’ connections (brain connections that monitor goal-relevant signals) and ‘actuator’ connections (brain connections that in turn affect the monitored signals). Another vital mechanism relates to the control system’s ability to search for functional (actuator) connectivity patterns that effectively modulate distributed neural computations to be more in line with the behavioral goal. It is likely that effective actuator connectivity patterns can be identified in several different ways.
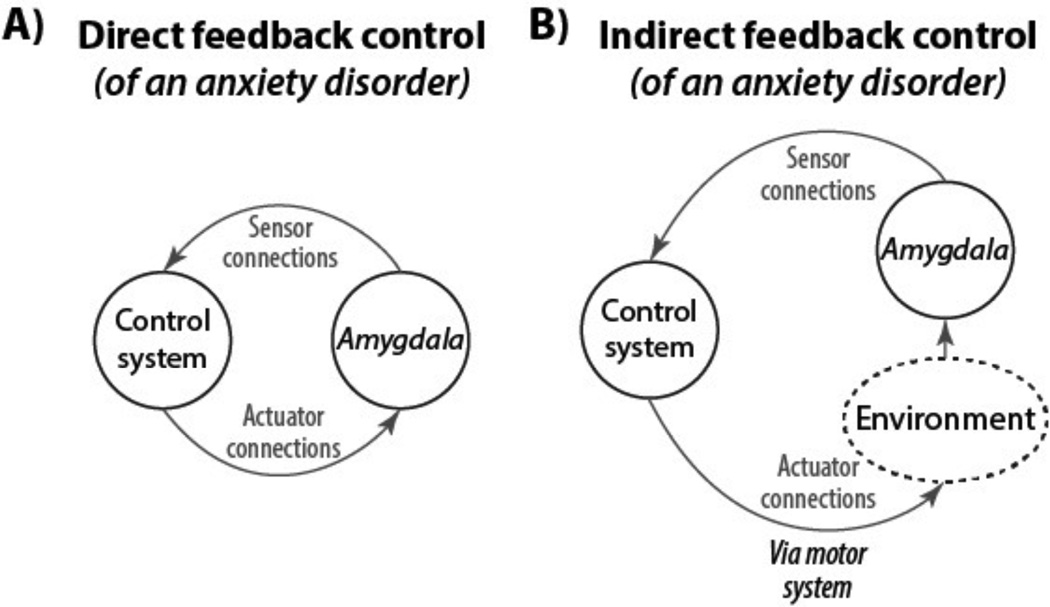
Perhaps the most straightforward means for actuator connectivity patterns to be identified during a search process is through direct feedback control (Figure 4A). This involves direct monitoring of the to-be-regulated system via brain connectivity with the control system. For example, direct feedback control might be used when you decide to move your right index finger to press a button: 1) The goal may be set in the control system via the basal ganglia predicting reward (e.g., because you are thirsty and the button delivers a bottle of water), 2) The goal-associated functional connectivity pattern is loaded from long-term memory via existing (automatic) associations and sent to motor cortex, 3) Information from the motor and proprioceptive/tactile systems do not match the goal pattern so the control system sends another signal in a search for the correct signal until the goal is achieved. Something similar may happen, for example, during the regulation of anxiety in anxiety disorders. For instance, in the case of social phobia: 1) The goal of enjoying a movie in a theater may be set in the control system via the basal ganglia predicting reward, 2) A series of actions get you to the theater and the movie begins, 3) Anxiety resulting from a hyperactive amygdala (Phan and others 2006) may get in the way of enjoying the movie (the goal), initiating a search by the control system to find a functional connectivity configuration that reduces hyperactivity in the amygdala (i.e., inhibition of the amygdala by the control system).
Figure 4. Direct and indirect feedback control.
An anxiety disorder (e.g., social phobia) is illustrated as an example of two ways that the control system could regulate symptoms of anxiety. A) Direct feedback control is illustrated by direct monitoring and inhibition of a hyperactive amygdala via brain connectivity. Note that direct feedback control can also involve brain connectivity over multiple synapses (e.g., control system to orbitofrontal cortex to amygdala). B) Indirect feedback control is illustrated by direct monitoring and indirect inhibition of a hyperactive amygdala via changes to the environment. These changes are implemented via the motor system. Examples include 1) deep breathing, which changes sympathetic vs. parasympathetic balance and indirectly influences amygdala, and 2) moving to a room that is comforting, thereby removing any environmental stimuli contributing to the hyper-active amygdala. A combination of direct and indirect feedback control strategies is likely the most effective, though implementing multiple control strategies would require a highly effective control system.
An alternative feedback control mechanism may also result in a reduction of amygdala hyperactivity. This mechanism – indirect feedback control (Figure 4B) – involves control of a process that 1) is accessible to the control system, and 2) is incompatible with the to-be-controlled process. In the social phobia example a control system strategy of regulating breathing (a process readily accessible by the control system) can result in reduced amygdala hyperactivity, perhaps due to strong connections in the limbic system between breathing and mental state. Indirect feedback control can also involve more explicit changes to the environment (e.g., leaving the theater to watch a movie at home) to achieve a goal. There are likely indirect feedback control processes that apply to other mental diseases as well.
Though two distinct feedback control mechanisms were described, they both involved the control system as central to responding to feedback in a goal-driven manner. This suggests there are likely numerous scenarios where an intact and robust control system would be highly useful for regulating the symptoms of mental disease.
Control system disruption as cause or consequence of mental disease
The flexible hub framework postulates that many mental diseases (especially ‘secondary control’ diseases) are not caused by control system disruption. Rather, consistent with an immune system analogy, the theory postulates that most cases of mental disease could be ameliorated or perhaps even prevented with an especially effective control system. This suggests that control system disruption may nonetheless play a causal and critical role in a variety of mental diseases, with implications for functional outcomes.
Critically, however, the reverse causal direction is likely also present: having a mental disease disrupts control system processes. We hypothesize this causal direction because the dedication of the control system to regulating symptoms likely reduces the system’s spare capacity in other domains, such as solving problems in daily life or on a test of cognitive/executive control abilities in a laboratory. Reduced ability to deal with the problems of daily life is of course itself a symptom. The predicted influence of reduced control system capacity on daily life is consistent with observations that cognitive control abilities are reduced in a variety of mental illnesses (Barch 2005; Bowie and others 2006; Lee and others 2012) and that such disruptions of cognitive control abilities profoundly affect daily life (Bowie and others 2006). Note that such indirect reductions in control system capacity due to attempts to implement any given feedback control strategy (e.g., suppress negative thoughts) likely reduces capacity for discovering more effective feedback control strategies (e.g., deep breathing, cognitive reappraisal). The consequence of this may – in some cases – be a kind of ‘stuck’ state, in which mental health is unlikely to improve without external intervention.
More generally, the reduction of control system capacity by symptom regulation demands is consistent with well-known capacity limits of cognitive control abilities (Schmeichel and others 2008) and the control system that implements those abilities (Mitchell and Cusack 2008; Buschman and others 2011). There are a variety of factors that influence cognitive control capacity. Some factors that reduce cognitive control capacity include excessive stress (Sato and others 2012), cognitive load (e.g., pursuit of other goals, rumination) (Brinker 2013), poverty (possibly via cognitive loading) (Mani and others 2013), and negative affect (Kleider and others 2009). Some factors that increase cognitive control capacity include motivation (reward prediction) (H.S. Locke and Braver 2008), optimal levels of stress (Vijayraghavan and others 2007), focused goals (E.A. Locke and Latham 2006), effective strategies for the goal at hand (Cole, Laurent, and others 2013), and adaptive habits (to reduce cognitive load) (Chein and Schneider 2012). The multitude of factors that influence control system capacity suggests that cognitive control capacity can vary substantially both within and across individuals as these factors change and interact. Further, this suggests there are multiple means for increasing control system capacity and therefore facilitate functional outcomes during treatment (see Figure 2).
Psychotherapy as augmenting the control system
Psychotherapy is often effective in improving mental health (Seligman 1995; Knekt and others 2013). We suggest this may be primarily due to improvements in goal pursuit – psychotherapy may enhance the control system by augmenting its feedback control mechanism (Figure 5A). It may be that if the control system is not successful in regulating aberrant representations in other systems a therapist can initiate a feedback control loop to facilitate the existing feedback control implemented by the control system. This may involve asking questions to gain information about the symptoms and previous attempts to regulate them, followed by instructions for strategies to try. This is the same goal-directed search process described above (Figure 1D), but augmented by a therapist’s knowledge and experience. Thus, a social phobia patient with especially high amygdala hyper-activity (Phan and others 2006) and/or a somewhat ineffective control system can be instructed on how to detect anxiety (improved selection of ‘sensor connections’) and to initiate a deep breathing strategy whenever anxiety is present (improved selection of ‘actuator connections’). This can speed up the search for a strategy (i.e., a set of sensor and actuator connections) to regulate symptoms.
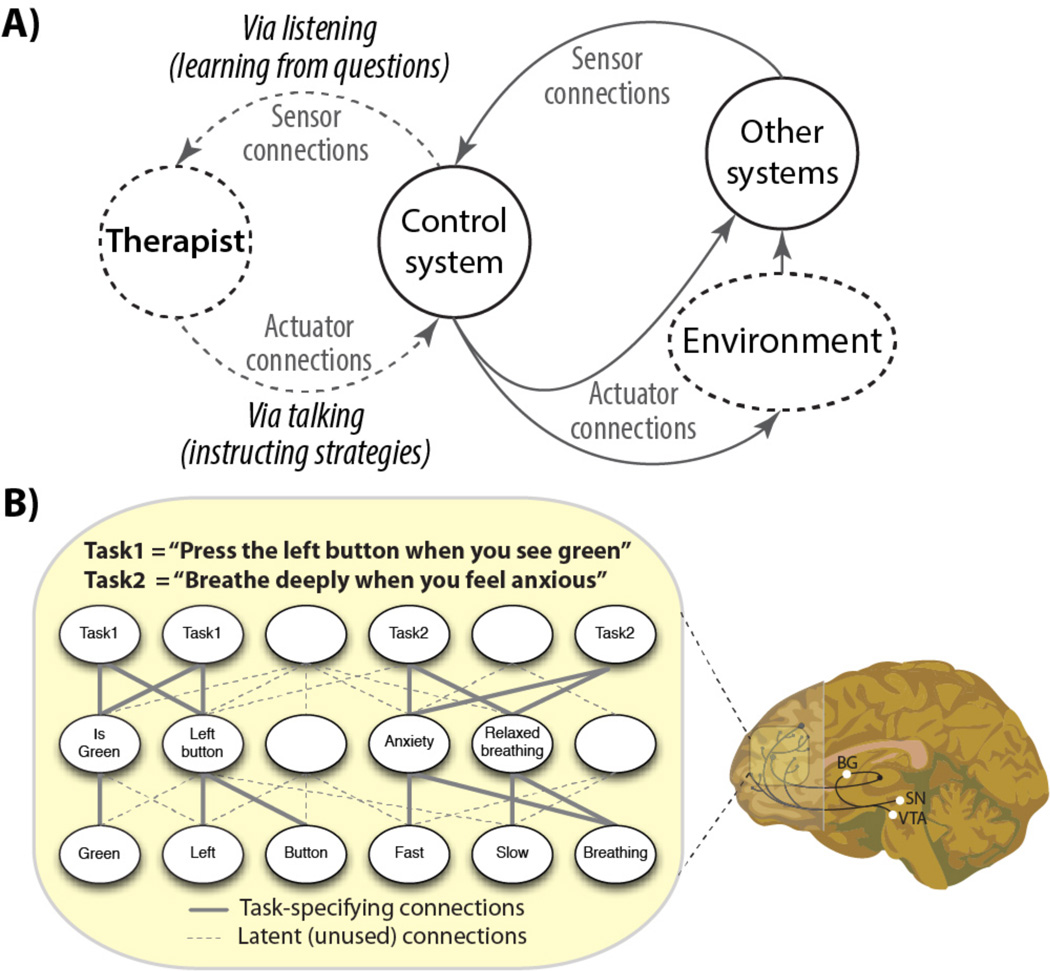
Figure 5. Rapid instructed task learning, flexible hubs, and psychotherapy.
A) Psychotherapy is illustrated as augmenting the control system’s search for ways to regulate symptoms. The therapist utilizes listening (with questions) to detect the nature of symptoms (and previous attempts to regulate them), which informs instructed strategies that are ultimately implemented by the control system (via rapid instructed task learning (Cole, Laurent, and others 2013)). A specific example of this therapeutic process may be exposure-response prevention with cognitive-behavioral therapy elements designed for obsessive-compulsive disorder (Abramowitz and Arch 2014). B) Potential neural population mechanisms within LPFC for rapid instructed task learning (Cole, Laurent, and others 2013). Verbal instructions activate primitive components (bottom) and their relations are built via local connectivity to build task procedures on the fly. Substantia nigra (SN), ventral tegmental area (VTA), and other basal ganglia (BG) (O'Reilly and Frank 2006) help update LPFC with new instructions (see Figure 1D). Task 1 is a typical cognitive laboratory task, involving an arbitrary stimulus-response association. Task 2 is an adaptive real-world strategy used in psychotherapy to reduce anxiety. Both tasks are illustrated using the same mechanisms. Note that the mechanisms are simplified for illustration (e.g., representations overlap across neural populations in LPFC) (Cole, Laurent, and others 2013; Rigotti and others 2013).
This account is highly compatible with recently developed theories of the neural mechanisms of psychotherapy (DeRubeis and others 2008; Clark and Beck 2010). These theories are based on recent observations that successful psychotherapeutic treatments of depression and anxiety disorders are associated with increased activity in control system regions and decreased activity in amygdala and other emotion processing regions (Goldin and others 2009; Browning and others 2010). These models of therapeutic mechanisms and the flexible hub framework are also consistent with observations that the control system is involved in goal-directed thought (including emotion) suppression (Wager and others 2008). Building upon these theories, however, the flexible hub framework includes more specific neural mechanisms while also providing a more general account across mental disorders and in the maintenance of mental health in healthy individuals. These differences may prove important for gaining further insight into the mechanisms of psychotherapy, as well as insight into the interaction of psychotherapy with psychopharmacology and personal efforts by patients to reduce their symptoms.
In addition to the global connectivity and feedback control mechanisms described above, the flexible hub framework postulates neural mechanisms at the level of local neural populations (Figure 5B). These mechanisms describe how novel procedures can be rapidly learned from instructions – rapid instructed task learning (Cole, Reynolds, and others 2013). This skill may be especially important for psychotherapy, given psychotherapy’s reliance on verbal instructions to achieve cognitive and behavioral change. The key idea is that we learn a set of cognitive components early in life (e.g., monitoring breathing, detecting anxiety, controlling breathing) that can be rapidly recombined via connectivity in the control system into novel procedures through instructions. This ability likely allows for the cognitive flexibility necessary for psychotherapy to be effective.
Note that there are some important nuances to this framework. First, it is possible for the control system itself to have faulty strategies that actually exacerbate symptoms rather than improve them. For instance, previously small reductions in negative affect from eating may result in the maladaptive control strategy of binge eating to regulate negative affect (Spitzer and others 1993), causing more negative affect in the long run. Similarly, control system attempts to regulate anxiety can result in panic attacks if attending to the symptom elevates anxiety (in a vicious cycle) (Pauli and others 1991). Such cases are likely especially difficult to correct given that the control system’s feedback control ‘program’ is itself compromised (e.g., like an autoimmune disorder). It is likely that hierarchical control can come into play in such cases – the higher-order control of strategies based on more generalized thinking, which may reflect the organization of the control system (Badre 2008). Individuals in these situations may benefit the most from ‘control system augmentation’ by a therapist, since this can help reprogram the control system to use more effective symptom regulation strategies.
As a second nuance, the framework might apply more for some forms of psychotherapy than others. In particular, it may apply best to cognitive-behavioral therapy (CBT) (Butler and others 2006), which combines cognitive therapy (focusing on regulating thoughts and emotions) and behavioral therapy (focused on regulating actions/behavior). This is because the two aspects of CBT correspond to utilizing both available types of actuator connections (Figure 4 & 5B) – direct control-to-symptom connections (via cognitive/affective regulation) and indirect control-to-symptom connections (via behavior/environment regulation). This framework may also apply to even highly distinct forms of psychotherapy, however. For instance, a variety of psychotherapies (e.g., acceptance and commitment therapy) use mindfulness meditation, which involves focusing attention and accepting events as they occur (Khoury and others 2013). Focusing attention is a core function of the control system, while emotional reappraisal to allow for acceptance of events is also thought to be mediated by the control system (Ochsner and others 2002). Consistent with this, the control system is active during mindfulness meditation (Chiesa and others 2013). Importantly, however, the control system becomes less active after extensive practice (Chiesa and others 2013), suggesting the ultimate goal of meditation is for the control system to train other systems to ‘automatically’ facilitate a mindful state (i.e., attention to the moment without judgment) – a state incompatible with a variety of harmful dysfunctions. We review evidence that the control system can train other systems to achieve automaticity in the next section.
Predictions of the flexible hub framework for future research
The flexible hub theory makes a variety of predictions cutting across mental disorders. We outline what we see as some of the more important predictions for future research to test, which may lead to discoveries that can improve understanding of disease mechanisms and lead to improved therapeutic outcomes.
Perhaps the clearest prediction is that control system disruptions will be associated with an even larger set of mental disorders than already covered here. Further, details regarding the nature of those control system disruptions are predicted by the flexible hub feedback control mechanism described above. For instance, the framework predicts that control system connectivity with a variety of systems – and especially those systems whose dysfunction is primarily causing the mental disorder – will be lower in most individuals with virtually any form of mental disorder. While this is a broad prediction, we suggest it will have a very specific manifestation depending on the nature of the behavioral symptoms involved.
We have emphasized control system disruption in terms of large-scale connectivity, yet the system could be disrupted in other ways as well. For instance, the control system could be disrupted in terms of local connectivity (J. Chen and others 2012), genetics (Esslinger and others 2009), local protein dysregulation (Drummond and others 2013), or electrophysiological activity dynamics (Wölwer and others 2012). It will be important for future research to investigate these different possible means of disruption (and possible treatments) in patients with a variety of mental diseases as well as in animal models.
A particularly important prediction for early intervention is that control system disruption early in life may be predictive of mental disorders later. This is in line with recent findings that reduced childhood cognitive control abilities are associated with worse outcomes in autism and attention deficit hyperactivity disorder (Johnson 2012), and are predictive of the development of borderline personality disorder (Ayduk and others 2008). Also consistent is the observation that cognitive control abilities early in life predict later mental illness more generally (Mischel and others 2011; Moffitt and others 2011). The key idea behind this prediction is that those with a strong control system throughout life are better able to regulate the onset of symptoms (i.e., aberrant brain activity). This protective aspect of the control system is also in line with the analogy that the control system is akin to an ‘immune system of the mind’.
A corollary of the previous prediction is that many healthy individuals likely have sub-clinical symptoms of mental disorders that remain sub-clinical only because the control system successfully regulates them (see lower right corner of Figure 2). This prediction, if true, has profound consequences for our understanding of mental illness and the (potentially minor) distinctions between those diagnosed with a mental disorder versus not. This prediction is also associated with even more specific hypotheses: that some healthy individuals likely have reduced cognitive control abilities due to the need to constantly regulate symptoms, and that many individuals likely experience symptoms of mental disorders at some point in life – especially while the control system is developing. Consistent with this expectation, it was recently found that most mentally healthy individuals have at least one symptom of a mental disorder prior to adulthood (Copeland and others 2011).
If the control system regulates symptoms as suggested here, it may seem as though such regulation would need to go on indefinitely once symptoms arise in a mental disorder. Importantly, however, there is evidence that the control system is able to ‘train’ posterior regions to ‘automatically’ implement simple goal-compatible processes over time (Miller and Cohen 2001; Chein and Schneider 2005; Chein and Schneider 2012). This suggests that an effective control system may be able to utilize feedback control to reduce neural dysfunctions over the long term via training to achieve automaticity. For example, the control system may reduce an anxious individual’s amygdala hyperactivity so often that long-term depression among amygdala synapses reduces the neural dysfunction. As another example, a control strategy to breath deeply whenever stressful situations arise (to reduce amygdala hyperactivity dynamically) may become ‘automatic’ and independent of the control system over time, as functional connectivity between stress-associated sensation representations and deep breathing representations get strengthened.
Finally, the flexible hub framework makes three predictions related to external manipulation of control systems. First, the theory predicts that increasing control system activity – possibly via cognitive training (Mackey and others 2013), transcranial stimulation, or pharmacology – would improve symptoms in a variety of mental diseases. Second, improving control system integrity (e.g., improving within-system or global connectivity) – again via cognitive training (Mackey and others 2013), transcranial stimulation, or pharmacology – would reduce symptoms in a variety of mental diseases. Third, neurofeedback (Weiskopf 2011) may be an especially effective approach to enhance control system feedback control to reduce symptoms in a variety of diseases. It will be important to test these possibilities experimentally as they make clear predictions regarding potential ways to enhance treatment of a broad range of mental diseases.
We have outlined an extension of the flexible hub theory (Cole, Reynolds, and others 2013) to account for recent findings in the neuroscience of a variety of mental diseases. This novel framework is compatible with a broad sampling of recent findings indicating a central role of the control system in mental disease. Nevertheless, more evidence is necessary to verify the framework by testing its predictions. For instance, more before-and-after treatment studies would be useful in establishing a causal role of control system integrity in mental disease and treatment outcomes. Similarly, longitudinal studies across diagnoses will be vital to arbitrate the causal role of the control system in different conditions. Further, direct manipulations of the control system, such as via transcranial stimulation (Fox and others 2012), pharmacology (Anticevic, Gancsos, and others 2012), or neurofeedback (Weiskopf 2011) could provide not only strong evidence for a causal role of the control system in mental disease but also potential novel treatments. Additionally, while the current account is positioned at the level of neural systems, linking the proposed framework across levels of analyses will be important for a complete understanding of the proposed mechanisms. This can be done, for instance, by incorporating detailed pharmacological probes (Krystal and others 2003) and biophysically-based computational modeling studies (Wang 2010) of specific cognitive processes into the experimental repertoire when testing this theory. Generally, discovering more about the basic mechanisms of the control system will provide critical tests and extensions of the framework, which can in turn facilitate clinical studies seeking to understand the exact computational dynamics by which the control system may facilitate regulation of symptoms in each of a variety of mental diseases.
Finally, the flexible hub theory suggests a potentially efficient means to achieving effective new treatments by focusing basic and clinical research on the control system, given the potential to apply our understanding of this system to improve treatment outcomes across a variety of mental diseases simultaneously.
Acknowledgements
We thank Diane Rosenbaum and Todd Braver for helpful feedback and suggestions during preparation of this manuscript.
Grant support:
This work was supported by the US National Institutes of Health under awards K99/R00 MH096801 (Cole) and DP5OD01210901 (Anticevic).
References
- Abramowitz JS, Arch JJ. Strategies for improving long-term outcomes in cognitive behavioral therapy for obsessive-compulsive disorder: insights from learning theory. Cognitive and Behavioral Practice. 2014;21:20–31. [Google Scholar]
- Adam D. Mental health: On the spectrum. Nature. 2013;496:416–418. doi: 10.1038/496416a. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. Global Prefrontal and Fronto-Amygdala Dysconnectivity in Bipolar I Disorder with Psychosis History. BPS. 2012:1–9. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The Role of Default Network Deactivation in Cognition and Disease. Trends Cogn Sci (Regul Ed) 2012:1–9. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global Resting-State Functional Magnetic Resonance Imaging Analysis Identifies Frontal Cortex, Striatal, and Cerebellar Dysconnectivity in Obsessive-Compulsive Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Krystal JH, Barch DM. A broken filter: Prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res. 2012:1–7. doi: 10.1016/j.schres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk Ö, Zayas V, Downey G, Cole AB, Shoda Y, Mischel W. Rejection sensitivity and executive control: Joint predictors of borderline personality features. Journal of Research in Personality. 2008;42:151–168. doi: 10.1016/j.jrp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro–caudal organization of the frontal lobes. Trends Cogn Sci (Regul Ed) 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci (Regul Ed) 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual review of clinical psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2012;43:85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163:418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Braver T, Cohen J. Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Brinker JK. Rumination, Mood and Cognitive Performance. PSYCH. 2013;04:224–231. [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. BPS. 2010;67:919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsett SLE, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJS. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2014;137:242–254. doi: 10.1093/brain/awt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun Y, Serugendo GDM, Gacek C, Giese H, Kienle H, Litoiu M, et al. Engineering self-adaptive systems through feedback loops. Springer: 2009. [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proceedings of the National Academy of Sciences. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler ACA, Chapman JEJ, Forman EME, Beck ATA. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clinical Psychology Review. 2006;26:15–15. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Chein J, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res Cogn Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. The Brain's Learning and Control Architecture. Current Directions in Psychological Science. 2012;21:78–84. [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, et al. Causal interactions between frontoparietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xu Y, Zhang K, Liu Z, Xu C, Shen Y, et al. Comparative study of regional homogeneity in schizophrenia and major depressive disorder. Am. J. Med. Genet. 2012;162:36–43. doi: 10.1002/ajmg.b.32116. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A, Jakobsen JC. Clinical Psychology Review. Clinical Psychology Review. 2013;33:82–96. doi: 10.1016/j.cpr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy ofanxiety and depression: Convergencewith neurobiological findings. Trends Cogn Sci (Regul Ed) 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable Global Dysconnectivity and Individual Differences in Schizophrenia. BPS. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Laurent P, Stocco A. Rapid instructed task learning: A new window into the human brain's unique capacity for flexible cognitive control. Cogn Affect Behav Neurosci. 2013;13:1–22. doi: 10.3758/s13415-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. NeuroImage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Costello EJ, Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2011;50:252–261. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. CONTROL OF GOAL-DIRECTED AND STIMULUSDRIVEN ATTENTION IN THE BRAIN. Nat Rev Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci (Regul Ed) 2010;14:31–39. doi: 10.1016/j.tics.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Miezin F, Cohen A, Wenger K, Dosenbach R, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophr Res. 2013 doi: 10.1016/j.schres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci (Regul Ed) 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605–605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research: Neuroimaging. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. BPS. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) NeuroImage. 2012:1–12. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich H-C, Wu M, Simon JJ, Herzog W. Neurocircuit function in eating disorders. Int. J. Eat. Disord. 2013;46:425–432. doi: 10.1002/eat.22099. [DOI] [PubMed] [Google Scholar]
- Goff DC, Hill M, Freudenreich O. Treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2011;72:e13. doi: 10.4088/JCP.9096tx6cc. [DOI] [PubMed] [Google Scholar]
- Goldin P, Manber-Ball T, Werner K, Heimberg R, Gross J. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. Journal of Affective Disorders. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Henriques GR. The Harmful Dysfunction Analysis and the Differentiation between Mental Disorder and Disease. The Scientific Review of Mental Health Practice: Objective Investigations of Controversial and Unorthodox Claims in Clinical Psychology, Psychiatry, and Social Work. 2002 [Google Scholar]
- Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Executive function and developmentaldisorders: the flip side of the coin. Trends Cogn Sci (Regul Ed) 2012;16:454–457. doi: 10.1016/j.tics.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Clinical Psychology Review. Clinical Psychology Review. 2013;33:763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kleider HM, Parrott DJ, King TZ. Shooting behaviour: How working memory and negative emotionality influence police officer shoot decisions. Appl. Cognit. Psychol. 2009;24:707–717. [Google Scholar]
- Knekt P, Lindfors O, Sares-Jäske L, Virtala E, Härkänen T. Randomized trial on the effectiveness of long-and short-term psychotherapy on psychiatric symptoms and working ability during a 5-year follow-up. Nordic Journal of Psychiatry. 2013;67:59–68. doi: 10.3109/08039488.2012.680910. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge MA. Journal of Affective Disorders. Journal of Affective Disorders. 2012;140:113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Locke EA, Latham GP. New directions in goal-setting theory. Current Directions in Psychological Science. 2006;15:265–268. [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Lynall M-E, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D, Laws KR, Mckenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta-analytical review of well-controlled trials. Psychol Med. 2009;40:9. doi: 10.1017/S003329170900590X. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Miller Singley AT, Bunge SA. Intensive Reasoning Training Alters Patterns of Brain Connectivity at Rest. Journal of Neuroscience. 2013;33:4796–4803. doi: 10.1523/JNEUROSCI.4141-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and Executive Systems Abnormalities in Adults with Childhood ADHD: A DTMRI Study of Connections. Cerebral Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty Impedes Cognitive Function. Science. 2013;341:976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, et al. “Willpower” over the life span: decomposing self-regulation. Social Cognitive and Affective Neuroscience. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, Cusack R. Flexible, capacity-limited activity of posterior parietal cortex in perceptual as well as visual short-term memory tasks. Cereb Cortex. 2008;18:1788–1798. doi: 10.1093/cercor/bhm205. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R, Frank M. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pauli P, Marquardt C, Hartl L, Nutzinger DO, Hölzl R, Strian F. Anxiety induced by cardiac perceptions in patients with panic attacks: A field study. Behaviour research and therapy. 1991;29:137–145. doi: 10.1016/0005-7967(91)90042-2. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Poljac E, Bekkering H. A review of intentional and cognitive control in autism. Front. Psychology. 2012;3:436. doi: 10.3389/fpsyg.2012.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front. Hum. Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, et al. The importance of mixed selectivity incomplex cognitive tasks. Nature. 2013:1–6. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Sato H, Takenaka I, Kawahara JI. The effects of acute stress and perceptual load on distractor interference. The Quarterly Journal of Experimental Psychology. 2012;65:617–623. doi: 10.1080/17470218.2011.648944. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. J Pers Soc Psychol. 2008;95:1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein J. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cognitive Science. 2003;27:525–559. [Google Scholar]
- Seligman ME. The effectiveness of psychotherapy: the Consumer Reports study. American Psychologist. 1995;50:965. doi: 10.1037//0003-066x.50.12.965. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, et al. Binge eating disorder: its further validation in a multisite study. Int. J. Eat. Disord. 1993;13:137–153. [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012:1–9. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant Frontal and Temporal Complex Network Structure in Schizophrenia: A Graph Theoretical Analysis. Journal of Neuroscience. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JC. The concept of mental disorder: diagnostic implications of the harmful dysfunction analysis. World Psychiatry. 2007;6:149–156. [PMC free article] [PubMed] [Google Scholar]
- Wang X-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiological reviews. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N. Real-time fMRI and its application to neurofeedback. NeuroImage. 2011:1–11. doi: 10.1016/j.neuroimage.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Wölwer W, Stroth S, Brinkmeyer J, Gaebel W. Electrophysiological correlates of planning and monitoring in first episode schizophrenia. Psychiatry Res. 2012;203:83–88. doi: 10.1016/j.pscychresns.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, et al. Disrupted Axonal Fiber Connectivity in Schizophrenia. BPS. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, et al. Disrupted Brain Connectivity Networks in Drug-Naive, First-Episode Major Depressive Disorder. BPS. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]