Abstract
Single nucleotide polymorphisms (SNP) in the genes for pituitary adenylyl cyclase-activating peptide (PACAP) and the PAC1 receptor have been associated with stress-related psychiatric disorders. Although from recent work we have argued that stress-induced PACAP expression in the bed nucleus of the stria terminalis (BNST) may mediate stress-related psychopathology, it is unclear whether stress-induced increases in BNST PACAP expression require acute or repeated stressor exposure, and whether increased BNST PACAP expression is related to stress-induced increases in circulating glucocorticoids. In the current work, we have used quantitative real-time polymerase chain reaction (qPCR) to assess transcript expression in brain punches from rats after stressor exposure paradigms or corticosterone injection. BNST PACAP and PAC1 receptor transcript expression was increased only after 7 days of repeated stressor exposure; no changes in transcript levels were observed 2 or 24 hours after a single restraint session. Moreover, repeated corticosterone treatment for 7 days was not sufficient to reliably increase BNST PACAP transcript levels, suggesting that stress-induced elevations in corticosterone may not be the primary drivers of BNST PACAP expression. These results may help clarify the mechanisms and temporal processes that underlie BNST PACAP induction for intervention in stress-related anxiety disorders.
Keywords: pituitary adenylate cyclase-activating peptide (PACAP), bed nucleus of the stria terminalis (BNST), stress, corticosterone, glucocorticoids
Introduction
The bed nucleus of the stria terminalis (BNST) integrates and coordinates the many physiological and behavioral responses to stress. These include heightened anxiety-like behavior, and activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic autonomic nervous system (Herman et al. 1996; Walker et al. 2003; Herman et al. 2005; Waddell et al. 2006; Choi et al. 2007; Ulrich-Lai and Herman 2009; Radley and Sawchenko 2011). Importantly, BNST function is augmented following repeated stressor or glucocorticoid exposure (for review, see Hammack et al. 2010). For example, repeated stressor or glucocorticoid treatment paradigms in rodents result in increased BNST volume and dendritic arborization in parallel with increased anxiety-like behavior (Vyas et al. 2003; Pego et al. 2008; Dinzi et al. 2011). Moreover, increased BNST corticotropin-releasing hormone (CRH) mRNA and anxiety-like behavior have been observed following repeated systemic or intra-amygdala corticosterone administration (Makino et al. 1994; Shepard et al. 2006). Hence, stress-induced changes in BNST morphology and neurochemistry may mediate HPA axis dysregulation and pathological anxiety-like behaviors associated with many stress-related psychopathologies.
Pituitary adenylate cyclase-activating peptide (PACAP) and its cognate G protein coupled PAC1 receptor are highly expressed in areas that modulate responses to stress, including the BNST (Arimura et al. 1991; Nomura et al. 1996; Shioda et al. 1997; for review see: Vaudry et al. 2009). Consistent with a role in stress responding, PACAP or PAC1 receptor null mice have altered corticosterone circadian rhythm, diminished HPA activation after prolonged stressor exposure (Hashimoto et al. 2009; Stroth and Eiden, 2010; Hattori et al. 2012), reduced anxiety-like behavior and fear, (Hashimoto et al. 2001; Otto et al. 2001; Girard et al. 2006), and impaired contextual fear conditioning (Otto et al. 2001). Intra-BNST PACAP infusion increases both anxiety-like behavior (Hammack et al. 2009) and anorexia in rats (Kocho-Schellenberg et al. 2014).
In recent studies, expression of PACAP and PAC1 receptor transcript were found to be increased in the BNST following repeated stress, and these changes may underlie the behavioral consequences of stressor exposure (Hammack et al. 2009; Hammack et al. 2010). In humans, circulating PACAP levels and a PAC1 receptor gene (ADCYAP1R1) single nucleotide polymorphism (SNP) were associated with post-traumatic stress disorder (PTSD) symptoms and diagnosis in women. Additionally, PAC1 receptor gene methylation status correlated with PTSD independent of gender, and PAC1 receptor expression and the PAC1 gene SNP were associated with fear conditioning and discrimination, as well as anxiety measures (Ressler et al. 2011). Hence, these studies suggest that changes in PACAP signaling may mediate stress-induced behaviors related to fear and anxiety.
Despite the involvement of PACAP in modulating function in stress responsive brain regions, the mechanisms underlying stress-induced PACAP expression are still not understood. The experiments reported here examine BNST PACAP and PAC1 receptor mRNA levels following a single or repeated stress paradigm, or treatment with the glucocorticoid corticosterone.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (250 – 275 g) were obtained from Charles River Laboratories (Canada). After delivery, rats were allowed to habituate in their home cages for at least one week before experimentation. Rats were single-housed and maintained on a 12-h light/dark cycle (lights on at 07:00 h). Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Experiment 1
The first experiment was conducted to determine if transcript expression changes that occur following repeated variate stress could also be observed 2 h (acute) or 24 h (single) after one stressor exposure. Rats were pseudo-randomly assigned to one of 4 groups: (1) a control group that did not receive any stressor exposure; (2) a repeated variate stress paradigm group (described below); (3) an acute stress group (stress and assessment 2 h later); and (4) a single stress group (stress and assessment 24 h later). There were no weight differences among the groups prior to experimental start. Repeatedly stressed rats were exposed to a 7 day variate stress paradigm in which a single stressor was presented on each day. These included: oscillation stress, in which rats were placed inside a plastic chamber 28 × 17 × 13 cm (L × W × H) secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ) for low/medium speed oscillation for 30 min.; forced swim, in which rats were placed in a cylindrical container 29 × 37 cm (D × H) filled with room temperature water to a depth that prevented the tail from touching the bottom for 5 min of monitored swimming; footshock, in which rats inside a Plexiglas conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 ×35 cm (L × W × H) received two 1.0 mA 5 sec scrambled footshocks with 1 min inter-trial interval; restraint stress, in which rats were placed in a cylindrical restraining device 9 × 15 cm (D × H) for 60 min; and pedestal standing stress, in which rats were placed on an elevated platform 20 × 20 cm (L × W) 60 cm from the floor for 30 min. Two of the stressors were repeated during the paradigm, and all rats received restraint on the 7th day. Immediately prior to stressor exposure and until the day of euthanasia, food, water and rat body weights were recorded daily for all groups. All stressors were delivered between 9 and 11 AM; 2 h or 24 h (experiment dependent) following the termination of the last stressor, rats were anesthetized with isoflurane and euthanized by decapitation. Control and stressed rats were euthanized in a counterbalanced fashion. The brains were rapidly removed, blocked using a rodent brain matrix (Ted Pella, Inc. Redding, CA) and the dorsal and ventral aspects of the anterolateral BNST (dlBNST and vBNST, respectively) were micropunched (Stoelting; Wood Dale, IL) for RNA extraction and quantitative PCR (qPCR) assays as described below.
Experiment 2
Experiment 2 evaluated whether repeated corticosterone injection is sufficient to increase PACAP and other stress-related gene transcripts in patterns similar to those observed after repeated stressor exposure. Rats were matched into experimental groups as described above. Rats in the treatment group received subcutaneous 2.5 mg/kg corticosterone injections (Cort; Sigma-Aldrich, St. Louis, MO) for 6 consecutive days between 9 and 11 AM; control groups received vehicle injections of the same volume during the same period. Corticosterone was prepared in phosphate buffered saline (40%) with 95% ethanol (16%) and propylene glycol (44%). The 2.5 mg/kg corticosterone dose was shown previously to provide peak blood corticosterone levels comparable to those 30 min after acute stress exposure, which return to baseline levels by 4 h (Kalman and Spencer, 2002; Barnum et al. 2008). Injection amount was calibrated on a daily basis from recorded body weight. Twenty-four hours after the last injection, all animals were euthanized and micropunched brain tissue was collected as described above. As BNST PACAP transcript levels after repeated 2.5 mg/kg corticosterone injections appeared to be increased without attaining significance (see Results), a separate experiment was performed in which the treated group received 10 mg/kg corticosterone in the same paradigm.
Behavioral testing
We reported previously that the repeated variate stress paradigm produces anxiety-like behavior as measured by acoustic startle responding in a light-enhanced startle task (Hammack et al. 2009). To determine whether repeated variate stress and/or corticosterone treatments produce anxiety-like behavior, the rats from the different experimental groups were placed on an elevated-plus maze 24 h after the last treatment (stress or corticosterone). The maze consisted of two opposing open and two opposing closed arms (60 cm long and 9 cm wide) and was elevated 74 cm from the floor. Illumination with a red bulb was at 6 lux. Open arm time and total distance during 5 min trials were measured using EthoVision XT version 6.1.326 (Noldus Information Technology, The Netherlands).
Corticosterone assay
Blood samples were collected for corticosterone measurements. Thirty min after corticosterone injection on the 1st and last day of study, the animals were restrained in a clear Plexiglas tube for blood sampling via tail clip. The entire collection procedure was less than 3 min to avoid heightened corticosterone release associated with cage removal. The blood samples were immediately centrifuged and plasma was stored at −20 C until assay. The corticosterone enzyme-linked immunoassay (CORT EIA, Enzo Life Sciences, Farmingdale, NY) demonstrated a sensitivity of 26.99 pg/ml and an inter-assay variance of 6.7%.
Quantitative PCR (qPCR)
QPCR was performed exactly as described previously (Girard et al. 2002; Girard et al. 2006; Hammack et al. 2009) for PACAP, PAC1 receptor, mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) transcripts. Total RNA from micropunched brain tissue samples were extracted using STAT-60 RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX); all RNA from same brain regions were reverse transcribed simultaneously using random hexamer primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA) to obviate variability. Real-time quantitative PCR was performed as described using SYBR Green I detection (Girard et al. 2002; Girard et al. 2006; Hammack et al. 2009). Briefly, cDNA templates were diluted 5-fold to minimize the inhibitory effects of the reverse transcription reaction components and assayed on an ABI Prism 7500 Fast Real -Time PCR System (Applied Biosystems, Foster City, CA) using SYBR Green I JumpStartTM Taq ReadyMix (Sigma, St. Louis, MO) containing 3.5 mM MgCl2, 200 :M dATP, dGTP, dCTP and dTTP, 0.64 U Taq DNA polymerase and 300 nM of each primer in a final 25 µl reaction volume. Oligonucleotide primer sequences were: PACAP (S) 5'-CATGTGTAGCGGAGCAAGGTT-3' (AS) 5'-GTCTTGCAGCGGGTTTCC-3'; PAC1 (S) 5’-AACGACCTGATGGGACTAAAC-3’ (AS) 5’-CGGAAGCGGCACAAGATGACC-3’; MR (S) 5’-CCACACATGCGAGCAACG-3’ (AS) 5’-TTAGGGAAAGGAACGTCGTG-3’; GR (S) 5’-TCAGCAGCCACGGGACCAC-3’ (AS) 5’-CACTTGACGCCCACCTAACA-3’; 18S (S) 5’-AGTCGCCGTGCCTACCAT-3’ (AS) 5’-GCCTGCTGCCTTCCTTG-3’. Following amplification, the melting profiles for the amplicons were performed to verify unique product generation. For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence (Girard et al. 2002; Girard et al. 2006). The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle (CT) was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. The transcript levels in each sample were calculated from the CT by interpolation from the standard curve to yield the relative changes in expression. Data were normalized to 18S RNA levels. Where possible for each target sequence, all samples from the same brain region were amplified together in the same assay to minimize variability.
Statistics
Weight change for repeatedly treated rats was expressed as percent weight gain from experiment start. Changes in mRNA transcript levels were calculated as fold-change from the respective control treatment group. Statistical analysis was performed on SPSS version 20 (IBM Software, Armonk, NY) and graphical representations were created on GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA). One-way ANOVA and student’s t-test were completed as appropriate for analyses of group differences. Outliers greater than two standard deviations from the group mean were removed from further analyses. Data are expressed as mean ± SEM.
Results
BNST PACAP/PAC1 receptor transcript levels after stressor exposure
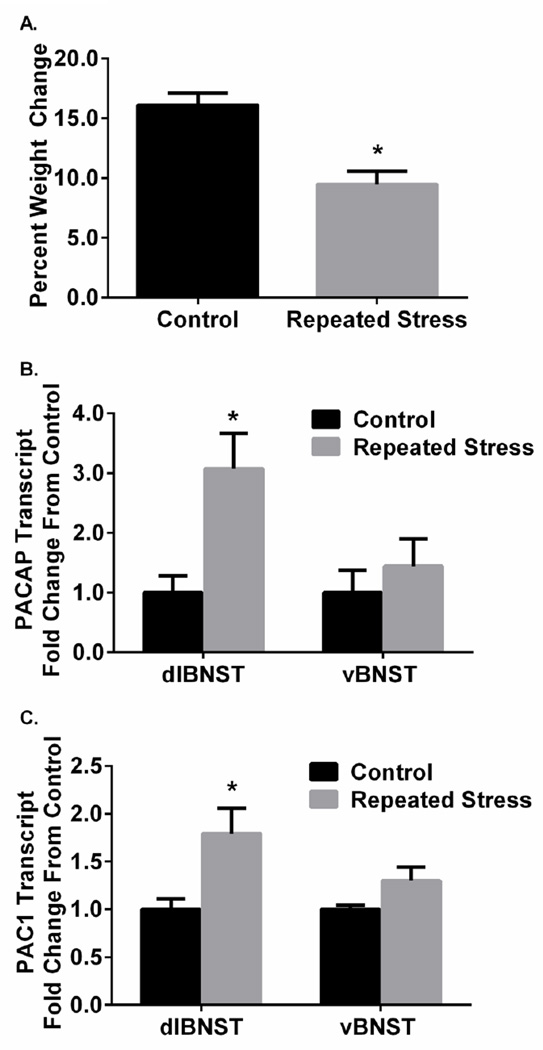
Our previous quantitative PCR approaches demonstrated that repeated variate stress increased PACAP and PAC1 receptor transcript expression specifically in the dlBNST among different CNS regions (Hammack et al. 2009). Consistent with previous work, rats in the repeated stress group gained less weight than non-stress control rats across the 7 day experimental period, (t(8) = 4.449, p < 0.05; Figure 1A). When brain punches from the same animals were examined for transcript levels, repeated variate stress increased dlBNST PACAP transcript expression (t(9) = −2.947, p < 0.05) and PAC1 receptor transcript levels (t(9) = −2.576, p < 0.05) compared to those in non-stressed control animals (Figure 1B, 1C). There were no apparent differences between repeated stress and control rats on vBNST PACAP transcripts (t(8) = −0.758, p = 0.47) or PAC1 receptor mRNA (t(8) = −1.977, p = 0.083; Figure 1B, 1C). These results were in good agreement with our previous observations (Hammack et al. 2009).
Figure 1.
Stressor exposure alters dlBNST PACAP transcript expression. (A), Repeated variate stress (7 day, see Materials and Methods) attenuated rat weight gain to demonstrate efficacy of the stress paradigm. Repeated stress increased both PACAP (B) and PAC1 (C) mRNA transcript expression within the dlBNST without affecting levels in the vBNST. dlBNST, dorsal anterolateral bed nucleus of the stria terminalis; vBNST, ventral anterolateral bed nucleus of the stria terminalis; PACAP, pituitary adenylate cyclase- activating peptide. Data represent mean SEM; *, significantly different from control at p < 0.05.
As previous studies have demonstrated that indices of BNST neuroplasticity may be sensitive to glucocorticoids, we examined GR and MR transcript level changes to determine if there was any relationship between PACAP/PAC1 transcript expression and GR or MR transcripts following stress. There were no differences between repeated-stress rats and control rats in dlBNST GR transcript expression (t(9) = −1.763, p = 0.112) or MR mRNA levels (t(9) = −1.084, p = 0.306); similarly, there was no different between groups for vBNST MR transcripts (t(8) = −0.836, p = 0.427) although there was a trend towards increased vBNST GR transcript expression after repeated stress (t(8) = −2.100, p = 0.069; data not shown). Because vBNST PACAP/PAC1 receptor transcript and dlBNST/vBNST MR/GR transcript levels were not altered following repeated stress, these transcripts were not assessed in subsequent experiments.
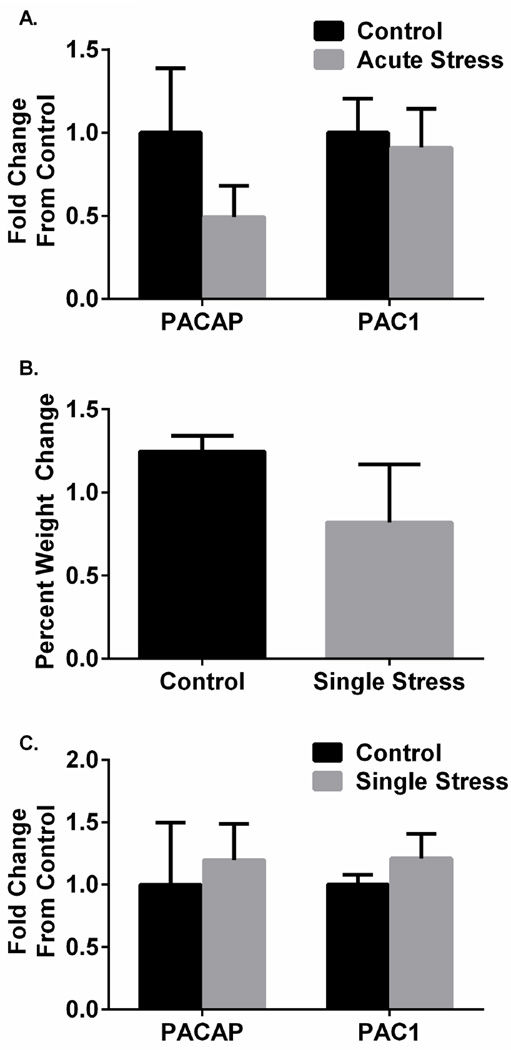
As other stress mediators including CRH or arginine vasopressin (AVP) can be regulated by acute stressor exposure (Ma et al. 1997) we assessed whether BNST PACAP and PAC1 receptor transcripts demonstrated similar rapid regulatory dynamics. However, dlBNST PACAP (t(10) = 1.17, p > 0.05) and PAC1 receptor (t(10) = 0.29, p = 0.778) transcript levels were not different 2 h following a single acute stressor exposure compared to control rats (acute stress, Figure 2A).
Figure 2.
dlBNST PACAP and PAC1 receptor expression are not altered by single or acute stress. No significant changes were observed in dlBNST PACAP or PAC1 mRNA transcript expression 2 h after restraint stress (A, acute stress). There was no difference in weight gain for rats that received a single stress during the 24 h following restraint compared to control rats (B). Neither dlBNST PACAP nor PAC1 mRNA transcript expression was altered in the dlBNST 24 h after restraint stress exposure (C). Data represent mean ± SEM.
We considered that the changes in PACAP or PAC1 receptor transcripts may not be immediately evident after acute stress but demonstrate a more protracted expression pattern. Hence, a different cohort of rats received a single (1 h) restraint stress for evaluations 24 h later. These rats did not demonstrate altered weight gain during the 24 h post-stress period compared to non-stress control rats (t(13) = 1.112, p > 0.05; Figure 2B). There was also no significant increase in dlBNST PACAP (t(15) = −0.351, p = 0.73) or PAC1 receptor transcript expression (t(15) = −0.949, p = 0.358) 24 h after restraint stress as compared to controls (Figure 2C). These BNST PACAP/PAC1 receptor results appeared consistent with previous data demonstrating that acute stress had no apparent effects on hypothalamic PACAP expression (Hannibal et al. 1995).
BNST transcript expression after exogenous corticosterone treatment
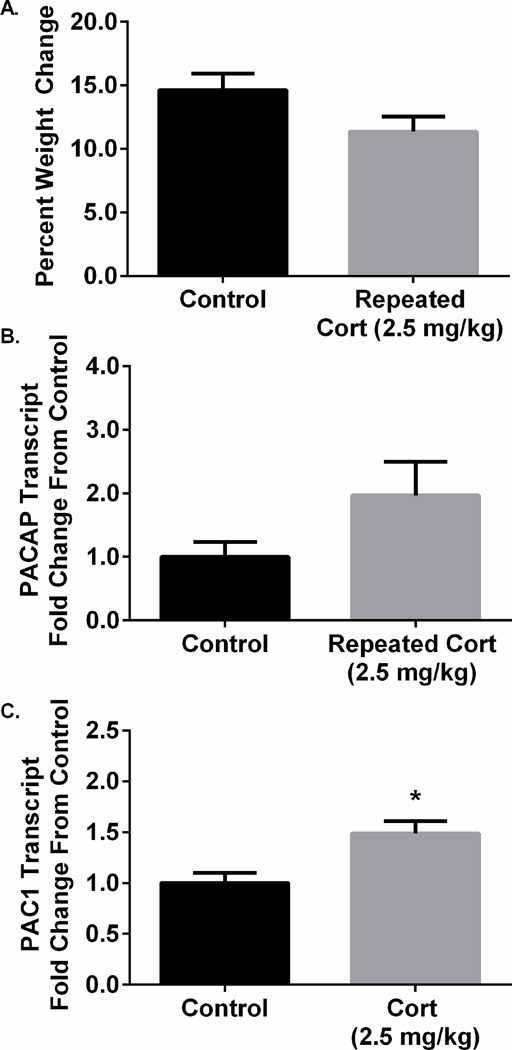
One possible mechanism underlying the increase in BNST PACAP and PAC1 receptor expression following repeated stressor exposure may be regulation by stress-induced adrenal corticosterone release. To examine the potential role of stress-induced glucocorticoid release on dlBNST PACAP expression, rats were injected with a dose of corticosterone (2.5 mg/kg) shown previously to elevate blood corticosterone levels comparable to those after a single stressor exposure (Kalman and Spencer, 2002; Barnum et al. 2008). Notably, the injections increased blood corticosterone levels more than 5-fold above baseline, comparable to the levels found in high stress conditions. Hence, if the changes in BNST PACAP transcript were secondary to stress-induced corticosterone release, the corticosterone injection dose used in this study would have been sufficient to drive its expression.
The corticosterone injections produced a strong trend towards rat weight change compared to vehicle treated controls (t(34) = 1.84; p = 0.07; Figure 3A). From these groups, there were no apparent effects of corticosterone treatment on dlBNST PACAP transcript levels (t(31) = −1.714, p > 0.05); however, there was a significant increase in dlBNST PAC1 transcript expression (t(17) = −3.072, p < 0.05; Figure 3B, 3C). To obviate the potential that the corticosterone dose was insufficient to regulate PACAP expression, a separate group of animals was injected with 10 mg/kg corticosterone using the same paradigm. Even under the higher dosing conditions, dlBNST PACAP transcript levels were not different from those in vehicle treated controls (t(14) = −0.902, p = 0.38; data not shown).
Figure 3.
Repeated corticosterone injection differentially regulates dlBNST PACAP and PAC1 transcript expression. Repeated corticosterone treatment (2.5 mg/kg) produced a trend towards attenuated weight gain in comparison to vehicle treated rats across 7 days of treatment (A). There was no effect of repeated or a single corticosterone treatment (2.5 mg/kg) on PACAP (B), but produced a significant increase in PAC1 receptor transcript expression in the dlBNST (C). Data represent mean ± SEM, *p < 0.05 compared to control.
Anxiety-like behavior following repeated variate stress or corticosterone injection
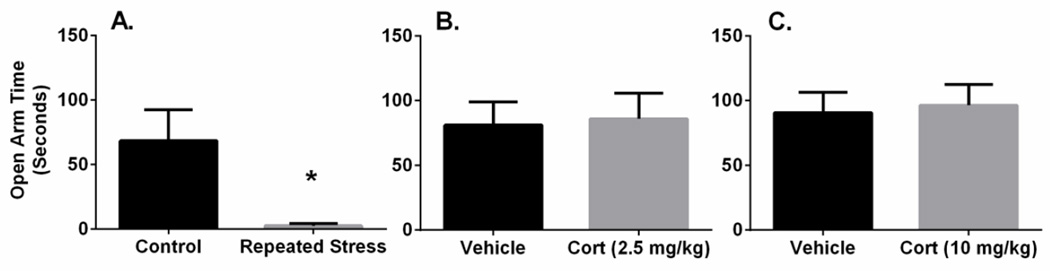
Repeated variate stress significantly reduced open arm exploration on the elevated-plus maze compared to non-stressed rats (t(7) = .2.719, p < 0.05; Figure 4A), consistent with our previous report demonstrating that 7 day stress paradigm increases anxiety-like behavior (Hammack et al. 2009). Interestingly, neither repeated injections with 2.5 mg/kg corticosterone (t(14) = 0.1818, p = 0.86) nor 10 mg/kg corticosterone (t(14) = 0.2611, p = 0.80) altered elevated-plus maze open arm exploration (Figure 4B, 4C), suggesting that 7 days of repeated corticosterone injections alone was insufficient to mimic the effects of repeated variate stress on anxiety-like behavior. Notably, these behavioral results paralleled the effects of stress and corticosterone injection on BNST PACAP transcript levels; repeated stress-induced dlBNST PACAP transcript expression correlated with heightened anxiety-like behavior, whereas corticosterone injections had no apparent effects on either measure.
Figure 4.
Repeated corticosterone treatment did not mimic repeated stress effects on anxiety-like behavior on the elevated plus maze. Rats that received 7 days of repeated variate stress (repeated stress) spent significantly less time on the open arms of the maze compared to non-stressed (control) rats. Rats that received 7 days of corticosterone (Cort) treatment at both the 2.5 mg/kg or 10 mg/kg doses did not display any differences in time spent on the open arms compared to vehicle treated rats. Data represent mean SEM * p < 0.05 compared to control.
Discussion
Our current work examines some of the outstanding questions related to stress-induced PACAP expression in the BNST. Our results demonstrate that repeated stressor exposure is necessary for increased PACAP and PAC1 receptor transcript expression in the dlBNST, that acute single stressor exposure alone appears insufficient to regulate BNST PACAP or PAC1 receptor mRNA expression regardless of temporal parameters, and that the regulation of dlBNST PACAP mRNA does not appear to be secondary to elevated blood corticosterone levels found in stress. Interestingly, unlike PACAP mRNA levels, repeated corticosterone injections increased dlBNST PAC1 receptor transcripts suggesting that hormonal stress mechanisms may regulate BNST PACAP signaling at the receptor level. These results agreed and much extended our previous results (Hammack et al. 2009); further, these observations may be significant in demonstrating that persistent stress challenges impact dlBNST PACAP/PAC1 receptor expression and function that may be upstream and largely distinct from classical HPA regulation.
The regulation of PACAP transcript expression by repeated than acute stress mechanisms contrasts sharply with the regulation of other stress regulators including hypothalamic CRH and AVP expression (Watts and Sanchez-Watts, 1995; Ma et al. 1997). BNST PACAP mRNA levels were not upregulated 2 or 24 hours following a single stressor exposure which appeared consistent with other studies demonstrating the inability for a variety of acute stressors to regulate hypothalamic PACAP transcripts (Hannibal et al. 1995). Hence, the upregulation of BNST PACAP may be more closely tied to anxiety-related behavioral states that develop only when stressor exposure is sustained. Congruent with this posit, our previous work also suggested that over the course of the repeated stress paradigm, BNST activity is gradually recruited such that the latter days of stressor exposure produced a more severe anorexic state than that observed in the early days of stress (Roman et al. 2012). BNST PACAP may critically regulate these effects; the upregulation of BNST PACAP expression is protracted by stress, and the ensuing excitatory and neurotrophic effects of PACAP signaling may drive the maladaptive neurocircuit plasticity to affect behavior. In sum, BNST PACAP upregulation may be a critical mediator of psychopathologies that are associated with chronic or repeated stressor exposure.
Heightened corticosterone levels from stress-induced HPA activation can dramatically alter neuronal survival, cytoarchitecture and transmitter/peptide production in vitro and in vivo (Packan and Sapolsky, 1990; Schulkin et al. 1998; McEwen, 2008; for review, see Groeneweg, 2011). In investigating whether corticosterone has similar regulatory effects on PACAP expression, we demonstrated that even with administration protocols that sustained blood corticosterone levels greater than those typically observed after stress, BNST PACAP transcript levels were not altered. Elevated corticosterone levels did not replicate the effects of repeated variate stress on PACAP transcripts even with substantially more statistical power than was required to produce the effects of stress, and had no apparent effects on anxiety-like behavior on an elevated-plus maze. The duration of our corticosterone treatments (7 days) was shorter than that of previous studies (>21 days) describing the corticosterone effects on anxiety states (Pego et al., 2008; Diniz et al. 2011); our corticosterone treatment paradigm may not have affected the functional or morphological plasticity to impact anxiety-like responses. Notably, the absence of corticosterone effects on anxiety-like behavior was mirrored by an absence of BNST PACAP transcript induction. These results also demonstrated that inductions in BNST PAC1 receptor transcript alone were insufficient to mediate the stress-mediated anxiety-like behavior, as corticosterone increased PAC1 receptor transcripts without anxiety-like behavior.
While intra-BNST GR activation has been shown to increase anxiety-like behavior (Shepard et al. 2009), there were no significant changes in either GR or MR transcript expression following stressor exposures in the current studies. Although the temporal induction of BNST GR or MR transcripts may be different from that for PACAP following stress or corticosterone administration, and the current qPCR methods did not permit cellular resolution of GR/MR mRNA analyses on PACAP neurons, these data may be consistent with our corticosterone study suggesting that stress-induced increases in dlBNST PACAP transcript levels were not dependent on GR or MR signaling.
The BNST has been argued to be an important regulator of HPA axis activity and to receive input from multiple regions including the medial prefrontal cortex, amygdala and hippocampus. Additionally, as the BNST has been argued to mediate the behavioral response to long-duration sustained threatening stimuli (Waddell et al. 2006) and context conditioning (Sullivan et al. 2004), all of the stressors utilized in our paradigm may have directly stimulated BNST activity to result in augmented PACAP/PAC1 receptor expression. Although our data suggested that BNST PACAP may not be immediately regulated by HPA corticosterone responses, stress-induced corticosterone may still be activating other genes and mediators upstream of PACAP resulting in the apparent temporal delay in its stress-regulated expression. BNST CRH is modulated by corticosterone (Makino et al. 1994; Watts and Sanchez-Watts, 1995; Shepard et al. 2006) and may be one candidate mediator upstream of PACAP expression although other studies have suggested PACAP regulation of CRH neurons (Kozicz et al. 1997; Legradi et al. 1998; Agarwal et al. 2005). The regulation of BNST PACAP expression may also be multifactorial requiring the aggregation of direct BNST stimulation, activation of other stressrelated neurocircuits and circulating glucocorticoid signaling.
In aggregate, the current set of studies suggest that BNST PACAP expression is differentially regulated from other stress mediators, including CRH, and appears to require activation of stress conditions or signals that are necessarily prolonged. While further studies are required to assess how PACAP mechanisms contribute to anxiety-like behavior, these studies further support BNST PACAP involvement in stress-related behavioral pathologies.
Acknowledgements
This work was supported by grants MH-97988, MH-072088, and MH-096764 from the National Institutes of Health. Portions of the work were also supported by National Alliance for Research on Schizophrenia and Depression (NARSAD) and the University of Vermont College of Arts and Sciences, as well as funds from the Center of Biomedical Research Excellence (COBRE) in Neuroscience at the University of Vermont (National Institute of Health NCRR P20RR16435).We are grateful to Terrence Deak and Cara Hueston for their assistance with this project.
References
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Research Molecular Brain Research. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Eskow KL, Dupre K, Blandino P, Jr, Deak T, Bishop C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta. Neuroscience. 2008;156:30–41. doi: 10.1016/j.neuroscience.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L, Dos Reis BB, de Castro GM, Medalha CC, Viana MB. Effects of chronic corticosterone and imipramine administration on panic and anxiety-related responses. Brazilian Journal of Medical and Biological Research. 2011;44:1048–1053. doi: 10.1590/s0100-879x2011007500117. [DOI] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y, El Meskini R, Ronnett GV, Waschek JA, May V. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. Journal of Neurochemistry. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regulatory Peptides. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. Journal of Endocrinology. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. Journal of Molecular Neuroscience. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Fahrenkrug J, Larsen PJ. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F, Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S, Baba A. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Hashimoto R, Shintani N, Tanaka K, Yamamoto A, Hatanaka M, Guo X, Morita Y, Tanida M, Nagai K, Takeda M, Baba A. Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. Journal of Neurochemistry. 2009;110:595–602. doi: 10.1111/j.1471-4159.2009.06168.x. [DOI] [PubMed] [Google Scholar]
- Hattori S, Takao K, Tanda K, Toyama K, Shintani N, Baba A, Hashimoto H, Miyakawa T. Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Frontiers in Behavioral Neuroscience. 2012;6:58. doi: 10.3389/fnbeh.2012.00058. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kalman BA, Spencer RL. Rapid corticosteroid-dependent regulation of mineralocorticoid receptor protein expression in rat brain. Endocrinology. 2002;143:4184–4195. doi: 10.1210/en.2002-220375. [DOI] [PubMed] [Google Scholar]
- Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E, Toufexis DJ, Braas KM, May V, Hammack SE. Pituitary adenylate cyclase activating peptide (PACAP) in the bed nucleus of the stria terminalis (BNST) produces anorexia and weight loss in male and female rats. Neuropsychopharmacology. 2014 in press. [Google Scholar]
- Kozicz T, Vigh S, Arimura A. Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Research. 1997;767:109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- Légrádi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neuroscience Letters. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. Journal of Endocrinology. 1997;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Research. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Ueta Y, Serino R, Kabashima N, Shibuya I, Yamashita H. PACAP type I receptor gene expression in the paraventricular and supraoptic nuclei of rats. Neuroreport. 1996;8:67–70. doi: 10.1097/00001756-199612200-00014. [DOI] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schutz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. Journal of Neuroscience. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Research. Molecular Brain Research. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Packan DR, Sapolsky RM. Glucocorticoid endangerment of the hippocampus: tissue, steroid and receptor specificity. Neuroendocrinology. 1990;51:613–618. doi: 10.1159/000125400. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. European Journal of Neuroscience. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. Journal of Neuroscience. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Kocho-Schellenberg M, Garret MA, Braas K, May V, Hammack SE. Excitotoxic lesions of the bed nucleus of the stria terminalis (BNST) attenuate the effects of repeated stress on weight gain: evidence for the recruitment of BNST activity by repeated, but not acute, stress. Behavioral Brain Research. 2012;227:300–304. doi: 10.1016/j.bbr.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behavioral Brain Research. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chambers CO, Busch C, Mount A, Schulkin J. Chronically elevated corticosterone in the dorsolateral bed nuclei of stria terminalis increases anxiety-like behavior. Behavioral Brain Research. 2009;203:146–149. doi: 10.1016/j.bbr.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Shioda S, Shuto Y, Somogyvári-Vigh A, Legradi G, Onda H, Coy DH, Nakajo S, Arimura A. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neuroscience Research. 1997;28:345–354. doi: 10.1016/s0168-0102(97)00065-5. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Review Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological Reviews. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. Journal of Physiology. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]