Abstract
Objective
To determine the extent to which OMERACT participants agree that instruments that have been used in clinical trials and measure OMERACT core outcome domains in acute gout fulfil the filter requirements of truth, discrimination and feasibility and to determine where future research efforts need to be directed.
Methods
The results of a systematic literature review and analysis of individual-level data from recent clinical studies of acute gout were presented to OMERACT participants. The information was discussed in breakout groups and opinion was defined by subsequent voting in a plenary session. Endorsement was defined as at least 70% of participants voting in agreement with the proposition (where the denominator excluded those participants who did not vote or who voted ‘don’t know’).
Results
The following measures were endorsed for use in clinical trials of acute gout: (1) 5-point Likert scale and/or VAS (0 to 100mm) to measure pain; (2) 4-point Likert scale for joint swelling; (3) 4-point Likert scale for joint tenderness; and (4) 5-point Likert scale for patient global assessment of response to treatment. Measures for the activity limitations domain were not endorsed.
Conclusions
Measures of pain, joint swelling, joint tenderness and patient global assessment in acute gout were endorsed at OMERACT-11. These measures should now be used in clinical trials of acute gout.
Key Indexing Terms: gout, outcome measures, psychometrics
Introduction
Gout is the most common inflammatory arthritis, occurring more commonly than rheumatoid arthritis, with most recent prevalence estimates of 3.9% in the U.S(1). At OMERACT-9 (2008) five core domains for acute gout studies were endorsed namely pain, joint swelling, joint tenderness, patient global assessment and activity limitations (2). In addition, several discretionary domains were identified including joint impairment, work disability, joint erythema, acute phase markers and physician global assessment. Similarly, core domains for chronic gout were also defined and endorsed.
At OMERACT-9 and -10 meetings (2010), data related to measures for several domains of chronic gout were presented, and measures for pain, activity limitation, health-related quality of life, patient global and serum urate were endorsed(3–8). The objective of a gout workshop at OMERACT-11 (2012) was to present data from randomized controlled trials (RCTs) and observational studies related to measures of acute domains at OMERACT-10 for each domain in acute gout and seek endorsement on specific instruments. In this report we summarize the results of the OMERACT voting, reports from the participant break-out sessions and discuss a research agenda.
At OMERACT-11, the focus of the gout workshop was to obtain endorsement of specific instruments that measure each of the five core domains identified as required outcomes in acute gout trials at OMERACT-9 (9). Two companion papers reviewed the existing literature and recent acute gout studies for psychometric properties of measures for each of these domains (INSERT REFERENCES TO Dalbeth et al. and Taylor et al. IN THE SAME ISSUE: the other two gout workshop papers). This information was used to inform OMERACT participants to assist with breakout discussion and plenary voting.
Methods
During a 2.5 hour gout workshop at OMERACT-11, we had detailed discussions related to acute gout instruments. The opening 30-minute presentation consisted of a brief introduction related to acute gout, followed by a patient’s description of his personal experience with gout and his life journey with the disease, followed by presentation of data analysis from RCTs and observational studies related to various measures for acute gout. Subsequently, we had four break-out sessions with each focused on detailed discussion related to the measures of: (1) pain; (2) joint swelling and tenderness; (3) patient global assessment; and (4) activity limitations. The reporters from each of the break-out groups presented their reports at the general session. This was followed by voting by all OMERACT participants for each measure for the 5 core domains of acute gout. A majority vote of ≥ 70% in agreement with the proposition is required for OMERACT endorsement. The OMERACT executive committee had decided that the percentage vote was to be calculated from participants voting ‘yes’ or ‘no’ (thereby excluding ‘don’t know’ or non-response from the denominator).
Results
Breakout discussions
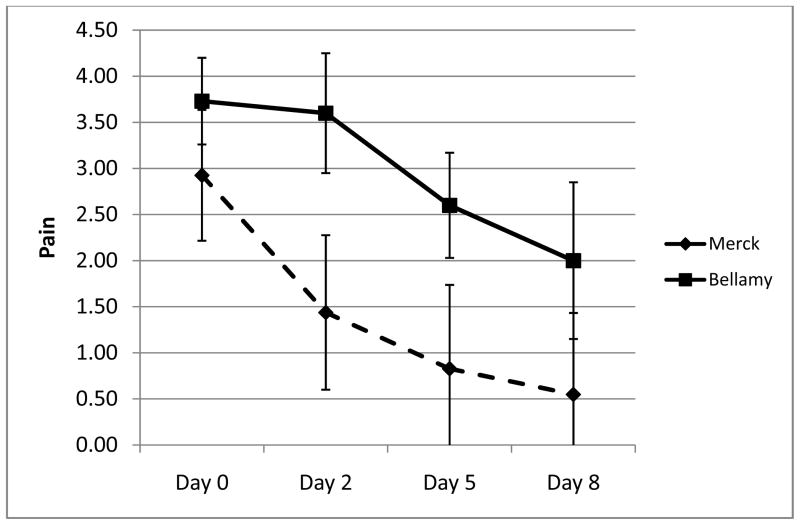
In relation to pain assessment, data had been presented from several trials that had applied a 5-point Likert scale, visual analog scale (VAS) and/or numeric rating scale (NRS) different measures to assess pain. Participants in the break-out group commented that the Likert pain scale has the advantages of convenience (particularly with electronic reporting diaries) compared to the VAS pain scale which allows for more granular measurement and trialists may choose either or both, since both appear to function very well in acute gout trials. Comments were also made for the future research agenda to consider the slope of pain improvement (that is, the rate of change in pain), the minimally acceptable state, time to pain resolution and achieving acceptable state, pain at rest versus pain on motion, measurement of pain behavior and pain impact, and to measure both change in pain as well as the achieved final state. Comments were made specifically to acknowledge that pain and other patient-reported outcomes as well as measures of inflammation (joint swelling and tenderness) change so rapidly with effective treatment options for acute gout that reliability has less relevance for the psychometric assessment of any acute gout measure. This can be observed in Figure 1, which shows pain scores from a reported study of untreated acute gout over 7-days (13) superimposed upon data from an interventional study of etoricoxib and indomethacin for acute gout over 8 days (12). It can be seen that even in untreated gout, pain improves over a matter of days. Most measures validated for acute gout domains have large effect sizes and discriminated well between groups and changes within a group, implying that these measures must be capable of demonstrating change beyond measurement error (even if the measurement error has not been formally quantified).
Figure 1.
Pain improves quickly in acute gout (with or without treatment). The plot shows the mean (±standard deviation) of pain scores (5-point Likert scale) from an interventional trial in acute gout (Merck, n=339) and a cohort study of untreated acute gout (Bellamy, n=11, reference #13).
Data from trials that used a physician-assessed 4-point Likert scale for joint sweling and tenderness were presented. Issues with regards to joint swelling and tenderness were also discussed in detail. Participants asked for clarification whether these measures as reported were physician-assessed or patient-reported, and it was clarified that these were all physician-assessed. A suggestion was made to assess patient reported joint swelling as another measure of this domain and perhaps this will need more validation data. Another suggestion was whether a sentinel joint should be monitored rather than multiple joints and how to assess swelling beyond a joint, when it affects the entire foot or leg.
We had presented data from acute gout trials that used a 5-point Likert scale patient global measure of change in response to treatment. The break-out groups debated the advantages and disadvantages of patient global disease measure (e.g. on a 0–10 or 0–100 scale with “no disease activity” and “severe/very severe disease activity” as anchors or a likert scale such as none, mild, moderate, severe, very severe) and patient global assessment of change (e.g. likert scale), and suggested that both be measured rather than one in clinical trials. The groups noted that in gout trials, only likert scales for global assessment of change were used, but similar to other rheumatic diseases, future trials need to include additional measures as discussed above. Participants also noted that patient global response to treatment scale was biased towards improvement, though they also recognized that improvement is almost the rule in the natural history of acute gout. They also suggested some other interesting versions of measures of return to pre-acute gout flare as considerations for global scales.
The current assessment of activity limitations in acute gout was generally viewed as unsatisfactory. The Health Assessment Questionnaire (HAQ) is specifically framed to assess the activity limitations over the previous week and contains many items related to upper extremity limitations. The break-out groups commented that since gout is a lower extremity predominant arthritis with acute gout flares affecting lower extremity, it was possible that HAQ needed to be modified for lower extremity functional limitations or another instrument that is more focused on lower extremity activity limitations should be used. Furthermore, it was observed that acute gout evolves quickly and that an activity limitation measures should have the time-frame of a day rather than a week for respondents to consider. Another issue raised was whether HAQ was a better measure of chronic joint disease rather than acute disease.
Plenary voting
The results of the voting are shown in Table 1. Overall, there was endorsement for the 5-point Likert scale and VAS (0 to 100mm) to measure pain in acute gout; the 4-point Likert scale for joint swelling and joint tenderness; and the 5-point Likert scale for patient global assessment of response to treatment. A measure for the activity limitations domain was not endorsed.
Table 1.
Voting results and endorsement of measures of acute gout for clinical trials in gout
| Domain | Measure | % voting Yes* | Endorsed for use |
|---|---|---|---|
| Pain | 5-point Likert or Visual Analog Scale | 87% | Yes |
| Joint swelling | 4-point Likert | 71% | Yes |
| Joint tenderness | 4-point Likert | 72% | Yes |
| Patient global | 5-point Likert | 86% | Yes |
| Activity limitation | Health Assessment Questionnaire (HAQ) | 29% | No |
from a total of 83 voting participants; the proportion is those voting yes divided by the sum of those voting yes or no; votes of “don’t know” were excluded from the denominator based on an executive decision prior to the meeting related to how to count the votes
Discussion
In this report, we describe the process for OMERACT endorsement of at least 1 measure for four of the five core domains that should be included in acute gout clinical trials. Valid measures for pain (Likert scale or VAS), joint swelling, joint tenderness and patient global assessment (all Likert scales) were shown to meet the OMERACT filter requirements and were endorsed by OMERACT. Development of new, high quality outcome measures in gout is a significant advance that should allow standardization of outcome reporting in clinical trials and other clinical studies of acute gout. However, no measure for activity limitation was endorsed. HAQ, which had the most amount of validation data, still lacked the evidence for between group differences. The endorsement of all other measures provides trialists with validated outcomes measures for consistent use in clinical trials of acute gout. The use of other instruments are not precluded but it is recommended that OMERACT endorsed instruments be concurrently assessed as a minimum. Since these are the measures of the core domains, it is recommended that at least one measure of each core domain be included in clinical trials or observation studies of acute gout.
Since no measure of activity limitation was endorsed, what should gout trialists do? Although we do not make a specific recommendation, we suggest that trialists consider inclusion of HAQ-DI, HAQ-II or PROMIS/Improved HAQ (10) in clinical trials, so that enough data can be collected and analyzed to assess whether this will be a valid measure of activity limitation in patients with acute gout. Another suggestion is to include other measures of activity limitation in addition to a version of HAQ to assess which of the two instruments (HAQ or alternate measure) will be more sensitive to change or have better distributional properties (floor and ceiling effects). One example of an instrument that might be better for the lower limb problems experienced by most acute gout patients is the Lower Extremity Functional Scale, a 20-item self-reported scale that reflects current problems rather than problems over a specific time-period (11).
In summary, this report provides analysis of data related to measures of acute gout domains. The OMERACT endorsed these measures of acute gout domains and these measures can now be included in clinical trials of acute gout. Future studies should assess and/or develop measures of activity limitation for acute gout clinical trials. In addition, other measures of non-core domains including work disability, joint erythema, acute phase markers and physician global assessment. should be validated using trial data, so that clear guidance regarding their use can be provided to clinical trialists.
Acknowledgments
Source of support:
Grants: This work was supported with the resources and the use of facilities at the Birmingham VA Medical Center, Alabama, USA (JAS) and University of Otago, New Zealand (WJT).
We thank the all the participants of OMERACT-11, especially patients, for providing valuable insights and discussions related to these measures.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- JAS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Ardea, Regeneron, Allergan, URL pharmaceuticals and Novartis. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology’s Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee.
- WJT declares no conflicts of interest.
- ND has acted as a consultant for Ardea Biosciences, Metabolex, Novartis and Takeda. Her institution has received funding from Fonterra and she is a named inventor on a patent related to milk products and gout.
- LSS has served on the Board of Directors for Savient Pharmaceuticals, and as a consultant for Takeda
- JS has received research support from Savient Pharmaceuticals, Regeneron, Ardea Biosciences, Metabolex, Nuon Therapeutics, Pharmos and has received consulting funds from Savient Pharmaceuticals, Regeneron, Novartis, Ardea Biosciences, Metabolex, Nuon Therapeutics, BioCryst, and Pharmos.
- RG declares no conflicts of interest.
- RA has received research grants from Novartis.
- LM declares no conflicts of interest.
- VS has received consultant fees Metabolex, Novartis, and Savient.
- GW declares no conflicts of interest.
- DK has received consultant fees from Ardea, Takeda, Novartis, and Savient, as well as have been a Speaker Bureau for Savient FM declares no conflict of interest.
- FM declares no conflicts of interest.
- NS has received research grants from Novartis, has worked on the Advisory Board for Novartis, Takeda, Savient, Enzyme Rx, URL Pharma and has also served on the Speakers Burear for Novartis, Takeda, and Savient, as well as received consultant fees from Novartis and Takeda.
- AB declares no conflict of interest.
- MB declares no conflict of interest.
- KS has received consultant fees from Ardea, Regenron and Takeda as well as served on the Data Safety Monitoring Board for BioCryst.
- HRS has received a grant from Takeda and is a consultant with Regeneron, Novartis, Ardea, Metabolex, Savient, Biocryst, Westward and Pfizer.
- NLE has received consultant fees from Takeda Pharmaceutical, Savient Pharmaceutical, Ardea Biosciences, Regeneron Pharmaceuticals, Mebabolex Pharmaceuticals and Biocryst Pharmaceuticals.
Contributor Information
William J Taylor, Email: will.taylor@otago.ac.nz.
Nicola Dalbeth, Email: n.dalbeth@auckland.ac.nz.
Lee S Simon, Email: lssconsult@aol.com.
John Sundy, Email: john.sundy@duke.edu.
Rebecca Grainger, Email: rebecca.grainger@otago.ac.nz.
Rieke Alten, Email: rieke.alten@schlosspark-klinik.de.
Lyn March, Email: lynmar@med.usyd.edu.au.
Vibeke Strand, Email: vstrand@aol.com.
George Wells, Email: gawells@ottawaheart.ca.
Dinesh Khanna, Email: medsubs-dkhanna@yahoo.com.
Fiona McQueen, Email: f.mcqueen@auckland.ac.nz.
Naomi Schlesinger, Email: schlesna@rwjms.rutgers.edu.
Annelies Boonen, Email: a.boonen@mumc.nl.
Maarten Boers, Email: m.boers@VUMC.NL.
Kenneth G. Saag, Email: ksaag@uab.edu.
H. Ralph Schumacher, Jr, Email: schumacr@mail.med.upenn.edu.
N. Lawrence Edwards, Email: larry.edwards@medicine.ufl.edu.
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. doi: 10.1002/art.30520. Epub 2011/07/30. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. J Rheumatol. 2009;36(10):2342–5. doi: 10.3899/jrheum.090370. Epub 2009/10/13. [DOI] [PubMed] [Google Scholar]
- 3.Taylor WJ, Singh JA, Saag KG, Dalbeth N, MacDonald PA, Edwards NL, et al. Bringing it all together: a novel approach to the development of response criteria for chronic gout clinical trials. J Rheumatol. 2011;38(7):1467–70. doi: 10.3899/jrheum.110274. Epub 2011/07/05. [DOI] [PubMed] [Google Scholar]
- 4.Dalbeth N, McQueen FM, Singh JA, MacDonald PA, Edwards NL, Schumacher HR, Jr, et al. Tophus measurement as an outcome measure for clinical trials of chronic gout: progress and research priorities. J Rheumatol. 2011;38(7):1458–61. doi: 10.3899/jrheum.110272. Epub 2011/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JA, Taylor WJ, Simon LS, Khanna PP, Stamp LK, McQueen FM, et al. Patient-reported outcomes in chronic gout: a report from OMERACT 10. J Rheumatol. 2011;38(7):1452–7. doi: 10.3899/jrheum.110271. Epub 2011/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh JA, Yang S, Strand V, Simon L, Forsythe A, Hamburger S, et al. Validation of pain and patient global scales in chronic gout: data from two randomised controlled trials. Ann Rheum Dis. 2011;70(7):1277–81. doi: 10.1136/ard.2010.144022. Epub 2011/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamp LK, Khanna PP, Dalbeth N, Boers M, Maksymowych WP, Schumacher HR, Jr, et al. Serum urate in chronic gout--will it be the first validated soluble biomarker in rheumatology? J Rheumatol. 2011;38(7):1462–6. doi: 10.3899/jrheum.110273. Epub 2011/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grainger R, Taylor WJ, Dalbeth N, Perez-Ruiz F, Singh JA, Waltrip RW, et al. Progress in measurement instruments for acute and chronic gout studies. J Rheumatol. 2009;36(10):2346–55. doi: 10.3899/jrheum.090371. Epub 2009/10/13. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher HR, Jr, Taylor W, Edwards NL, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome Domains for Studies of Acute and Chronic Gout. J Rheumatol. 2009;36:2342–5. doi: 10.3899/jrheum.090370. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF, Krishnan E, Rose M, Lingala B, Bruce B. Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Res Ther. 2011;13(5):R147. doi: 10.1186/ar3461. Epub 2011/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binkley J, Stratford P, Lott S, Riddle D The North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale: Scale development, measurement properties, and clinical application. Phys Ther. 1999;79:4371–383. [PubMed] [Google Scholar]
- 12.Schumacher HR, Jr, Boice JA, Daikh DI, Mukhopadhyay S, Malmstrom K, Ng J, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488–92. doi: 10.1136/bmj.324.7352.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987;24(1):33–6. doi: 10.1111/j.1365-2125.1987.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]