Abstract
Natural aging is accompanied by a dysregulation of the host immune response that has well-known clinical consequences but poorly defined underlying causes. It has previously been reported that advancing age is associated with an increase in membrane cholesterol level in T cells. The aim of this study was to investigate whether high-density lipoprotein (HDL) can modulate the age-related accumulation of membrane cholesterol in T cells and impact on their subsequent responsiveness. Our data reveal that cholesterol metabolism, influx, and efflux are altered in T cells with aging, which may in part explain the increase in membrane cholesterol level observed in T cells in elderly individuals. HDL was unable to promote reverse cholesterol transport in T cells from elderly subjects with the same efficiency as was observed in T cells from young subjects besides unchanged ABCA-1 and SR-BI expressions. HDL exhibited a short-acting co-stimulatory effect by enhancing T cell production of interleukin-2 (IL-2). Moreover, HDL from healthy normolipemic individuals exerted differential effects on T cell proliferation that depended on the age of the HDL donor. Finally, HDL modulated TCR/CD28 activation by inducing sustained signaling through pLck, pERK, and pAkt. These data suggest that HDL has immunomodulatory effects on T cells that are influenced by age.
Keywords: Aging, Immunosenescence, HDL, Inflamm-Aging, Metabolic pathways
Introduction
Age-related increases in the incidence of infections, cancers, neurodegenerative diseases, and chronic inflammatory diseases such as atherosclerosis represent a substantial burden on health care provision in the developed world (Larbi et al. 2008). Although the underlying causes that link human aging to increased risk of pathology is not fully understood, it is clear that alterations in immune functions play a critical role (Pawelec 2012; Fulop et al. 2005; López-Otín et al. 2013). Numerous studies of age-related immune dysfunction, or “immunosenescence,” have been published (Fülöp et al. 2013; Larbi et al. 2013), but the conclusions drawn from these investigations remain controversial. The direct clinical consequences of age-related immune dysfunction seem clear and are well demonstrated by the increased rate and severity of infections, cancers, and autoimmune disorders observed in the elderly. While age-related changes in the immune response are clearly multi-factorial, the central defect underlying declining immune function with age is thought to be an overwhelming decrease in T cell function (Pawelec 2012). Since impaired T cell function is a hallmark of immunosenescence in the elderly, and membrane lipid rafts (LRs) are an integral component of the signaling apparatus in T lymphocytes, it is possible that altered activation of T cells in the elderly may be related to changes in the cholesterol content and composition of membrane LRs.
Aging is associated with global alterations in immune function (Larbi et al. 2013), but the immune system is a complex interactive system composed of many different cell types and cell sub-populations that are not altered to the same extent and do not contribute equally to immunological aging. Immunosenescence should perhaps be considered to represent the dysregulation of a system that is constantly trying to adapt and maintain homeostasis in the face of diverse inputs and outputs that are still only crudely defined (Pawelec 2012). Studies of elderly humans and animal models have revealed that production of interleukin-2 (IL-2) and clonal expansion of effector and memory T cells are the aspects of the immune system which are the most susceptible to age-related alterations (Fülöp et al. 2013). It is therefore possible that changes in cell membrane biology and disruption of the proximal signaling events may contribute to these altered functions (Larbi et al. 2011; Goronzy et al. 2012; Fulop et al. 2014). The major histocompatibility (MHC)-restricted activation of T cells by antigen-presenting cells (APCs) requires intimate communication between the stimulating APCs and responding T cells through the formation of an immune synapse (Reichardt et al. 2010). It is now widely accepted that immune synapse formation is mediated by specialized microdomains of the T cell plasma membrane known as lipid rafts (LRs) that initiate assembly of the complex machinery of T cell signal transduction (Simons and Gerl 2010; Simons and Sampaio 2011). LRs are enriched in cholesterol, sphingolipids, and proteins and are associated with several key biological processes including cell signaling and protein transport and adhesion (Kabouridis 2006).
A series of investigations in the late 1980s suggested that biochemical and biophysical alterations at the level of the cell plasma membrane could be associated with the altered immune response with aging (Maczek et al. 1998). It was noted that there were age-related modifications in the lipid composition of the lymphocyte plasma membrane and that these modifications directly affected membrane fluidity. Recent data from our own laboratories have shown that the levels of plasma membrane cholesterol, the major stabilizing component of LRs, were increased twofold in T lymphocytes from elderly individuals, resulting in a plasma membrane that was less fluid than that observed in T cells from young individuals (Larbi et al. 2006a). Alterations in the assembly of T cell signaling components in LRs have been reported to be associated with aging. These observations may to some extent reflect a persistent, low-grade immune activation in elderly subjects that has been proposed by the “Inflamm-Aging” model (Franceschi et al. 2000; Larbi et al. 2004a). We have also used confocal microscopy to demonstrate that aging is associated with altered coalescence of LRs in T cells activated with a combination of anti-TCR and anti-CD28 antibodies. LRs exhibited weak coalescence in CD4+ and CD8+ T cells from elderly subjects although the alterations were less pronounced in the CD8+ T cell compartment. Alterations in the composition of the T cell plasma membrane as a consequence of aging therefore seem to play a central role in these impaired lymphocyte functions.
The role played by lipoproteins in modulating membrane cholesterol content has recently been subject to extensive investigations and has led to the suggestion that these molecules may have immunomodulatory functions (Norata et al. 2012; Murphy et al. 2008, 2011; Carpintero et al. 2010; Gruaz et al. 2010; Landry et al. 2006). In particular, high-density lipoproteins (HDLs) have been shown to modulate the innate immune response by decreasing the production of inflammatory mediators, as well regulating adaptive immunity either by inhibiting antigen presentation or by altering the cholesterol content of LRs to alter T cell activation and signaling. Although most studies on the immunomodulatory properties of HDL were carried out in relation to atherosclerosis (Badimon and Vilahur 2012), accumulating evidence shows that HDL levels are decreased in many chronic inflammatory diseases, including rheumatoid arthritis and lupus (Borba et al. 2006). With aging, the quantity and the quality of HDL are significantly altered (Park and Cho 2011). We have shown that HDL is oxidatively altered with aging and exhibits reduced anti-oxidant and anti-inflammatory activity, as this is also a characteristic in chronic inflammatory diseases (Jaouad et al. 2006; Berrougui and Khalil 2009), and consistent with the predictions of the Inflamm-Aging model (Khalil et al. 2012; Rea et al. 2004). Whether these age-related changes in HDL biology contribute to T cell immunosenescence is currently unknown. To our knowledge, there is no known molecular mechanism that can account for the increased cholesterol content of the T cell plasma membrane in normolipemic elderly subjects, and it is unclear how these changes in membrane composition impact on T cell function. In the present study, we investigated cholesterol metabolism in T cells and tested the ability of HDL to modify the cholesterol content of the plasma membrane in order to shed light on the mechanisms that drive immune dysfunction in elderly subjects.
Materials and methods
Subjects
Fifteen elderly volunteers aged 65 to 78 years (mean, 76.2 ± 6.5 years) participated in the study. The cohort of 15 young healthy subjects was 19 to 25 (mean, 23.4 ± 1.4) years old. The research protocol was approved by the local institutional ethics committee of the Research Center on Aging. All subjects gave written informed consent. The volunteers were in good health, normolipemic (Table 1), and satisfied the inclusion criteria of the SENIEUR protocol for immune investigations of human elderly subjects (Lighart 2001). None of the participating subjects were taking medication or suffered from diseases that may affect lipoproteins metabolism or HDL levels.
Table 1.
Clinical parameters in young and elderly subjects
| Parameters (normal range) | Young (n = 15) | Elderly (n = 15) | p value |
|---|---|---|---|
| Age | 23.4 ± 1.4 | 76.2 ± 6.5 | 0.0001 |
| Gender | 11F/4M | 10F/5M | NS |
| TC (3.0–6.2 mmol/l) | 4.2 ± 0.2 | 4.6 ± 0.3 | NS |
| Triglycerides (0.3–2.4 mmol/l) | 0.9 ± 0.5 | 1.0 ± 0.5 | NS |
| HDL-C (>0.9 mmol) | 1.5 ± 0.2 | 1.7 ± 0.3 | NS |
| LDL-C | 1.7 ± 0.3 | 2.4 ± 0.4 | NS |
| CRP (0–8 mg/l) | <3 | <3 | NS |
| Cortisol (85–618 nmol/l) | 436.3 ± 94.4 | 433.7 ± 53.7 | NS |
TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CRP C-reactive protein, NS non-significant
Isolation of HDL
Isolation of whole HDL (HDL2 and HDL3) was performed within 2 h according to the method of Sattler et al. (1994), using the Beckman Optima TLX ultracentrifuge in the presence of ethylenediaminetetraacetic acid (EDTA, 0.4 mg/ml) as already described (Khalil et al. 2012). In brief, HDLs (1.063 < d < 1.19) were separated by 2 h of ultracentrifugation at 15 °C at 100,000 rpm in a TLA 100.4 rotor. After separation, the HDLs were obtained directly by needle aspiration of the HDL band by piercing the wall with a 23-gauge flat-headed needle syringe. HDLs were dialyzed overnight at 4 °C against a 10 mM sodium phosphate buffer (pH 7). HDL concentrations are expressed in terms of total protein contents (μg protein/ml). Proteins were measured by commercial assay (Pierce method, Rockford, IL, USA).
T cell separation
Blood obtained by venipuncture was collected in heparinized tubes and diluted twofold with phosphate-buffered saline (PBS). Peripheral blood mononucleated cells (PBMCs) were isolated by Ficoll Paque plus density sedimentation, as described (Larbi et al. 2006a). The buffy coat was recovered; the cells were washed (PBS) and counted.
Cell viability was greater than 95 % (Trypan blue exclusion). Identical numbers of cells from young and aged donors were used in each comparative experiment. T cell purification: PBMCs were freed of monocyte by adhesion to plastic tissue culture flasks coated with autologous serum (1 h, 37 °C), and B cells and phagocytic cells by nylon wool retention, as described (Fülöp et al. 2001). Purified T cells were greater than 98 % CD3+ cells with less than 1 % surface IgM (B cells)-, CD16 (NK cells)-, and CD14 (monocytes)-positive contaminating cells. Cell viability was greater than 97 % (Trypan blue exclusion). Identical numbers of T cells from young and aged donors were used in each comparative experiment. The number of T cells used for the cholesterol loading experiments was 5 × 105, unless stated otherwise.
Lymphocyte proliferation and cytokine expression
For proliferation, PBMCs (2 × 105 cells/well) were exposed to anti-CD3 (5 μg/ml) and/or anti-CD28 (5 μg/ml) for 72 h in 96-well flat-bottomed microcultures (Microtest, Becton Dickinson) in a final volume of 200 μl of RPMI 1640 medium containing 10 % fetal bovine serum (FBS), streptomycin (100 μg/ml), and penicillin G (100 U/ml) at 37 °C in an atmosphere of 95 % air, 5 % CO2, and 90 % relative humidity. Cell proliferation was quantified either by measuring [3H]-thymidine incorporation, as described (Fülöp et al. 1999), or by the CFSE (2.5 μM) dilution assay. PBMCs (1 × 106/ml) were stimulated with PMA (50 ng/ml) and Ionomycin (750 ng/ml) for 6 h in the presence of Brefeldin A (GolgiPlug®, BD Biosciences). After stimulation, cells were washed two times in PBS containing 10 % FCS prior to surface marker staining (CD3, CD4, CD8). Cells were then fixed and permeabilized with Fix/Perm® and Perm/Wash® buffers according to the manufacturer’s instructions. Cells were stained for intracellular IFN-γ and TNF-α expression, washed two times, and analyzed by FACS.
Isolation of lipid rafts and western blots
T cells were kept for 1 h in RPMI medium at 37 °C. The cells (20 × 106 lymphocytes) were then exposed to a combination of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) mAb for various periods of time at 37 °C, as described (Larbi et al. 2006a), or were left untreated (control). Lipid raft isolation on sucrose density gradients was done as described (Larbi et al. 2006a). Lipid rafts were distributed in fractions 1 to 3 of the gradient whereas nonlipid rafts corresponded to fractions 5 to 8. Western blotting analyses were done using pooled fractions 1–3 and pooled fractions 5–7, as described (Larbi et al. 2006a).
Proteins from total cell lysates (20 μg) or pooled lipid raft fractions (30 μl) were sized by SDS-PAGE under reducing conditions, transferred to PVDF membranes, and revealed by Western blotting, as described (Larbi et al. 2006a). Densitometric analyses were performed using the image analyzer Chemigenius2 Bio Imaging System (Syngene, Frederick, MD) or the Java-based ImageJ freeware (http://rsbweb.nih.gov/ij/).
Cholesterol efflux/influx measurements
Both cholesterol efflux and cholesterol influx (cholesterol uptake) were measured in the presence of T cells.
For the measurement of cholesterol efflux, T cells (5 × 105) were incubated in fresh medium containing 2 μCi/ml [3H]-cholesterol for 24 h. Labeled T cells were washed and equilibrated in serum-free medium containing 1 % bovine serum albumin (BSA) for an additional 12 h. After the equilibration period, the [3H]-cholesterol-enriched T cells obtained from young or elderly subjects were washed three times and incubated between 30 min and 24 h with whole HDL (50 μg/ml). At the end of the incubation time, cells were lysed in 0.1 M NaOH. The counts per minute (cpm) in the supernatant and cell lysates were determined using a liquid scintillation counter. Cholesterol efflux (radiolabeled cholesterol released from cells) was calculated using the following formula: (radioactivity (cpm) in supernatant / radioactivity (cpm) in cells + medium) × 100.
For the measurement of cholesterol influx (cholesterol uptake), T cells were incubated with 3H-cholesterol for 24 h (Berrougui et al. 2009). The medium was then collected from the culture plates. The cells were washed two times with PBS and lysed. The cholesterol influx was measured by determining the percentage of radiolabeled cholesterol incorporated (% cholesterol influx) using the following formula: (cpm in the cell / cpm in the cell + medium) × 100.
Membrane cholesterol and fluidity measurement
Cholesterol levels within membrane were determined by HPLC analysis after extraction of sucrose gradient fractions. We used the HPLC method described by de Mello et al. (2004) and Katsanidis and Addis (1999) with some modifications (Larbi et al. 2006a).
Cell membrane anisotropy (r) of T cells was determined using the fluorescent probe diphenylhexatriene (DPH), as described (Larbi et al. 2006a). Fluidity (f) was calculated using the inverse value of anisotropy (f = 1/r).
Flow cytometry experiments
Phenotyping and functional analysis of CD4+ and CD8+ T cells were performed by flow cytometry. PBMCs from participants were stained with an antibody cocktail consisting of anti-CD3, anti-CD4, anti-CD8, anti-CD45RA, anti-CD27, and a live/dead exclusion dye. Samples were stained for 20 min at 4 °C and washed two times in PBC containing 1 % FBS. Other experiments include anti-CD25, anti-SR-BI, and anti-ABCA-1 antibodies used in combination with an anti-CD3 antibody to assess their expression in T cell.
In the case of each experimental measurements of cytokines (IL-2, TNF-α, and IFN-γ), the cells (1 × 106 cells/ml) were placed in Eppendorf tubes, washed by brief centrifugation with cold PBS, fixed by treatment (20 min in the dark, 4 °C) with 250 μl of 4 % (w/v) paraformaldehyde (BioLegend, Burlington, ON), and then washed with a mixture of PBS (1 ml) and diluted (PBS) permeabilization buffer (250 μl) containing FBS and saponin (PermWash buffer, BD Pharmingen). After washings, staining was done with permeabilization wash buffer (200 μl) containing conjugated anti-human IL-2, TNF-α, or IFN-γ Ab (0.2 μg/ml) for 30 min at 4 °C, in the dark. The stained cells were washed once with permeabilization wash buffer and resuspended in PBS (250 μl). The samples were analyzed within 24 h by flow cytometry using FACSCalibur or Fortessa LSRII instruments (Beckton Dickinson). A minimum of 10,000 events was acquired in each analysis, as described (Larbi et al. 2006a).
Statistical analyses and power calculation
Values are expressed as means ± SEM. Data were analyzed using Student’s t test for continuous variables and chi-square test for categorical ones. Statistical analyses were performed using SigmaStat software (Systat Software Inc., Chicago, IL). A p value of <0.05 was considered statistically significant.
With 15 subjects by group, we will have 80 % power for detection of a large effect size of 1 using a two-tailed t test with alpha level of 0.05.
Results
T cell cholesterol balance is altered with age
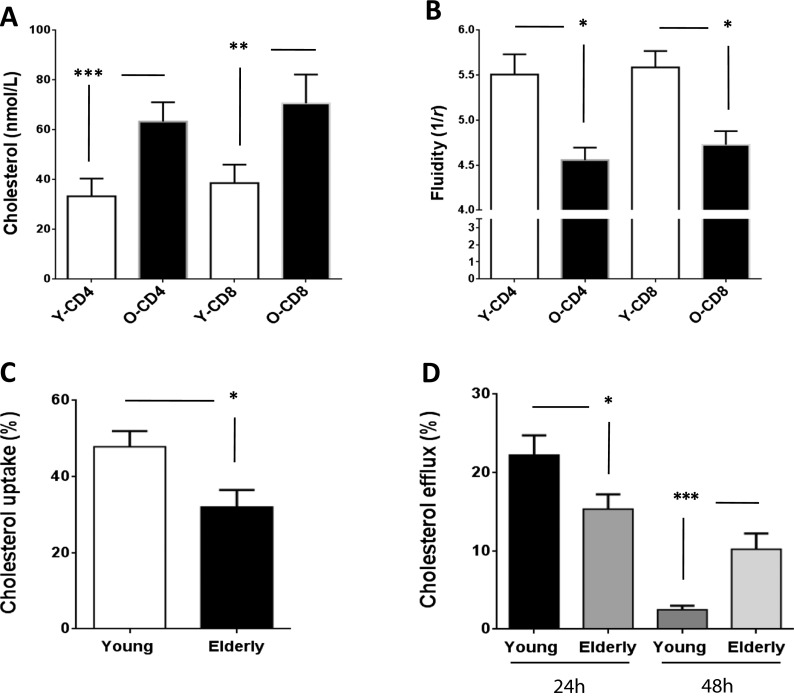
Previous studies have clearly linked hyper-lipidemia and associated co-morbidities (such as cardiovascular diseases) with a pro-inflammatory status. We selected only healthy normolipemic elderly and young donors in order to exclude this confounding factor from our study of “immunological aging.” As described in Table 1, levels of cholesterol, triglycerides, and lipoproteins were comparable between elderly (72.6 ± 6.5 years) and young individuals (23.4 ± 1.4 years). All values measured were within normal ranges, and CRP levels were <3 mg/L for all participants, thereby excluding individuals with a recent infection or inflammatory conditions. Since 90 % of cellular cholesterol is located in the outer membrane, we measured the total free cholesterol content in the membrane and in the lipid rafts (LRs) of T cells from young and elderly subjects. The LR cholesterol content in CD4+ and CD8+ T cells was significantly increased in elderly subjects (Fig. 1a). The cholesterol concentration of CD4+ T cells as measured by HPLC was 32.34 ± 7.09 nmol/l in young subjects and 63.33 ± 7.76 nmol/l in elderly subjects (p < 0.0001). In the case of CD8+ T cells, the cholesterol content was 38.67 ± 7.37 nmol/l for young subjects and 70.67 ± 8.59 nmol/l for elderly subjects (p < 0.001). Similar results were obtained in our analyses of the total cell membrane (Larbi et al. 2006a). These increases in membrane cholesterol had functional consequences, since we observed a significant decrease in membrane fluidity in T cells from elderly individuals (p < 0.05; Fig. 1b). The membrane fluidity (1/r) observed in CD4+ T cells was 5.51 ± 0.23 for young individuals and 4.56 ± 0.14 for elderly individuals. In the case of CD8+ T cells, membrane fluidity was 5.59 ± 0.19 for young subjects and 4.73 ± 0.15 for elderly subjects. Thus, altered cell metabolism leading to increased membrane cholesterol was associated with decreased fluidity, suggesting that age-related changes in the lipid composition of the T cell membrane may have functional consequences in terms of host protection.
Fig. 1.
Age-related alterations in T cell membrane cholesterol exchange. The cholesterol content (a) as well as the fluidity (b) of T cells from young (white bars) and elderly individuals (black bars) is shown. Significant difference is displayed with ***p < 0.0001 and **p < 0.001. The ability of T cells to uptake and release cholesterol at steady state was tested by using radiolabeled cholesterol. T cells from young individuals were able to better uptake cholesterol from the extracellular milieu (c, *p < 0.05) and also to release it faster (24 h) that T cells from elderly individuals (d, *p < 0.05). Cholesterol efflux (radiolabeled cholesterol released from cells) was calculated using the following formula: (radioactivity (cpm) in supernatant / radioactivity (cpm) in cells + medium) × 100. The cholesterol influx was measured by determining the percentage of radiolabeled cholesterol incorporated (% cholesterol influx) using the following formula: (cpm in the cell / cpm in the cell + medium) × 100
We next measured cholesterol turnover by assessing both the cholesterol uptake (influx) by T cells when incubated with 3H-cholesterol-enriched LDL and cholesterol efflux from T cells pre-loaded with 3H-cholesterol and incubated with HDL. When compared with T cells from young subjects, the uptake of 3H-cholesterol (expressed in %) was significantly reduced in T cells obtained from elderly subjects (elderly, 32.02 ± 4.50; young, 47.83 ± 4.10; Fig. 1c). In parallel, the percentage of 3H-cholesterol initially loaded in cells but detected in the culture media after 24 h (cholesterol efflux) was significantly higher in the case of young individuals (Fig. 1d). T cells from elderly individuals released the radiolabeled cholesterol only after 48 h. The ratio of T cell cholesterol efflux at 24 h relative to that observed at 48 h was 8.8 in the case of young subjects and 1.5 in the case of elderly subjects. These findings suggested that T cell cholesterol efflux slows down or becomes inefficient/passive with aging, contributing to an increase in the total level of T cell cholesterol.
High membrane cholesterol content is associated with altered T cell phenotype and function
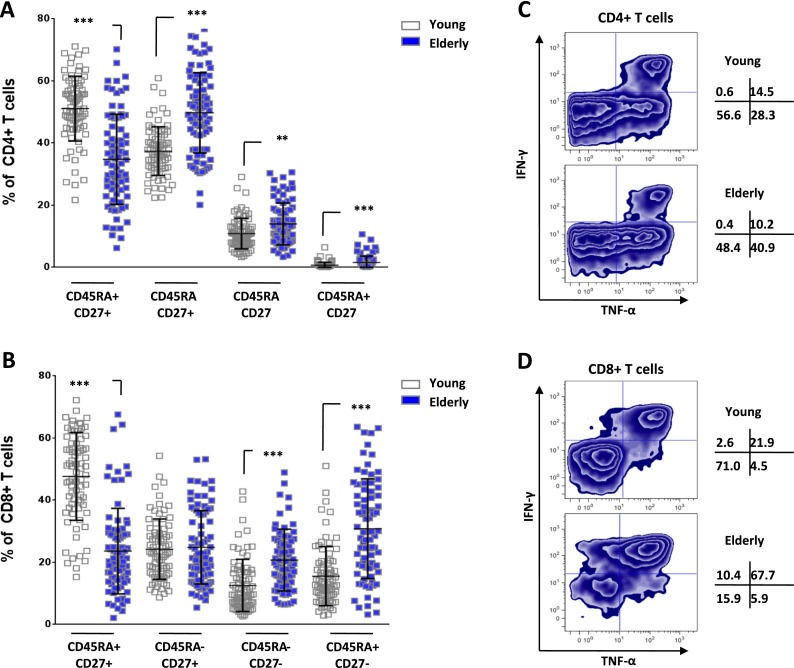
In order to assess whether the high cholesterol content detected in T cells from aged subjects was associated with altered cell function, we next investigated the phenotype of CD4+ and CD8+ T cells obtained from both young and elderly individuals. Peripheral blood mononuclear cells were separated and stained with directly conjugated antibodies against CD3, CD4, CD8, CD27, and CD45RA. Cell populations are classified using the accepted definition of naïve (CD45RA+CD27+), central memory (CD45RA−CD27+), effector memory (CD45RA−CD27−), and terminally differentiated (CD45RA+CD27−) in T cells (Di Mitri et al. 2011). In these analyses, the frequencies of all populations were significantly different for the CD4+ T cells. The reduced frequency of naïve T cells was accompanied with increased frequency of central memory, effector memory, and late differentiated T cells with aging (Fig. 2a). Similarly, the frequency of CD45RA + -CD27- and CD45RA-CD27- cells in the CD8+ compartment was also significantly increased in the elderly compared with that in young individuals (p < 0.0001; Fig. 2b). The frequency of naïve CD8+ T cells was significantly lower in elderly, but no change was observed for central memory cells. The CD8+ compartment displays more marked changed toward the most differentiated subsets. We next investigated the function of CD4+ and CD8+ T cells by stimulating PBMC with PMA/ionomycin in the presence of Brefeldin A for 4 h before assessing intracellular levels of TNF-α and IFN-γ by flow cytometry. In Fig. 2c, we display a representative experiment showing that the capacity of CD4+ T cells to produce cytokines was not reduced in elderly subjects. In contrast, the frequency of CD8+ T cells that were unable to support cytokine responses was in fact lower in elderly individuals (15.9 %) than in the young population (71.0 %) suggesting that CD8+ T cells may actually increase their capacity to produce cytokines with aging (Fig. 2d). This finding is consistent with the changes in T cell phenotype typically observed with aging (increase in memory cells capable of producing cytotoxic cytokines). Most of TNF-α+IFN-γ+ cells were lacking expression of co-receptors (CD28 and CD27) while only a minute fraction of the responding cells show a naïve profile (data not shown) as expected from the literature. Taken together, these data confirm that age-related changes occur in CD4+ and CD8+ T cell phenotypes, cytokine producing capacity, and membrane cholesterol content.
Fig. 2.
Impact of aging on T cell phenotype and function. PBMCs were separated from blood collected from young (n = 15) and elderly (n = 15) individuals. Cells were stained for CD3, CD4, CD8, CD27, and CD45RA expression using directly conjugated antibodies and analyzed by flow cytometry. a The CD4+ T cell subset distribution (based on CD45RA and CD27 expression) of young (empty bars) and elderly individuals (filled bars) is shown with significant differences (**p < 0.01 and ***p < 0.0001). b The same analysis and significance were reported for the CD8+ population. c PBMCs were stimulated with PMA/ionomycin for 4 h and stained for surface CD3, CD4, CD8, and intracellular TNF-α and IFN-γ. We provide a representative plot of CD4+ T cells from young and elderly individuals. d The same analysis was performed for CD8+ T cells on the same donors as in c. Numbers shown represent the distribution of TNF-α and IFN-γ producing cells
HDL-mediated cholesterol efflux from T cells
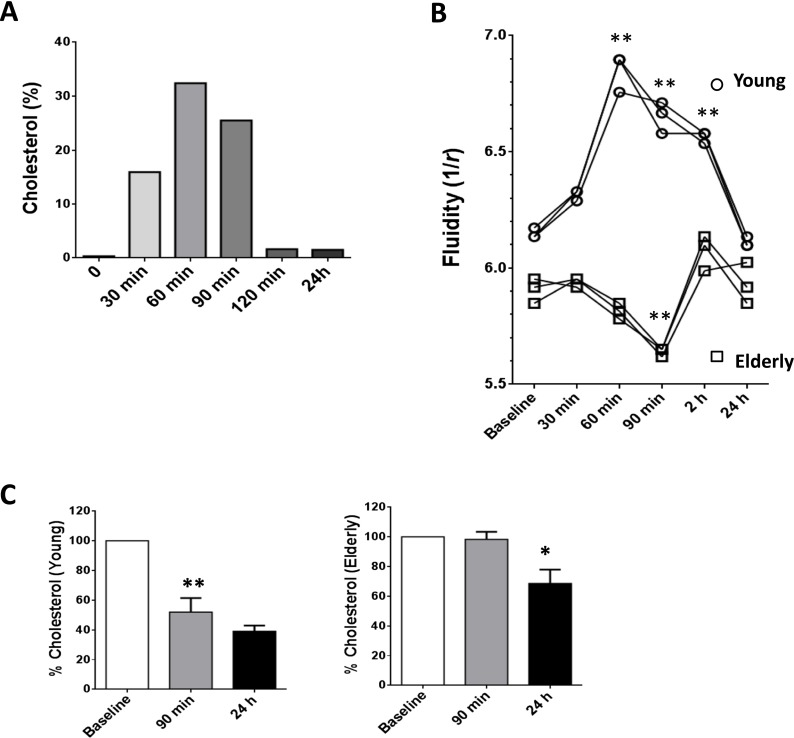
Cholesterol efflux occurs via three independent pathways: (1) aqueous diffusion, (2) nonspecific efflux via SR-BI receptors, and (3) specific efflux via cholesterol-responsive members of the ABC superfamily. Whereas aqueous diffusion and scavenger receptor class B, type I (SR-BI)-mediated efflux transport free cholesterol to a wide variety of cholesterol acceptors (particles containing phospholipids, HDL, and lipidated apo-lipoproteins; LDL, etc.), the ABCA1 pathway mediates the transport of cholesterol in a unidirectional manner, mainly to lipid-poor apoA-I. In contrast, the ABCG1 pathway is responsible for the transport of cholesterol to all the subfamily members of HDL. Cholesterol efflux to HDL is the first rate-limiting step of reverse cholesterol transport, a process that contributes to maintain of cholesterol homeostasis. Thus, we investigated the efficiency of HDL purified from either young or elderly subjects, to mediate extraction of cholesterol from the membrane LRs, on age-matched allogeneic T cells. As shown in Fig. 3a, HDL incubated for 60 min with T cells showed the highest efficiency for cholesterol extraction when compared to other incubation times tested (Fig. 3a). After 90 min, HDL influx/efflux appeared to have been equilibrated, suggesting that HDL extraction of membrane cholesterol occurs rapidly in T cells. We used this time point (90 min) as the basis for the following experiments. We hypothesized that HDL extraction of membrane cholesterol from allogeneic T cells was less efficient if the HDL was obtained from elderly subjects. We were therefore interested to investigate whether T cell cholesterol extraction by HDL from elderly subjects had functional repercussions. Indeed, the membrane fluidity of T cells obtained from young individuals was significantly increased after 60-min exposure to HDL (p < 0.01) and returned to basal levels after 24h (Fig. 3b) confirming a reduction in membrane cholesterol levels as suggested in Fig. 3a. Mean membrane fluidity rose from 6.15 to a maximum of 6.85 by 60 min after exposure to HDL, which represented an ∼11 % increase in T cell membrane fluidity in T cells from young individuals. HDL from elderly subjects were unable to increase the membrane fluidity of allogeneic T cells obtained from other elderly subjects. We also observed a transient yet significant decrease in T cell membrane fluidity during the 90-min period after HDL treatment of T cells from elderly subjects (**, p < 0.01, Fig. 3b). While T cells from young subjects recovered membrane fluidity after 24h, T cells from elderly subjects exhibited fluctuations in membrane fluidity over the time course tested here. These data confirmed that HDL from elderly subjects are unable to support efficient cholesterol efflux (Berrougui et al. 2007) and may contribute to functional alterations in the T cell membrane.
Fig. 3.
Aging affects HDL-dependent cholesterol efflux in T cells. a T cells were incubated with HDL for increasing periods of time, and the amount of cholesterol extracted from the membrane of T cells is displayed. b Membrane fluidity expressed by anisotropy (r) was tested for the same experimental conditions as in a with three different donors in each group. c We confirmed that membrane fluidity alterations were due to cholesterol extraction. The fraction of cholesterol extracted over time is expressed in percentage of the initial labeled cholesterol content loaded with significant differences between elderly and young with *p < 0.05 and **p < 0.01
We next compared the capacity of HDL from young and elderly subjects to extract 3H-cholesterol from pre-loaded T cells. We observed that HDL purified from young subjects efficiently extracted cholesterol from T cell membranes via cholesterol efflux (52.0 ± 9.5 % 3H-cholesterol remaining in the membrane at 90-min time point; p < 0.01, Fig. 3c, left panel) whereas no significant difference was observed in membrane cholesterol content between 90 min and 24 h (p = 0.0951). In sharp contrast, HDL from elderly subjects was unable to extract cholesterol within the same 90-min period (97.3 ± 4.3 % 3H-cholesterol remaining in the membrane, i = 0.7649), and significant HDL extraction of membrane cholesterol was observed only after 24 h (fraction of 3H-cholesterol in the membrane decreased from 97.3 to 68.7 % after 24 h; p < 0.05, Fig. 3c, right panel). These data suggest that the reverse cholesterol transport (RCT) function of HDL is inefficient in elderly subjects and is unable to extract cholesterol from cell membranes except over extended periods of time.
Effect of HDL on T cell function
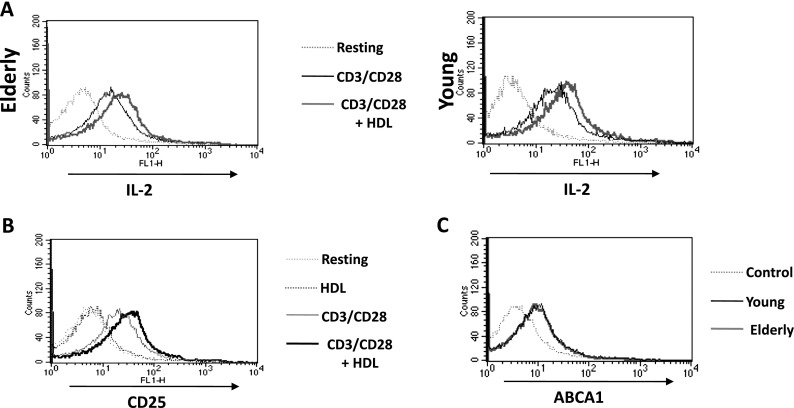
We next studied whether HDL influences T cell functions. We measured T cell production of IL-2, which is known to be impaired with aging (Larbi et al. 2006a). First, intracellular IL-2 production was assessed by flow cytometry in the whole CD3+ T cell population. Intracellular levels of IL-2 were measured after 12-h stimulation with anti-CD3/CD28 either with or without 50 μg/ml HDL. A representative experiment is shown demonstrating the expected higher level of IL-2 production in T cells from young subjects (MFI 50.4 ± 6.2) compared with T cells from elderly subjects (MFI 34.9 ± 5.4, p < 0.05). HDL treatment significantly increased the intracellular production of IL-2 in T cells from young subjects (MFI increased from 50.4 ± 6.2 to 83.5 ± 8.7; p < 0.01; Fig. 4a). HDL treatment also significantly increased the intracellular production of IL-2 in T cells from elderly subjects (MFI increased from 34.9 ± 5.4 to 52.7 ± 10.8; p < 0.05). It is notable that αCD3αCD28 activated T cells pre-treated with HDL increased IL-2 production by stimulated T cells from elderly donors to levels comparable with those of T cells from young donors stimulated with αCD3αCD28 alone. We next investigated the expression of CD25 as an activation marker of T cells and observed that HDL from young subjects significantly increased CD25 expression of αCD3αCD28-activated T cells (MFI increased from 48.9 ± 7.3 to 68.1 ± 7.5; p < 0.05). It is of note that HDL alone did not have any discernable effect on CD25 expression (Fig. 4b). Moreover, there was no significant difference in T cell expression of ABCA1 (the most important receptor involved in HDL and ApoA1-mediated RCT) when comparing between elderly subjects and young subjects (ABCA1 MFI 21.6 in the young population, 19.8 in the elderly population; Fig. 4c). There were no significant differences between the expression of SR-BI in T cells of young and elderly subjects (data not shown).
Fig. 4.
Immunomodulatory role of HDL. a T cells from elderly and young individuals were tested for IL-2 production in response to anti-CD3/CD28 stimulation with/without HDL by Flow Cytometry. The fluorescent intensity for IL-2 is displayed for CD3+ gated cells. Values in the flow plots represent mean fluorescence intensities that should represent the level of expression of the marker tested. b Expression of CD25 (activation marker) was also monitored under the same conditions, as well as c ABCA1 expression in T cells from young and elderly individuals
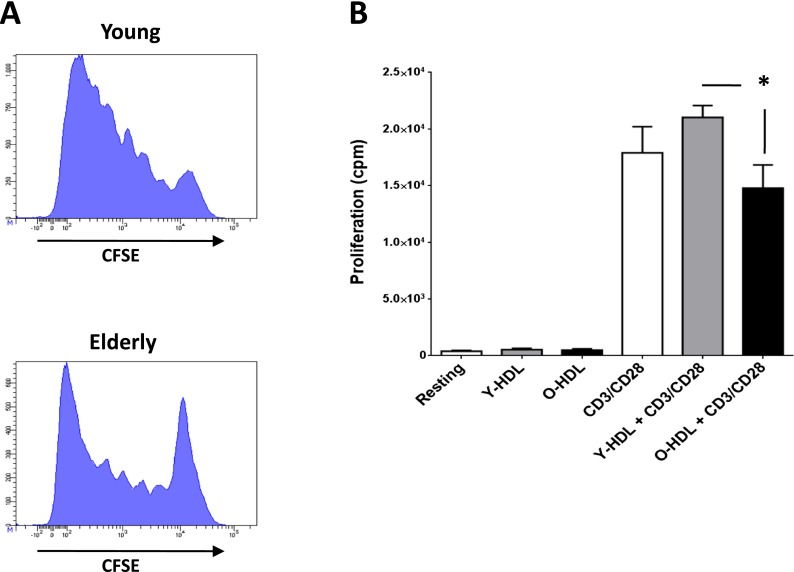
Since our data indicated possible HDL immunomodulatory effects on T cells from elderly subjects in respect of impaired IL-2 production, we next studied the effect of HDL on T cell proliferation. It is well known that the proliferative capacity of T cells is decreased in the elderly compared with young subjects (Douziech et al. 2002), and we were able to confirm the reduced proliferative activity of these cells in our analysis of total CD3+ T cells stimulated with αCD3αCD28 for 5 days (Fig. 5a). The CFSE dilution assay shows that many more T cells in the elderly are not able to divide while T cells from young individuals exhibit strong proliferative capacity. When added in the absence of stimulation, HDL from either young or elderly individuals did not induce any proliferation in T cells (Fig. 5b). When added in conjunction with stimulation, HDLs from young individuals mildly improve (≈+18 %) the proliferation of T cells while HDLs from the elderly mildly affect the proliferation (≈−17 %) of T cells in response to CD3/CD28 stimulation, but this is not significant. However, there was a significant reduced proliferative response (≈−30 %) in T cells from elderly compared to young subjects in presence of the corresponding HDL (p < 0.02). This suggests that the qualitative and quantitative composition of HDL may impact on key T cell functions including proliferation (Berrougui and Khalil 2009).
Fig. 5.
Impact of aging and HDL on T cell proliferation. a As aging has been associated with loss of functionalities, we tested the proliferative capacity of CD3+ T cells from young and elderly individuals and show the CFSE dilution assay of a typical experiment. PBMCs were stimulated with αCD3αCD28 antibodies for 5 days and stained for CD3 before FACS analysis. T cells from young individuals show a high proliferative response that is lower in the case of elderly individuals. b The proliferative response of T cells from elderly individuals following αCD3αCD28 stimulation was also tested in the presence of HDL as a putative immunomodulatory agent. Proliferation was tested by addition on 3H-thymide for the last 4 h of the proliferation assay and represented as counts per minute (cpm). The only significant difference observed (*p < 0.05) is the difference between T cells treated with HDL from young versus old individuals
Effect of HDL on TCR-mediated signaling
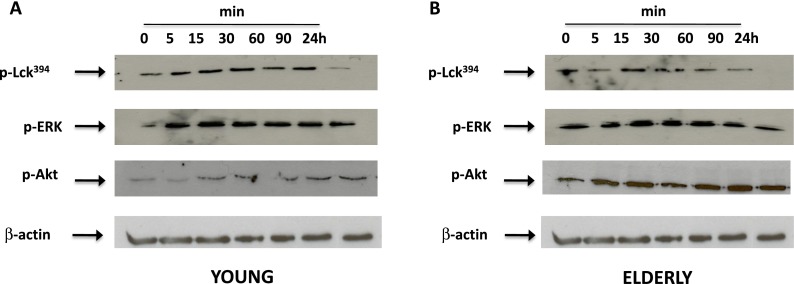
We next assessed whether the effects of HDL on T cell function were mediated at the TCR/CD28 signaling level. We have already shown that the activation of Lck, ERK, and Akt in T cells is altered with aging (Fülöp et al. 2012; Larbi et al. 2004b). We therefore investigated whether the phosphorylation status of these key signaling molecules can be modulated by HDL to explain the functional effects described above. T cells were pre-incubated with HDL for 90 min and were then stimulated with CD3/CD28 for various periods of time (Fig. 6). Western blot studies showed that exposure to HDL did not alter the activation kinetics of the early signaling molecules in T cells from young subjects (Fig. 6a), although HDL did increase the overall duration of signaling molecule phosphorylation. It has been reported that Lck, ERK, and Akt activation kinetics peak between 30 s and 30 min following CD3/CD28 stimulation. The activation of pAkt was prolonged up to 90 min in T cells pre-treated with HDL while it may normally last up to 30/60 min only based on the literature. Lck phosphorylation is one of the earliest events of TCR signaling cascade and may occur in the first seconds/minutes of TCR ligation. We observe its prolonged phosphorylation status induced by HDL, which may strongly influence the signaling cross talks that occur during the TCR/CD28 signaling cascade. The prolonged activation status of Lck and Akt suggests a stronger interference with the CD28-dependent signaling pathway. At a basal level, the phosphorylation status of Lck, ERK, and Akt was higher in T cells from elderly subjects compared with that from young subjects, which was in accordance with the Inflamm-Aging model reported to characterize elderly subjects and resembling data already reported for pLck (Larbi et al. 2006a). In the case of the elderly population, the modulating effect of HDL was not significant for Lck since the phosphorylation status of this molecule returned to initial levels within just 30 min. The effect of HDL on ERK and Akt phosphorylation kinetics was less clear for elderly individuals. The high basal level of signaling molecule phosphorylation in the elderly cohort hindered our assessment of the modulatory effects of HDL as observed in young individuals. Nonetheless, we still observed a significant upregulation of p-Akt levels in HDL-treated T cells from elderly subjects after CD3/CD28 stimulation (Fig. 6b).
Fig. 6.
Immunomodulatory role of HDL at the signaling level. T cells from young and elderly individuals were reincubated with 50 μg/ml corresponding HDL for the indicated time points and tested by Western blotting for the phosphorylation status of key TcR/CD28 signaling molecules. The phosphorylation of Lck, ERK1/2, and Akt was tested in young (a) and elderly individuals (b)
Discussion
The present study demonstrates that aging is associated with altered cholesterol metabolism in T cells, resulting in increased cholesterol levels in lipid rafts and decreased membrane fluidity. These changes were further accompanied by age-related changes in T cell subset distribution. The natural shift from naive cells to memory cells is a result of exposure to a vast numbers of different antigens during one’s lifespan. In elderly individuals, these exposures are reflected in the altered proliferative capacity and cytokine responsiveness of the various T cell subsets. Currently, we do not know whether the increased cholesterol level detected in T cells from elderly subjects is primarily a feature of memory cells or whether this is a more general age-related phenomenon that occurs throughout the T cell compartment. In this connection, we have recently shown that the altered signaling is general T cell feature and not related to the increased memory cell proportion with aging (Le Page et al. 2014).
All donors recruited for our study were normolipemic (Table 1) with no history of lipid dysfunction. Previous studies have shown that middle-aged individuals may display intermediate frequencies of memory cells, but we did not observe any significant increase in cholesterol levels in T cells from healthy, middle-aged individuals (35–50 years, data not shown). High-density lipoproteins (HDLs) via apolipoproteins (apoA-1) are responsible for the reverse cholesterol transport activity that allows maintaining cholesterol homeostasis. This process is initiated by the cholesterol efflux from cells (Khalil et al. 2012). HDL has been extensively shown to confer protection against atherosclerotic cardiovascular disease. In addition, HDL’s influence on apoA-1, component lipids, and key enzymes (PON1) can modulate endothelial functions and exert anti-oxidant and anti-inflammatory effects (Khera et al. 2011). It is also clear that by modulating cellular cholesterol levels, HDL plays an important modulatory role in innate and cellular immunity (Perrin-Cocon et al. 2012; Wang et al. 2012). Emerging evidence suggests that the anti-inflammatory and modulatory effects of HDL may in fact extend beyond their capacity to mediate cholesterol efflux to involve additional mechanisms (Norata et al. 2011). It is also an open question as to whether the quantity or the quality of HDL is the most important factor in determining immunological outcomes. We have used HDL from young and elderly individuals and T cells loaded with radiolabeled [3H]-cholesterol to test the impact of HDL on various cellular functions. We identified that incubation with HDL for 60 min yielded significant cholesterol extraction from T cell membranes. After 90-min exposure to HDL, the T cells did not display any distinct morphological changes or alterations in membrane character, and we cannot exclude that HDL alters the composition of membrane rafts. Our results confirm that HDL is able to extract the cholesterol from the membrane/LRs of T cells from young subjects, thereby modulating membrane fluidity, whereas this was not the case for T cells from elderly subjects, besides the SR-BI and ABCA1 expressions did not change with aging. This suggests that in elderly subjects, HDL is unable to mediate cholesterol efflux from T cells and this contributes to the increased membrane rigidity. It could be speculated then that the HDL apolipoprotein composition as well as the functional status of these apolipoproteins is altered with aging such as changes in the composition and structure of HDL, especially the phosphatidylcholine/sphingomyelin ratio, the fluidity of the phospholipidic layer, the concentration of apoA-I, and the activity of PON1 (Berrougui and Khalil 2009; Khalil et al. 2012). This contention is supported by our previous studies in which an alteration in HDL-driven cholesterol efflux was identified in macrophages from aged individuals (Khalil et al. 2012). Since HDLs were shown to be anti-oxidant and exert anti-inflammatory effects in addition to mediating cholesterol efflux, we assessed whether HDL could be used to modulate T cell production of IL-2 and potentially reverse the decline in cytokine output observed in aged subjects. Our data did not allow us to draw unequivocal conclusions as to which pathways might be influenced by HDL to alter T cell function; however, the possibility that HDL permits sustained cell signaling may play a role, especially via modulation of CD28 co-receptor signaling (as underlined by the increased IL-2 production we observed in T cells from elderly subjects).
We previously attempted to modulate the levels of plasma membrane cholesterol in T cells from elderly subjects to determine whether this in vitro manipulation could reestablish efficient T cell signaling/functions. This intervention has been termed the “rejuvenation approach.” Initial experiments using the cholesterol sequestering compound methyl β-cyclodextrin (MBCD) did not yield the expected results (Larbi et al. 2004c). It was observed that, besides decreasing plasma membrane cholesterol, MBCD had an independent signal-disrupting activity. We also used inhibitors of cholesterol synthesis (lovastatin) in Jurkat T cells. In this case, significantly reduced cell growth and induced apoptosis were observed at high concentrations, in agreement with the fact that only a few studies have demonstrated the immunomodulatory role of statins in human T cells (Larbi et al. 2006b). Alternatively, we attempted to “age” T cells from young subjects by increasing the cholesterol content. Mimicking this phenomenon in vitro by increasing the cholesterol content in T cells from young donors to the levels found in T cells from elderly subjects led to a decreased proliferative response as well as impaired IL-2 secretion, suggesting that homeostatic concentrations of plasma membrane cholesterol are important for T cell activation (Fülöp et al. 2012).
Based on our data, we suggest that the modulation of T cell functions by HDL is more closely related to its qualitative and quantitative composition (including apoA1 and PON1 content) rather than to its cholesterol-modulating role (Jaouad et al. 2006). We have previously used HDL to modulate the “immunosenescent” attributes of T cells, using IL-2 production as a marker of overall functionality (since IL-2 output was previously found to be constantly changing with age). This approach is in-line with the concept that HDL has anti-inflammatory and modulatory effects that can influence innate immunity by interfering with TLR activation, decreasing antigen presentation, and modulating TCR signaling. Furthermore, levels of HDL are notably decreased in immune-mediated disorders such as rheumatoid arthritis and systemic lupus erythematosis (Borba et al. 2006). Furthermore, the HDL physico-chemical structure is altered in acute inflammation and in chronic inflammatory diseases (Norata et al. 2012) as we have demonstrated in aging (Jaouad et al. 2006); the ApoA1 is displaced, and inflammatory molecules such as serum amyloid A are increased, thus profoundly altering the immune-modulating capacities of the parent HDL (Coetzee et al. 1986). These data are further supported by our finding that HDL from elderly subjects mainly tends to decrease the proliferation of T cells compared with HDL from young subjects. However, it is unclear which component parts of HDL are responsible for this ability to modify the function of immunosenescent T cells. Our results do not permit us to unequivocally conclude that HDL alters TCR and CD28 signaling to augment T cell functions in the elderly, but it seems likely that HDL influences how signaling is sustained in activated T cells. Having already demonstrated an alteration in phosphatase activities associated with aging (Fortin et al. 2006), we also measured the effects of HDL on phosphatase activities in T cells obtained from young and elderly subjects, and we found significant age-related differences in the early signaling pathways (manuscript in preparation). We cannot completely exclude the possibility that HDL is able to modulate cholesterol at the nanoscale level, but the ability of HDL to influence T cell functions is clear.
Cholesterol is a fundamental component of the cell membrane and more specifically of the nanoscale microdomains, designated as lipid rafts (LRs). LRs are very important mediators of many essential cell functions including signaling and protein transport and adhesion. We show here that the cholesterol metabolism is altered in T cells from elderly subjects compared to young subjects, since both uptake and efflux were significantly decreased in the elderly, resulting in an accumulation of cholesterol in the T cell membrane and LRs. The buildup of membrane cholesterol leads to decreased fluidity, which is absolutely essential for LRs to coalesce into the signalosome in order to form the immune synapse. This suggests that manipulation of membrane lipid composition by agents such as HDL or others could be an efficient and natural means of influencing the function of many different cell types. Further studies will be necessary to understand the possible role of HDL in regulating membrane raft composition. Ultimately, because of the high bio-availability of HDL, the implication of these findings is that HDL may alter many different cellular functions in parallel, including (i) cell-cell communication, (ii) regulation of cell activation, (iii) regulation of circulating lipid levels, (iv) the secretory phenotype of cells, (v) tight junction stability, (vi) interaction with the extracellular matrix, and (vii) higher lipid content in tissues such as muscles and the thymus. Further studies will be needed to unravel the molecular mechanisms underlying the HDL effect on altered T cell functions in the elderly, and to elucidate the correlation between age-dependent changes in HDL composition and immune dysfunction. These investigations may eventually lead to the development of lipid-based immunotherapies or adjuvants that can be used to boost host protection in the elderly. In particular, it is important to point out that the present study cannot determine if the effect observed is specific for a particular subset of T cells (Fülöp et al. 2013) even if our recent published data suggest that the CD45RA or CD45RO T cell subpopulations cannot explain changes in the signal transduction of T cells with aging (Le Page et al. 2014). Nevertheless, the data provide support for an important role for HDL in the altered response of T cells in aging, revealing additional clues to unravel the underlying mechanisms of immunosenescence which are under current investigations in our laboratories especially addressing this question in T cell subpopulations.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (No. 106634 and No. 106701), the Université de Sherbrooke, the Research Center on Aging, and the Agency for Science Technology and Research (A*STAR), Singapore. Anis Larbi is part of the ISAC Scholar program. The manuscript was edited by Neil McCarthy of Insight Editing London.
Competing interests
The authors declare that they do not have any competing interests.
Author contributions
Conceived and designed the experiments: AL, AK, GD, TF. Performed the experiments: CF, AL, HB. Analyzed the data and wrote the manuscript: TF, AL, GD, AK.
Contributor Information
Anis Larbi, Email: Anis_Larbi@immunol.a-star.edu.sg.
Tamas Fulop, Email: tamas.fulop@usherbrooke.ca.
References
- Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Ann N Y Acad Sci. 2012;1254:18–32. doi: 10.1111/j.1749-6632.2012.06480.x. [DOI] [PubMed] [Google Scholar]
- Berrougui H, Khalil A. Age-associated decrease of high-density lipoprotein-mediated reverse cholesterol transport activity. Rejuvenation Res. 2009;12:117–26. doi: 10.1089/rej.2009.0840. [DOI] [PubMed] [Google Scholar]
- Berrougui H, Isabelle M, Cloutier M, Grenier G, Khalil A. Age-related impairment of HDL-mediated cholesterol efflux. J Lipid Res. 2007;48:328–36. doi: 10.1194/jlr.M600167-JLR200. [DOI] [PubMed] [Google Scholar]
- Berrougui H, Grenier G, Loued S, Drouin G, Khalil A. A new insight into resveratrol as an atheroprotective compound: inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis. 2009;207:420–7. doi: 10.1016/j.atherosclerosis.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Borba EF, Carvalho JF, Bonfá E. Mechanisms of dyslipoproteinemias in systemic lupus erythematosus. Clin Dev Immunol. 2006;13:203–208. doi: 10.1080/17402520600876945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpintero R, Gruaz L, Brandt KJ, Scan A, Faill D, Combes V, Grau GE, Burger D. HDL interfere with the binding of T cell microparticles to human monocytes to inhibit pro-inflammatory cytokine production. PLoS One. 2010;5:e11869. doi: 10.1371/journal.pone.0011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- de Mello CV, Nguyen D, Giri B, Bunbury A, Schaffer E, Taub DD. Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol. 2004;5:2. doi: 10.1186/1471-2172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, Dupuis G, Fulop T. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 2002;37:369–87. doi: 10.1016/S0531-5565(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79:1061–72. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fülöp T, Gagné D, Goulet AC, Desgeorges S, Lacombe G, Arcand M, Dupuis G. Age-related impairment of p56lck and ZAP-70 activities in human T lymphocytes activated through the TcR/CD3 complex. Exp Gerontol. 1999;34:197–216. doi: 10.1016/S0531-5565(98)00061-8. [DOI] [PubMed] [Google Scholar]
- Fülöp T, Douziech N, Goulet AC, Desgeorges S, Linteau A, Lacombe G, Dupuis G. Cyclodextrin modulation of T lymphocyte signal transduction with aging. Mech Ageing Dev. 2001;122:1413–1430. doi: 10.1016/S0047-6374(01)00274-3. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- Fülöp T, Le Page A, Garneau H, Azimi N, Baehl S, Dupuis G, Pawelec G, Larbi A (2012) Aging, immunosenescence and membrane rafts: the lipid connection. Longevity and Healthspan. MS ID : 1582891234741859 [DOI] [PMC free article] [PubMed]
- Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Curr Opin Immunol. 2014;29C:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells—a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruaz L, Delucinge-Vivier C, Descombes P, Dayer JM, Burger D. Blockade of T cell contact-activation of human monocytes by high-density lipoproteins reveals a new pattern of cytokine and inflammatory genes. PLoS One. 2010;5:e9418. doi: 10.1371/journal.pone.0009418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaouad L, de Guise C, Berrougui H, Cloutier M, Isabelle M, Fulop T, Payette H, Khalil A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydryl groups. Atherosclerosis. 2006;185:191–200. doi: 10.1016/j.atherosclerosis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kabouridis PS. Lipid rafts in T cell receptor signalling. Mol Membr Biol. 2006;23:49–57. doi: 10.1080/09687860500453673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanidis E, Addis PB. Novel HPLC analysis of tocopherols, tocotrienols, and cholesterol in tissue. Free Radic Biol Med. 1999;27:1137–1140. doi: 10.1016/S0891-5849(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Khalil A, Berrougui H, Pawelec G, Fulop T. Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL anti-inflammatory activity in Alzheimer’s disease. Mech Ageing Dev. 2012;133:20–29. doi: 10.1016/j.mad.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J Biol Chem. 2006;281:36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- Larbi A, Dupuis G, Douziech N, Khalil A, Fulop T. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. Ann NY Acad Sci. 2004;2004(1030):125–133. doi: 10.1196/annals.1329.016. [DOI] [PubMed] [Google Scholar]
- Larbi A, Douziech N, Dupuis G, Khalil A, Pelletier Guerard KP, Fülöp T. Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukoc Biol. 2004;75:373–381. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- Larbi A, Douziech N, Khalil A, Dupuis G, Gheraïri S, Guérard KP, Fülöp T. Effects of methyl-beta-cyclodextrin on T lymphocytes lipid rafts with aging. Exp Gerontol. 2004;39:551–558. doi: 10.1016/j.exger.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Larbi A, Dupuis G, Khalil A, Douziech N, Fortin C, Fülöp T. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18:1017–1030. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Larbi A, Muti E, Giacconi R, Mocchegiani E, Fülöp T. Role of lipid rafts in activation-induced cell death: the fas pathway in aging. Adv Exp Med Biol. 2006;584:137–155. doi: 10.1007/0-387-34132-3_11. [DOI] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Larbi A, Rymkiewicz P, Asudev A, Low I, Shadan BN, Mustafah S, et al. The immune system in the elderly: a fair fight against diseases. Aging Health. 2013;9:1–13. doi: 10.2217/ahe.12.78. [DOI] [Google Scholar]
- Le Page A, Fortin C, Garneau H, Allard N, Tsvetkova K, Tan CT, Larbi A, Dupuis G, Fülöp T. Downregulation of inhibitory SRC Homology 2 Domain-containing Phosphatase-1 (SHP-1) leads to recovery of T cell responses in elderly. Cell Commun Signal 9. 2014;12(1):2. doi: 10.1186/1478-811X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighart GH. The SENIEUR protocol after 16 years: the next step is to study the interaction of ageing and disease. Mech Ageing Dev. 2001;122:136–140. doi: 10.1016/S0047-6374(00)00242-6. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczek C, Bock G, Jurgens G, Schonitzer D, Dietrich H, Wick G. Environmental influence on age-related changes of human lymphocyte membrane viscosity using severe combined immunodeficiency mice as an in vivo model. Exp Gerontol. 1998;33:485–498. doi: 10.1016/S0531-5565(98)00011-4. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, Chin-Dusting JP. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Catapano AL. HDLs, immunity, and atherosclerosis. Curr Opin Lipidol. 2011;22:410–416. doi: 10.1097/MOL.0b013e32834adac3. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Park KH, Cho KH. High-density lipoprotein (HDL) from elderly and reconstituted HDL containing glycated apolipoproteins A-I share proatherosclerotic and prosenescent properties with increased cholesterol influx. J Gerontol A Biol Sci Med Sci. 2011;66:511–520. doi: 10.1093/gerona/glr016. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immun Ageing. 2012;9:15. doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin-Cocon L, Diaz O, Carreras M, Dollet S, Guironnet-Paquet A, André P, Lotteau V. High-density lipoprotein phospholipids interfere with dendritic cell Th1 functional maturation. Immunobiology. 2012;217:91–99. doi: 10.1016/j.imbio.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Rea IM, McKeown PP, McMaster D, Young IS, Patterson C, Savage MJ, Belton C, Marchegiani F, Olivieri F, Bonafe M, Franceschi C. Paraoxonase polymorphisms PON1 192 and 55 and longevity in Italian centenarians and Irish nonagenarians. A pooled analysis. Exp Gerontol. 2004;39:629–35. doi: 10.1016/j.exger.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Reichardt P, Dornbach B, Gunzer M. APC, T cells, and the immune synapse. Curr Top Microbiol Immunol. 2010;340:229–249. doi: 10.1007/978-3-642-03858-7_12. [DOI] [PubMed] [Google Scholar]
- Sattler W, Mohr D, Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol. 1994;233:469–489. doi: 10.1016/S0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nature Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Yuan SG, Peng DQ, Zhao SP. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 2012;225:105–114. doi: 10.1016/j.atherosclerosis.2012.07.029. [DOI] [PubMed] [Google Scholar]