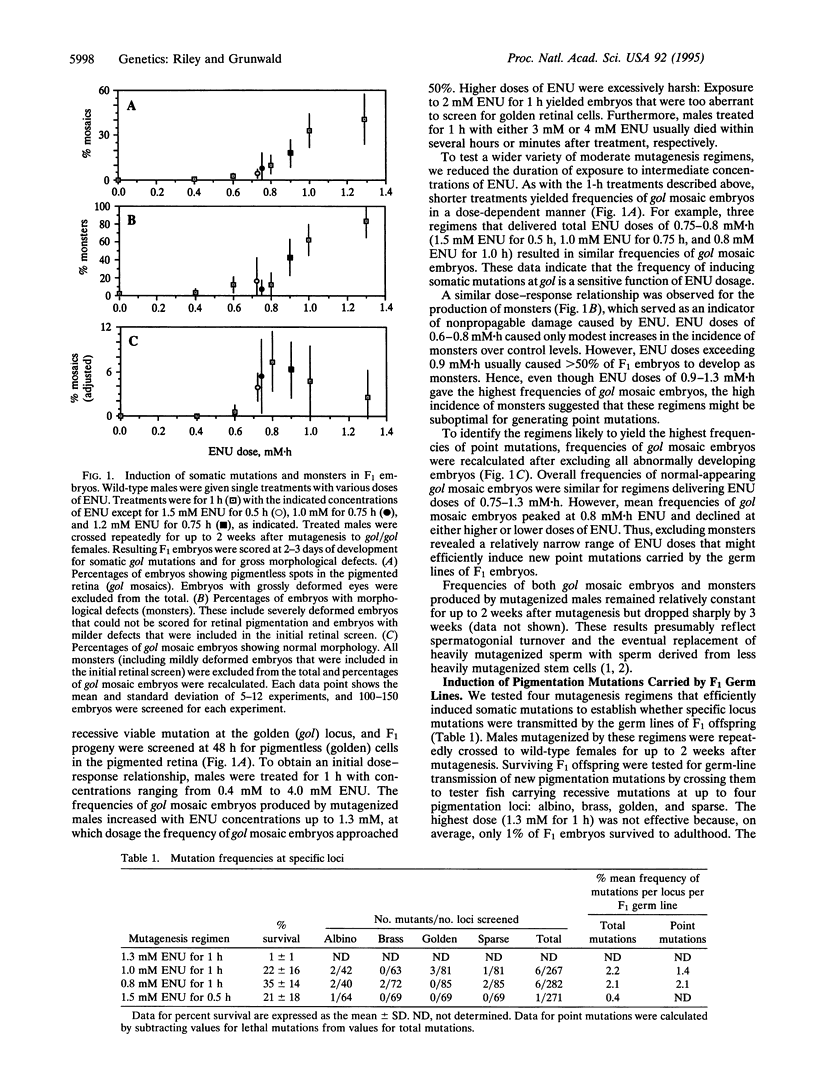
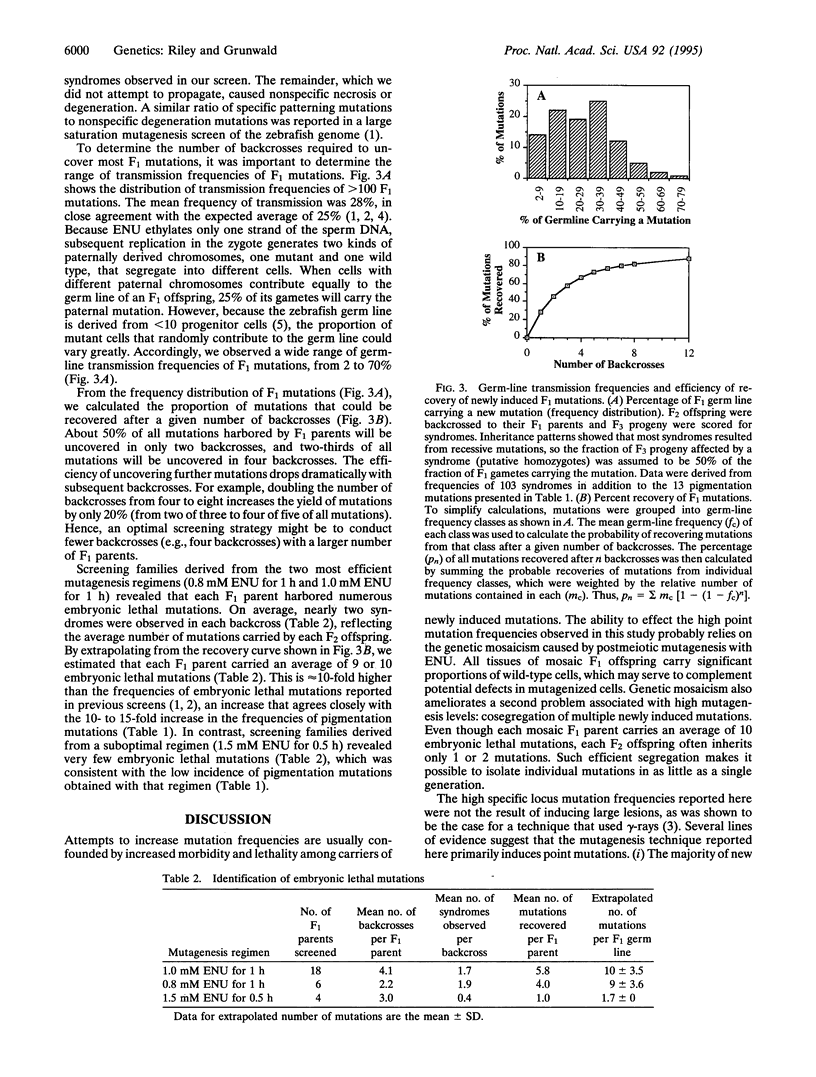
Abstract
A technique is described that greatly increases the efficiency of recovering specific locus point mutations in zebrafish (Danio rerio). Founder individuals that were mosaic for point mutations were produced by mutagenizing postmeiotic gametes with the alkylating agent N-ethyl-N-nitrosourea. Under optimal conditions, each founder carried an average of 10 mutations affecting genes required for embryogenesis. Moreover, approximately 2% of these founders transmitted new mutations at any prespecified pigmentation locus. Analyses of new pigmentation mutations confirmed that most were likely to be point mutations. Thus, mutagenesis of postmeiotic gametes with N-ethyl-N-nitrosourea yielded frequencies of point mutations at specific loci that were 10- to 15-fold higher than previously achieved in zebrafish. Our procedure should, therefore, greatly facilitate recovery of multiple mutant alleles at any locus of interest.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroian R. V., Sternberg P. W. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics. 1991 Jun;128(2):251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J., Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987 Nov 20;51(4):589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beddington R. S., Rashbass P., Wilson V. Brachyury--a gene affecting mouse gastrulation and early organogenesis. Dev Suppl. 1992:157–165. [PubMed] [Google Scholar]
- Beitel G. J., Clark S. G., Horvitz H. R. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990 Dec 6;348(6301):503–509. doi: 10.1038/348503a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Streisinger G., Singer F., Walker C. Frequency of gamma-Ray Induced Specific Locus and Recessive Lethal Mutations in Mature Germ Cells of the Zebrafish, BRACHYDANIO RERIO. Genetics. 1983 Jan;103(1):109–123. doi: 10.1093/genetics/103.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R. J., Schüpbach T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics. 1989 Dec;123(4):771–787. doi: 10.1093/genetics/123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. The Drosophila sex determination signal: how do flies count to two? Trends Genet. 1993 Nov;9(11):385–390. doi: 10.1016/0168-9525(93)90138-8. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Wieschaus E. Dosage requirements for runt in the segmentation of Drosophila embryos. Cell. 1986 Apr 25;45(2):289–299. doi: 10.1016/0092-8674(86)90393-4. [DOI] [PubMed] [Google Scholar]
- Gertler F. B., Bennett R. L., Clark M. J., Hoffmann F. M. Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell. 1989 Jul 14;58(1):103–113. doi: 10.1016/0092-8674(89)90407-8. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Streisinger G. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res. 1992 Apr;59(2):103–116. doi: 10.1017/s0016672300030317. [DOI] [PubMed] [Google Scholar]
- Han M., Aroian R. V., Sternberg P. W. The let-60 locus controls the switch between vulval and nonvulval cell fates in Caenorhabditis elegans. Genetics. 1990 Dec;126(4):899–913. doi: 10.1093/genetics/126.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkemeyer M., West S. R., Gertler F. B., Hoffmann F. M. A novel tyrosine kinase-independent function of Drosophila abl correlates with proper subcellular localization. Cell. 1990 Nov 30;63(5):949–960. doi: 10.1016/0092-8674(90)90498-4. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Haffter P., Nüsslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994 Mar 1;4(3):189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Priess J. R., Schnabel H., Schnabel R. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell. 1987 Nov 20;51(4):601–611. doi: 10.1016/0092-8674(87)90129-2. [DOI] [PubMed] [Google Scholar]
- Rogge R. D., Karlovich C. A., Banerjee U. Genetic dissection of a neurodevelopmental pathway: Son of sevenless functions downstream of the sevenless and EGF receptor tyrosine kinases. Cell. 1991 Jan 11;64(1):39–48. doi: 10.1016/0092-8674(91)90207-f. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Schier A. F., Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994 Apr;136(4):1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Coale F., Taggart C., Walker C., Grunwald D. J. Clonal origins of cells in the pigmented retina of the zebrafish eye. Dev Biol. 1989 Jan;131(1):60–69. doi: 10.1016/s0012-1606(89)80038-7. [DOI] [PubMed] [Google Scholar]
- Walker C., Streisinger G. Induction of Mutations by gamma-Rays in Pregonial Germ Cells of Zebrafish Embryos. Genetics. 1983 Jan;103(1):125–136. doi: 10.1093/genetics/103.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]




