Abstract
Bacillus thuringiensis (Bt) is popularly known as insecticidal bacterium. However, non-insecticidal Bt strains are more extensively available in natural environment than the insecticidal ones. Parasporin (PS) is a collection of genealogically heterogeneous Cry proteins synthesized in non-insecticidal isolates of Bt. An important character generally related with PS proteins is their strong cytocidal activity preferentially on human cancer cells of various origins. Identification and characterization of novel parasporin protein which are non-hemolytic and non-insecticidal but having selective anticancer activity raise the possibility of a novel application of Bt in medical field. In the present study, seven new indigenous isolates (T6, T37, T68, T98, T165, T186, and T461) of Bt showed variation in colony morphology, crystal characters and protein profiles with each other. Out of the seven new isolates screened for parasporin (ps) and cry genes, two of the new indigenous isolates (T98 and T186) of Bt showed the presence of ps4 gene. Partial ps4 gene was cloned from the two new isolates and the sequence of partial ps4 gene showed high homology with its holotype ps4Aa1. These two isolates were characterized based on the proteolytic processing of the inclusion proteins and the proteolytic products were found to be comparable to the PS4 reference strain A1470. The two isolates of Bt did not show toxicity toward Spodoptera litura and Helicoverpa armigera. Based on the results of this study, it can be concluded that the isolates T98 and T186 are parasporin producers.
Keywords: Bacillus thuringiensis, Parasporin, δ-endotoxin, Non-insecticidal inclusions, Cytocidal protein
Introduction
Bacillus thuringiensis (Bt) is an aerobic gram-positive and endospore-forming bacterium, first isolated in Japan from diseased larvae of the silkworm, Bombyx mori, as an entomopathogenic bacterium (Ishiwata 1901). It produces large crystalline parasporal inclusions in sporangia during sporulation (stationary phase of its growth cycle). This character is used to discriminate two taxonomically closely related species, B. thuringiensis and B. cereus (Logan 2005; Ohba et al. 2009). The parasporal inclusions often contain δ-endotoxin proteins that are specifically toxic to agriculturally and medically important insect pests of several orders, including Lepidoptera, Diptera, and Coleoptera (Beegle and Yamamoto 1992) and to even nematodes, mites, and protozoa (de Maagd et al. 2001), but are not pathogenic to mammals, birds, amphibians, or reptiles (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/) (Schnepf et al. 1998). This makes B. thuringiensis, a promising microbial agent in the control of insect pests in agriculture, forestry, veterinary, and public health management (Schnepf et al. 1998).
Meanwhile, non-insecticidal B. thuringiensis strains are ubiquitous in natural environments and are more widely distributed than insecticidal ones (Ohba 1996). It is remarkable that the non-insecticidal isolates frequently account for more than 90 % of the natural populations from soils (Ohba et al. 2002; Yasutake et al. 2007; Mizuki et al. 1999a, b). This raises the query whether non-insecticidal inclusions have any biological activity which is yet to be undiscovered (Ohba et al. 1988). An extensive effort to screen Cry proteins for biological activity other than insecticidal toxicity was initiated in 1996. This led to the discovery of a unique activity, which is preferential for certain human cancer cells (Mizuki et al. 1999a). The protein was first categorized and defined as bacterial parasporal proteins and these proteins are non-hemolytic but cytocidal to human cancer cells (Mizuki et al. 1999a, 2000). Globally, six different parasporin types, PS1–PS6 have been identified in countries, viz. Japan, Vietnam, India, Canada, and Caribbean Islands (Gonzalez et al. 2011) and classified by the Committee of Parasporin Classification and Nomenclature (http://parasporin.fitc.pref.fukuoka.jp/list.html). In view of potential application of these proteins, this study was undertaken to characterize new isolates of Bt collected from Western Ghats, India, based on colony and crystal morphology, protein profile, screening for presence of cry or parasporin genes by PCR and insect bioassay.
Materials and methods
Bacterial strains and plasmids
The bacterial strains used in this study were B. thuringiensis soil isolates (T6, T37, T68, T98, T165, T186, and T461) from Western Ghats of Tamil Nadu State, India (Ramalakshmi and Udayasuriyan 2010), and maintained in the Department of Plant Biotechnology, CPMB&B, Tamil Nadu Agricultural University, Coimbatore. The reference strains for parasporin (ps) genes, A1190 (ps1), A1547 (ps2), A1462 (ps3), and A1470 (ps4), provided by Dr. Natsuko Kurata, Biotechnology and Food Research Institute, Fukuoka Industrial Technology Centre, Japan, were used in this study. Bt strains, HD1 (cry1 and cry2) and 4Q7 (acrystalliferous) were used as reference strains. Escherichia coli (DH5α) was used as a host for cloning the gene. The vector, pTZ57R/T (Fermentas Inc., Canada) was used to clone parasporin gene fragments amplified from new isolates of Bt. The antibiotic concentration used for selection of E. coli transformants was 100 μg/ml of ampicillin.
Culture conditions
Bacillus thuringiensis culture was grown on T3 medium (Martin and Travers 1989) at 30 °C at 200 rpm for 2–8 days and the bacterial sporulation was monitored through phase contrast microscope for 2–8 days. E. coli was grown on LB medium for 24 h at 37 °C at 200 rpm.
Characterization of isolates for colony and crystal morphology
The B. thuringiensis isolates streaked on T3 agar plates were incubated at 30 °C for 2–8 days. Colony morphology was studied on single colonies developed on T3 agar plates. The Bt isolates inoculated in 5 ml of T3 broth were incubated at 30 °C at 200 rpm for 2–8 days, and the bacterial sporulation was monitored through phase contrast microscope at 100×. After about 90 % of cell lysis, a smear of 10 μl lysed culture was made on glass slide and heat fixed. After heat fixing, drops of the Coomassie Brilliant Blue stain (0.133 % Coomassie Brilliant Blue G250 in 50 % acetic acid) were added and kept as such for 1 min. Then, the smear was washed gently in running tap water. After blot drying with blotting paper, the stained cultures were observed through bright field microscopy for presence of crystalline inclusions (Ramalakshmi and Udayasuriyan 2010).
Preparation of inclusion proteins
The spore–crystal mixture was isolated from seven new isolates of Bt and reference strain A1470, as described by Lenin et al. (2001). Single colony of Bt strains was inoculated into 5 ml T3 broth and incubated in a rotary shaker, maintained at 30 °C at 200 rpm for 2–8 days, and the bacterial sporulation was monitored through phase contrast microscope. When more than 90 % of cells were lysed, the sporulated broth culture was transferred to 4 °C, at least half-an-hour before harvesting. The T3 broth containing spore–crystal mixture was centrifuged for 10 min at 10,000 rpm at 4 °C. The pellet was washed once with 5 ml of ice-cold 1× Tris–EDTA buffer [Tris 10 mM, EDTA 1 mM, pH 8.0 with 1 mM phenyl methyl sulphonyl fluoride (PMSF)], once with 5 ml of ice-cold 0.5 M NaCl followed by two more washes with 5 ml of Tris–EDTA buffer containing 1 mM PMSF by centrifuging at the same speed and time. Finally, the spore–crystal pellet was suspended in 100 μl of sterile distilled water containing 1 mM PMSF and stored at −20 °C.
Screening of parasporin and cry genes
Screening of the test isolates for ps and cry genes was carried out in a 25-μl PCR reaction. Total genomic DNA isolated from Bt strains using Genei pure bacterial DNA purification kit (Genei, Bangalore, India) was used as template for PCR screening. The PCR was accomplished using an Eppendorf thermal cycler with a reaction mixture containing 50–100 ng of total genomic DNA of Bt, 1× PCR buffer (10 mM Tris–HCl; pH 9.0, 50 mM KCl, 1.5 mM MgCl2), 75 μM each of dNTPs, 50 ng each of forward and reverse primers (Table 1) and 1.5 U of Taq DNA polymerase.
Table 1.
Primers used for screening of Bt isolates for different cry and ps genes
| Primer sequences | Annealing °C | Gene | Amplicon size (bp) | Primer position in ORF | Reference | |
|---|---|---|---|---|---|---|
| FP | RP | |||||
| F: CATGATTCATGCGGCAGATAAAC | 62 | cry1 | 278 | 2,783 | 3,060 | Ben-Dov et al. (1997) |
| R: TTGTGACACTTCTGCTTCCCATT | ||||||
| F: GTTATTCTTAATGCAGATGAATGGG | 64 | cry2 | 702 | 570 | 1,271 | Ben-Dov et al. (1997) |
| R: CGGATAAAATAATCTGGGAAATAGT | ||||||
| F: ATCAAGAATTTTCCGATAATC | 50 | ps1 | 1,136 | 154 | 1,289 | Yasutake et al. (2007) |
| R: CCAAAAGTGCCAGAATG | ||||||
| F: TGTTGGGACTGTTCAGTACGT | 56 | ps2 | 503 | 341 | 843 | * |
| R: CGTCACGGTACCTCTTAGTGT | ||||||
| F: GGAATCCAGGTGCACTGCT | 67 | ps3 | 701 | 264 | 964 | * |
| R: GTCCCGGATCATACGTTGGA | ||||||
| F: AGTGGTCTCCAGGCTCATACTGG | 59 | ps4 | 681 | 81 | 761 | * |
| R: TGATATTCCCGAACCTGCCCT | ||||||
* Designed in this study using Fast PCR 6.0
Template DNA was preheated at 94 °C for 2 min. Then it was denatured at 94 °C for 1 min, annealed to primers for 45 s and extensions of PCR products were achieved at 72 °C for 1 min. The PCR was performed for 30 cycles. The PCR products were analyzed on a 1.2 % agarose gel. Amplified product was ligated in pTZ57R/T PCR cloning kit and transformed into E. coli DH5α.
Proteolytic processing of inclusion proteins
Spore–crystal mixture isolated from parasporin producing isolate was washed thrice with 1 M NaCl and resuspended in sterile water and transferred to a microfuge tube. After centrifugation at 13,000 rpm for 5 min at 4 °C, the pellet containing purified inclusions was solubilized in 50 mM Na2CO3 (pH 10.0) containing 1 mM EDTA and 10 mM dithiothreitol for 1 h at 37 °C (200 μl/25 mg pellet). After centrifugation at 13,000 rpm for 5 min at 4 °C, the supernatant was passed through 0.2-μm filter to remove unsolublized materials. The pH of the filtrate was adjusted to 8.0 and split into two equal aliquots. One of the aliquots of solubilized proteins was treated with proteinase K (final conc. 60 μg/ml), in 50 mM Na2CO3 (pH 10.0) for 90 min at 37 °C. After proteinase K treatment, 1 mM PMSF was added to the mixture to stop the proteolytic reaction. Both the aliquots (solubilized and proteinase K-treated inclusions) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (Okumura et al. 2006; Saitoh et al. 2006).
Toxicity analysis of new isolates
The laboratory cultures of S. litura and H. armigera reared on a semi-synthetic diet (Patel et al. 1968) were used to determine the insecticidal activity of the isolates T98 and T186 using diet surface contamination method. Approximately 1 ml of the semi-synthetic diet was dispensed into 1.8 ml cryovials (Tarson®; 1 cm dia.) and allowed to cool for an hour. After solidification of the diet, 10 μl spore–crystal mixture was coated on the diet surface and allowed to air dry for 30 min. Neonate larvae of S. litura and H. armigera were released using a soft hairbrush and the tube closed with a screw cap. All the above steps were carried out in a laminar airflow chamber. Vials without crystal mixture served as a control. Each treatment was replicated four times and ten vials were maintained for each replication. Larval mortality was recorded for 7 days and subjected to ANOVA. All the experiments were carried out in a room with a photoperiod of 14:10 (L:D) at an average temperature of 27 °C and 60 % relative humidity.
Results and discussion
Bacillus thuringiensis formed white rough colonies which spread out and expanded over the plate quickly. Seven new isolates of Bt and six reference strains were observed for colony morphology on T3 plates. All the seven isolates produced creamy white colonies after 24 h of inoculation on T3 agar plates. The colony characteristics of test isolates showed slight variation with each other (Table 2). Chaterjee et al. (2006) also found similar variation in the morphological characteristics of Bt isolates on nutrient agar medium and reported circular, white, flat, and undulate colonies of the Bt isolates of West Bengal, India. The time taken for 90 % cell lysis in T3 broth was also observed for the new isolates along with the reference strains. The reference strains, HD1 and 4Q7 took 2–3 days, while the parasporin reference strains and the seven new isolates took 6–8 days for 90 % of cell lysis.
Table 2.
Morphological characteristics of new isolates
| Isolate | Color of colonies | Shape of colony | Margin of colony | Elevation of colony | Shape of inclusion |
|---|---|---|---|---|---|
| T6 | Creamy white | Irregular | Undulated | Raised | Spherical |
| T37 | Creamy white | Circular | Entire | Raised | Irregular |
| T68 | Creamy white | Circular | Entire | Raised | Spherical |
| T98 | Creamy white | Irregular | Undulated | Flat | Irregular |
| T165 | Creamy white | Irregular | Undulated | Flat | Spherical |
| T186 | Creamy white | Circular | Entire | Raised | Irregular |
| T461 | Creamy white | Irregular | Undulated | Raised | Spherical |
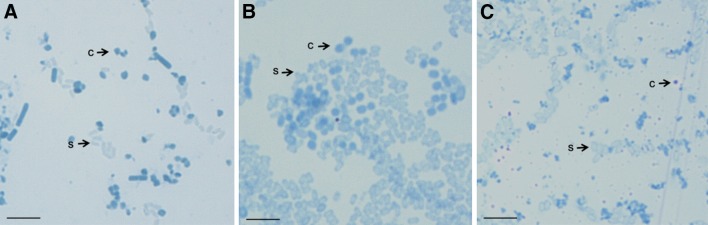
Morphology of the parasporal inclusion bodies of Bt was reported to be heterogeneous (Ohba et al. 2001). Crystal morphology of Bt isolates are of cuboidal, spherical, rhomboidal, and irregular shapes (Bernhard et al. 1997). However, four distinct crystal morphologies are apparent; the bipyramidal crystals are related to Cry1 proteins (Aronson and Fritz-James 1976), cuboidal inclusions related to Cry2 proteins and usually associated with bipyramidal crystals (Ohba and Aizawa 1986); square crystals related to Cry3 proteins (Herrnstand et al. 1986; Lopez-Meza and Ibarra 1996); amorphous and composite crystals related to Cry4 and Cyt proteins (Federici et al. 1990). There is a striking correlation between shape of crystal and spectrum of toxicity (Chambers et al. 1991; Ramalakshmi and Udayasuriyan 2010). Recent reports show parasporin protein inclusions which do not have insecticidal properties also exhibit variation in crystal morphology. The crystal morphology of parasporin producers varies from spherical, bipyramidal to irregular (Kitada et al. 2006). In the present study, four new isolates (T6, T68, T165, and T461) showed spherical inclusions. The isolates T37, T98, and T186 showed irregular-shaped inclusions (Table 2). The crystal shape of the isolates T98 and T186 is similar to the reference strain of ps4, A1470 (Fig. 1). Variation in crystal morphology may indicate the diversity of crystal proteins in isolates (Schnepf et al. 1998; Rampersad and Ammons 2005; Ibarra et al. 2003).
Fig. 1.
Bright field microscopic observation of crystal morphology from parasporin producing Bt isolates. a Parasporin reference strain A1470. b, c Bt isolates T98 and T186, respectively. c crystal, s spore. Scale bar 20 μm
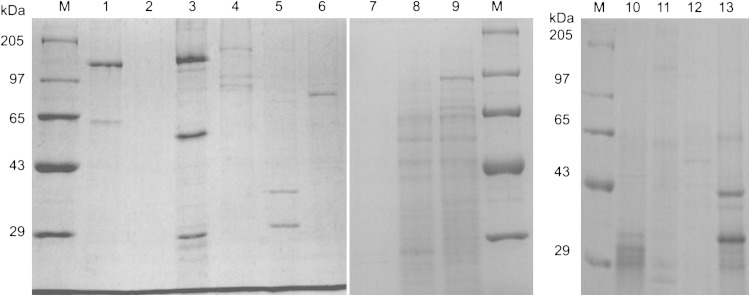
Grouping of Bt isolates according to crystal protein(s) profile analyzed through SDS-PAGE will give a prelude for the presence of diversity in cry and ps genes. Crystal protein profile of the seven new isolates, reference strains for Cry1, Cry2 (HD1), reference strains for PS1–PS4 (A1190, A1547, A1462, and A1470) and the acrystalliferous reference strain (4Q7) were compared. The reference strain HD1 showed a prominent 135-kDa protein of cry1 gene and 65-kDa protein of cry2. The Bt strain HD1 was included as one of the reference strains, even though it is known to be toxic to lepidopteran insects (Hofte and Whiteley 1989), to observe whether the new isolates produced distinct protein similar to that of HD1 or not. The acrystalliferous reference strain of Bt 4Q7 did not show prominent protein bands as reported earlier (Schnepf et al. 1985; Adang et al. 1985; Widner and Whiteley 1989). The reference strains of ps1, ps2, ps3, and ps4 showed various sized proteins ranging from 29 to 140 kDa as reported earlier workers (Mizuki et al. 2000; Kim et al. 2000; Yamashita et al. 2005; Okumura et al. 2004). All the new isolates had different protein profile when compared to reference strains (Fig. 2). Protein profile of the test isolates T68, T461, and T98 showed prominent multiple bands, whereas the isolates T37, T165, and T186 showed faint multiple bands. The new isolate T6 did not show any prominent protein band. All the new isolates and reference strains of Bt differed from each other suggesting that the parasporin and crystal proteins of the new isolates could be the novel one.
Fig. 2.
SDS-PAGE analysis of spore–crystal mixture of Bt strains. M Genei Protein marker (Higher Range #105977). Lanes 1–2 reference strain HD1 and 4Q7. Lanes 3–6 reference strains of parasporin (PS4, PS3, PS2 and PS1). Lanes 7–13 Bt isolates, T6, T37, T68, T461, T165, T186 and T98
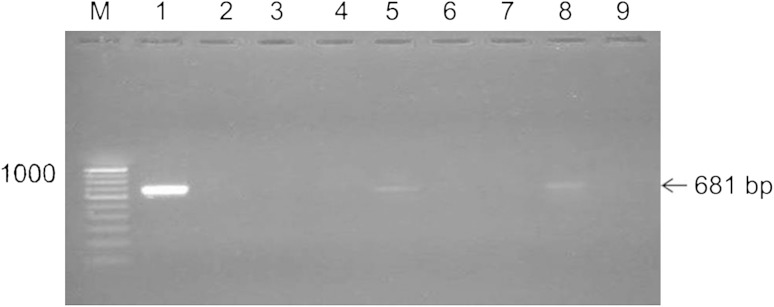
Among several methods available for characterization of Bt strains, such as PCR, RFLP, Southern blot analysis, and bioassay (Kronstad and Whiteley 1986), PCR is rapid and highly sensitive method for detecting and identifying novel Bt genes. Carrozi et al. (1991) proposed PCR as an accurate and rapid method for identification of novel strains with unknown crystal producing genes. The efficacy of PCR for cry genes and ps genes identification relies on the alternation of conserved and variable nucleotide regions. All the seven new isolates of Bt were screened for the presence of cry genes (cry1 and cry2) and ps genes (ps1, ps2, ps3, and ps4) by PCR. Primers specific for cry1 and cry2 family genes gave amplification of expected size in the reference strain HD1 only. None of the seven new isolates gave amplification to both these gene families, indicating the absence of cry1 and cry2 family genes. Primers specific for ps1, ps2, and ps3 genes gave amplification of expected sizes in the respective reference strains of Bt only. Primers specific to ps4 gene gave amplification of expected size in the reference strain (A1470) and two new isolates, T98 and T186 (Table 3; Fig. 3). This result suggested the presence of ps4 gene(s) in the two new isolates.
Table 3.
PCR screening of new isolates of Bt for cry and ps genes
| Isolate | cry1 | cry2 | ps1 | ps2 | ps3 | ps4 |
|---|---|---|---|---|---|---|
| T6 | – | – | * | * | – | – |
| T37 | – | – | – | * | – | – |
| T68 | – | – | – | – | – | – |
| T98 | – | – | * | – | – | + |
| T165 | – | – | – | – | – | – |
| T186 | – | – | – | * | * | + |
| T461 | – | – | – | – | – | – |
+ Present, − absent
* Unexpected size
Fig. 3.
Amplification of ps4 gene from the test isolates of Bt. M 100 bp ladder. Lane 1 Reference strain of ps4 A1470. Lanes 2–8 Bt isolates, T6, T37, T68, T461, T165, T186 and T98. Lanes 9 water control
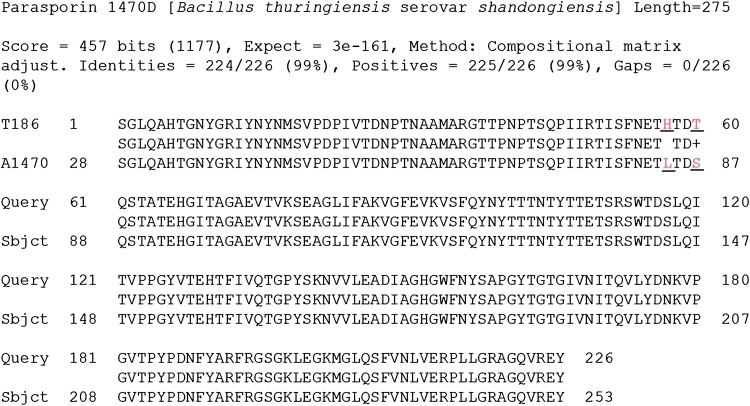
The partial ps4 gene (681 bp) fragment amplified by gene-specific primers from the new isolates T98 and T186 were cloned into pTZ57R/T (T/A) cloning vector. The transformants of E. coli were screened by PCR. The nucleotide sequence from the positive clone was generated from Eurofins Genomics India Pvt. Ltd., Bangalore. Sequence similarity analysis of nucleotide sequences of the partial ps4 gene (681 bp) cloned from the new isolates T98 (KC832499) and T186 (KC832500) with that of ps4Aa1 showed 100 and 99 % homology, respectively. Comparison of deduced amino acid sequence of T186 with that of ps4Aa1 showed variation in two positions. At position 84, leucine is replaced by histidine, and at position 87 serine by threonine (Fig. 4). Thus, the sequence of partial ps4Aa gene cloned from the two new isolates showed high homology with its holotype ps4Aa1. It confirms the presence of ps4Aa type gene in the two isolates, T98 and T186.
Fig. 4.
Comparison of deduced amino acid sequence of T186 and Ps4Aa1
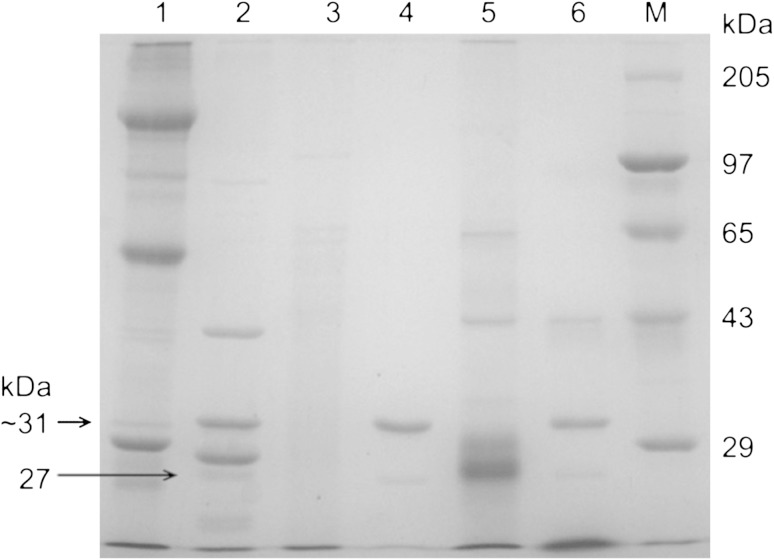
A study on proteolytic processing of crystal proteins from the ps4 harboring isolates T98, T186, and reference strain of ps4 (A1470) by SDS-PAGE, showed a major polypeptide of 40-kDa; two prominent bands: one at >29 kDa and another at <29 kDa; and a faint band at 27-kDa in the solubilized protein of reference strain of ps4 (Fig. 5) as reported earlier (Saitoh et al. 2006). Similar to that of the ps4 reference strain, the proteinase K-treated protein of new isolates (T98, T186) also showed a faint band at 27-kDa and a prominent band of >29 kDa (31-kDa). The protein of PS4 and the proteinase K are of same molecular weight ~31 kDa (Saitoh et al. 2006). Hence the prominent band at 31-kDa in proteolytic processed samples corresponds to proteinase K. As reported earlier, 31-kDa protoxin of PS4 will be digested to a 27-kDa toxin upon proteolytic processing. Therefore, it can be suggested that the faint band of 27-kDa polypeptide in the isolates (T98 and T186) may be proteolytic product of the 31 kDa PS4 protein. This gives the evidence that the test isolates may be parasporin producers. In addition, a prominent band of 43-kDa is also seen in the isolate T98 which discriminates the isolate from T186.
Fig. 5.
Proteolytic processing of inclusion proteins of Bt strains. M Genei Protein marker. Lanes 1, 3, 5 solublized inclusion protein. Lanes 2, 4, 6 proteinase K-treated solubilized protein. Lanes 1, 2 reference strain of ps4 A1470. Lanes 3 and 4, 5 and 6 Bt isolates T186 and T98, respectively
Generally, the parasporin protein producing strains of Bt do not produce any insecticidal protein (Kitada et al. 2006; Mizuki et al. 1999a). The two new isolates of the present study, T98 and T186 (which showed presence of ps4 gene) did not show toxicity on S. litura and H. armigera. Growth inhibition of insect larvae was also not observed in both the new isolates. The reference strains A1470 and 4Q7 also recorded the same results; whereas, the reference strain of cry1 and cry2 genes (HD1) showed 100 % mortality on both S. litura and H. armigera. Mizuki et al. ( 1999a) also reported that PS4 producers do not have insecticidal activity on lepidopteran (Plutella xylostella and Bombyx mori) and dipteran pests (Aedes aegypti, Culex pipiens molestus, Anopheles stephensi, Telmatoscopus albipunctatus, and Musca domestica).
Conclusion
Based on protein profile, PCR screening, nucleotide sequencing and insect bioassay, it is evident that the parasporin producing strains are members in B. thuringiensis populations occurring in natural environments of India. Cloning and characterization of complete gene (ps4) and evaluation of these isolates for their anticancer properties are required for identifying potential use of parasporin proteins in anticancer medical research.
Acknowledgments
We thank Dr. Natsuko Kurata, Biotechnology and Food Research Institute, Fukuoka Industrial Technology Centre, Japan, for providing the reference strains of Bt for parasporin genes.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Adang MJ, Staver MJ, Rocheleau TA, Leighton J, Barker RF, Thompson DV. Characterized full-length and truncated plasmid clones of the crystal protein of Bacillus thuringiensis subsp. kurstaki HD-73 and their toxicity to Manduca sexta. Gene. 1985;36:289–300. doi: 10.1016/0378-1119(85)90184-2. [DOI] [PubMed] [Google Scholar]
- Aronson AI, Fritz-James P. Structures and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976;40:360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beegle CC, Yamamoto T. History of Bacillus thuringiensis Berliner research and development. Can Entomol. 1992;124:587–616. doi: 10.4039/Ent124587-4. [DOI] [Google Scholar]
- Ben-Dov E, Zaritsky A, Dahan E, Barak Z, Sinai R, Manasherob R, Khamraev A, Troitskaya E, Dubitsky A, Berezina N, Margalith Y. Extended screening by PCR for seven cry group genes from field-collected strains of Bacillus thuringiensis. Appl Environ Microbiol. 1997;63:4883–4890. doi: 10.1128/aem.63.12.4883-4890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K, Jarrett P, Meadows M, Butt J, Ellis DJ, Roberts GM, Pauli S, Rodger P, Burges HD. Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization and activity against insect pests. J Invertebr Pathol. 1997;70:59–68. doi: 10.1006/jipa.1997.4669. [DOI] [Google Scholar]
- Carrozi NB, Kramer VC, Warren GW, Evola S, Koziel MG. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl Environ Microbiol. 1991;57:3057–3061. doi: 10.1128/aem.57.11.3057-3061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JA, Jelen MP, Gilbert T, Johnson B, Gawron CB. Isolation and characterization of a novel insecticidal crystal protein gene from Bacillus thuringiensis subsp. aizawai. J Bacteriol. 1991;173:3966–3976. doi: 10.1128/jb.173.13.3966-3976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaterjee SN, Bhattacharya T, Dangar TK, Chandra G. Ecology and diversity of Bacillus thuringiensis in soil environment. Afr J Biotechnol. 2006;6:1587–1591. [Google Scholar]
- de Maagd RA, Bravo A, Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001;17:193–199. doi: 10.1016/S0168-9525(01)02237-5. [DOI] [PubMed] [Google Scholar]
- Federici BA, Lthy P, Ibarra JE. The parasporal body of Bacillus thuringiensis subsp. israelensis: structure, protein composition and toxicity. In: de Barjac H, Sutherland DJ, editors. Bacterial control of mosquitos and blackflies: biochemistry, genetics and applications of Bacillus thuringiensis and Bacillussphaericus. New Brunswick: Rutgers University Press; 1990. pp. 16–44. [Google Scholar]
- Gonzalez E, Granados JC, Short JD, Ammons DR, Rampersad J. Parasporin from a Caribbean Island: evidence for a globally dispersed Bacillus thuringiensis. Curr Microb. 2011;164:3–8. doi: 10.1007/s00284-011-9905-5. [DOI] [PubMed] [Google Scholar]
- Herrnstand C, Soares CG, Wilcox ER, Edwards DI. A new strain of Bacillus thuringiensis with activity against coleopteran insects. Biotechnology. 1986;4:305–308. doi: 10.1038/nbt0486-305. [DOI] [Google Scholar]
- Hofte H, Whiteley HR. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53(2):242. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra JE, Rincon MC, Orduz S, Noriega D, Benintende G, Monnerat R, Regis L, Claudia MF, de Oliveria M, Lanz H, Rodriguez MH, Sanchez J, Pena G, Bravo A. Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Appl Environ Microbiol. 2003;69:5269–5274. doi: 10.1128/AEM.69.9.5269-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S. On a kind of severe flacherie (sotto disease) Dainihon Sanshi Kaiho. 1901;114:1–5. [Google Scholar]
- Kim HS, Yamashita S, Akao T, Saitoh H, Higuchi K, Park YS, Mizuki E, Ohba M. In vitro cytotoxicity of non-Cyt inclusion proteins of a Bacillus thuringiensis isolate against human cells, including cancer cells. J Appl Microbiol. 2000;89:16–23. doi: 10.1046/j.1365-2672.2000.01087.x. [DOI] [PubMed] [Google Scholar]
- Kitada S, Abe Y, Shimada H, Kusaka Y, Matsuo Y, Katayama H, Okumura S, Akao T, Mizuki E, Kuge O, Sasaguri Y, Ohba M, Ito A. Cytocidal actions of parasporin-2, an antitumor crystal toxin from Bacillus thuringiensis. J Biol Chem. 2006;281:26350–26360. doi: 10.1074/jbc.M602589200. [DOI] [PubMed] [Google Scholar]
- Kronstad J, Whiteley HR. Three classes of homologous Bacillus thuringiensis crystal-protein genes. Gene. 1986;43:29–40. doi: 10.1016/0378-1119(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Lenin K, Mariam MA, Udayasuriyan V. Expression of cry2Aa gene in an acrystalliferous Bacillus thuringiensis strain and toxicity of Cry2Aa against H. armigera. World J Microbiol Biotechnol. 2001;1:273–278. doi: 10.1023/A:1016674417728. [DOI] [Google Scholar]
- Logan NA. Bacillus anthracis, Bacillus cereus, and other aerobic endospore-forming bacteria. In: Borriello SP, Murray PR, Funke G, editors. Topley & Wilson’ Microbiology & Microbial Infections. Bacteriology. 10. London: Hodder Arnold; 2005. pp. 922–952. [Google Scholar]
- Lopez-Meza JE, Ibarra JE. Characterization of a novel strain of Bacillus thuringiensis. Appl Environ Microbiol. 1996;62:1306–1310. doi: 10.1128/aem.62.4.1306-1310.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PAW, Travers RS. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol. 1989;55:2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki E, Ohba M, Akao T, Yamashita S, Saitoh H, Park YS. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: in vitro cell killing action on human cancer cells. J Appl Microbiol. 1999;86:477–486. doi: 10.1046/j.1365-2672.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- Mizuki E, Ichimatsu T, Hwang SH, Park YS, Saitoh H, Higuchi K, Ohba M (1999b) Ubiquity of Bacillus thuringiensis on phylloplanes of arboreous and herbaceous plants in Japan. J Appl Microbiol 86:979–984
- Mizuki E, Park YS, Saitoh H, Yamashita S, Akao T, Higuchi K, Ohba M. Parasporin, human leukemic cell-recognizing parasporal protein of Bacillus thuringiensis. Clin Diagn Lab Immunol. 2000;7:625–634. doi: 10.1128/cdli.7.4.625-634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M. Bacillus thuringiensis populations naturally occurring on mulberry leaves: a possible source of the populations associated with silkworm-rearing insectaries. J Appl Microbiol. 1996;80:56–64. [Google Scholar]
- Ohba M, Aizawa K. Distribution of Bacillus thuringiensis in soils of Japan. J Invertebrate Pathol. 1986;47:277–282. doi: 10.1016/0022-2011(86)90097-2. [DOI] [Google Scholar]
- Ohba M, Yu YM, Aizawa K. Occurrence of non-insecticidal Bacillus thuringiensis flagellar serotype 14 in the soil of Japan. Syst Appl Microbiol. 1988;11:85–89. doi: 10.1016/S0723-2020(88)80052-3. [DOI] [Google Scholar]
- Ohba M, Vasano N, Mizuki E. Bacillus thuringiensis soil populations naturally occurring in the Ryukyus, a subtropic region of Japan. Appl Environ Microbiol. 2001;72(2):412–415. doi: 10.1016/S0944-5013(00)80017-8. [DOI] [PubMed] [Google Scholar]
- Ohba M, Tsuchiyama A, Shisa N, Nakashima K, Lee DH, Ohgushi A, Wasano N. Naturally occurring Bacillus thuringiensis in oceanic islands of Japan, Daito-shoto and Ogasawara-shoto. Appl Entomol Zool. 2002;37:477–480. doi: 10.1303/aez.2002.477. [DOI] [Google Scholar]
- Ohba M, Mizuki E, Uemori A. Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res. 2009;29:427–434. [PubMed] [Google Scholar]
- Okumura S, Akao T, Higuchi K, Saitoh H, Mizuki E, Ohba M, Inouye K. Bacillus thuringiensis serovar shandongiensis strain 89-T-34-22 produces multiple cytotoxic proteins with similar molecular masses against human cancer cells. Lett Appl Microbiol. 2004;39:89–92. doi: 10.1111/j.1472-765X.2004.01544.x. [DOI] [PubMed] [Google Scholar]
- Okumura S, Saitoh H, Wasano N, Katayama H, Higuchi K, Mizuki E, Inouye K. Efficient solubilization, activation, and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as inclusion bodies in Escherichia coli. Protein Expr Purif. 2006;47:144–151. doi: 10.1016/j.pep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Patel RC, Patel JK, Patel PB, Singh R. Mass breeding of Heliothis armigera (H.) Indian J Entomol. 1968;30:272–280. [Google Scholar]
- Ramalakshmi A, Udayasuriyan V. Diversity of Bacillus thuringiensis isolated from Western ghats of Tamil Nadu State, India. Curr Microbiol. 2010;61:13–18. doi: 10.1007/s00284-009-9569-6. [DOI] [PubMed] [Google Scholar]
- Rampersad J, Ammons D. A Bacillus thuringiensis isolation method utilizing a novel stain, low selection and high throughput produced typical results. BMC Microbiol. 2005;5:52–63. doi: 10.1186/1471-2180-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H, Okumura S, Ishikawa T, Akao T, Mizuki E, Ohba M. Investigation of a novel Bacillus thuringiensis gene encoding a parasporal protein, parasporin-4, that preferentially kills human leukemic T cells. Biosci Biotechnol Biochem. 2006;12:2935–2941. doi: 10.1271/bbb.60352. [DOI] [PubMed] [Google Scholar]
- Schnepf HE, Wong HC, Whiteley HR. The amino acid sequence of a crystal protein from Bacillus thuringiensis deduced from the DNA base sequence. J Biol Chem. 1985;260:6264–6272. [PubMed] [Google Scholar]
- Schnepf E, Crickmore N, Van rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner WR, Whiteley HR. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. kurstaki possess different host range specificities. J Bacteriol. 1989;171:965–974. doi: 10.1128/jb.171.2.965-974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Katayama H, Saitoh H, Akao T, Park YS, Mizuki E, Ohba M, Ito A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J Biochem. 2005;138:663–672. doi: 10.1093/jb/mvi177. [DOI] [PubMed] [Google Scholar]
- Yasutake K, Uemori A, Kagoshima K, Ohba M. Serological identification and insect toxicity of Bacillus thuringiensis isolated from the island Okinoerabu-jima, Japan. Appl Entomol Zool. 2007;42:285–290. doi: 10.1303/aez.2007.285. [DOI] [Google Scholar]