Abstract
Infectious diseases caused by antimicrobial-resistant microbes (ARMs) and the treatment are the serious problems in the field of medical science today world over. The development of alternative drug line to treat such infectious diseases is urgently required. Researches on ARMs revealed the presence of membrane proteins responsible for effusing the antibiotics from the bacterial cells. Such proteins have successfully been treated by plant-derived antimicrobials (PDAms) synergistically along with the commercially available antibiotics. Such synergistic action usually inhibits the efflux pump. The enhanced activity of plant-derived antimicrobials is being researched and is considered as the future treatment strategy to cure the incurable infections. The present paper reviews the advancement made in the researches on antimicrobial resistance along with the discovery and the development of more active PDAms.
Keywords: Antimicrobial-resistant microbes, Efflux pumps, Antimicrobial resistance, Plant antimicrobial compounds
Introduction
Increasing antimicrobial resistance (AMR) among microbes caused the emergence of new resistant phenotypes and further caused the development of new antimicrobial compounds (Goossens 2013). Infectious diseases caused by antimicrobial-resistant microbes (ARM) have been frequently reported since last few years (Vila and Pal 2010). About 440,000 new cases of multidrug-resistant tuberculosis (MDR-TB) are recorded annually, causing approximately 150,000 deaths all over the world. Recently, a joint meeting of medical societies, the first ever in India was held to tackle the challenges of antimicrobial resistance in developing world (Ghafur 2013). As a result of this conference “Chennai declaration” came into existence, initiating efforts through a national policy to control the rising trend of AMR in India and abroad (Ghafur et al. 2012).
Multidrug-resistant (MDR) microbes are resistant to three or more antibiotics (Styers et al. 2006), however; strains of Mycobacteriumtuberculosis, resistant to virtually all classes of antimicrobials have also been identified in the Kwa Zulu Natal Province of South Africa (Gandhi et al. 2006), a typical example of Extremely Drug-Resistant Tuberculosis (XDR TB) reported in 64 countries to date (World Health Organization 2011). The global emergence of MDRs is increasingly limiting the effectivity of the existing antibiotic drugs (Hancock 2005) for e.g. methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci spp. (Norrby et al. 2005). The development of resistance among the microbes is the result of continuous selection pressure of antibiotics and their surroundings causing genetic alterations (Bush 2004) which, are transferred to the next generation and reach out to the wider range of other geographical regions through the transfer of genetic information exchange between microbes (Amábile-Cuevas 2003) (Table 1 presents the examples of some of the common MDRs). In this review, attempt has been made to understand specific issues such as factors causing resistance, the role of developing world with a quick overview of plant-derived antimicrobials (PDAm) and synergistic compounds as an alternative drug line.
Table 1.
Examples of plasmids carrying integron integrase carrying gene cassettes imparting resistance against antimicrobials
| Plasmid gene cassette | Resistance against | Microbes (isolation) | Conjugative transfer | References |
|---|---|---|---|---|
| pVN84 | MDR | Vibrio spp. | ✓ | Rajpara et al. (2009) |
| MLSB [erm(B) & erm(C)] | Erythromycin | Staphylococcus spp. | ✘ | Schlegelova et al. (2008) |
| grlA or gyr A | Ciprofloxacin | Staphylococcus spp. | ✘ | Campion et al. (2004) |
| pbp2X | β-Lactam | Staphylococcus spp. | ✘ | Coffey et al. (1991) |
| CTX-M | ||||
| aac(6′)-Ib | Aminoglycoside | Klebsiella pneumoniae | ✓ | Soge et al. (2006) |
| emr(B) | Macrolide-lincosamide-streptogramin B | K. pneumoniae | ✓ | |
| pla TEM-1 | Ampicillin | K. pneumoniae | ✓ | |
| dfr | Trimethoprim | K. pneumoniae | ✓ | |
| p3iANG | ||||
| dfrA15 | Trimathoprim | Vibrio cholerae | ✓ | Ceccarelli et al. (2006) |
| bla PI | β-Lactam | V. cholerae | ✓ | |
| qacH | Quaternary ammonia-compounds | V. cholerae | ✓ | |
| aadA8 | aminoglycosides | V. cholerae | ✓ | |
| mecA | Methicillin (MDR) | S. aureus | ✘ | Hiramatsu et al. (2002) |
| qnr (carried on class 1 integron) | Ciprofloxacin | V. Cholerae | ✘ | Fonseca et al. (2008) |
| bla MDL-1 | Carbapenem | Enterobacteriaceae | ✓ | Kumarasamy et al. (2010) |
Factors causing AMR
Microbes comprise 50 % of total living biomass and are well-survived life forms on earth. There exists a sharp distinction between microbes as pathogenic and non-pathogenic although; one-way exchange of genetic elements (Amábile-Cuevas 2003) may confer the pathogenic characters to the non-pathogenic microbe. Pathogenic microbes cause infectious diseases in humans and animals and are treated with antibiotics. Antibiotics also known as antimicrobials are chemical substances, toxic for most of the life forms. Irrational and deliberate use of antibiotics, migration of infected individuals to other communities (Memish et al. 2003), prolonged use of medical health care systems in hospitals, hunger and malnutrition are some of the main causes of the development of resistance against antibiotics in the microbes (Byarugaba 2004; Vila and Pal 2010). Antimicrobial use in veterinary practices especially as food additives is one of the causes of development of AMRs in zoonotics that may spread to humans (Memish et al. 2003) through the food chain. In this connection, reports of Schlegelova et al. (2008) suggest, least chances of spreading of a resistant strain through the dairy products, however; improperly processed raw meat is strongly discouraged for human consumption in developed nations (Threlfall 2002).
Molecular understanding of AMR
Microbes attain resistance very rapidly against most of the currently available antibiotics because of the adaptability feature conferred by plasmids. Table 1 presents the examples of such plasmids carrying integron and gene cassettes in most common MDRs which on transfer, widespread the resistance (Kumarasamy et al. 2010). Gram-negative (Kumarasamy et al. 2010) and Gram-positive bacteria (Grohman et al. 2003) both exhibit conjugative transfer of plasmids, a natural way of horizontal gene transfer for e.g. the horizontal transfer of plasmid in between Vibrio fluvialis and Vibrio cholerae conferring resistance to V. fluvialis (Rajpara et al. 2009). Recent cases of AMR development include Pseudomonasaeruginosa and Acinetobacterbaumannii resistant to nearly all antibiotics including the carbanems (Huang and Hsueh 2008). Antibiotic inactivation (degradation of antibiotics by the microbial enzymes e.g. transferase and β-lactamase) causes resistance in microbes (Wright 2005; Jacoby and Munoz-Price 2005), more than 1,000 such β-lactamases are identified till date (Bush and Fisher 2011). Different antibiotics have different mode of actions, therefore, their use is largely dependent on variety of traits other than resistance (Amábile-Cuevas 2010) which either undergo rapid enzymatic degradation or actively effused by the resistant bacteria. Efflux pump in MDRs was first described by Roberts (1996) for tetracycline and macrolide antibiotics. In general, efflux pumps act through membrane proteins of substrate specificity, effuse the antibiotics from the bacterial cell, resulting in a low intracellular ineffective concentration of the drug (Gibbons 2004; Thorrold et al. 2007) altering the permeability of membrane. In a study, staphylococcal accessory regulator (sarA) was reported to contribute promising role, imparting resistance in S. aureus (Riordan et al. 2006). In addition, Kuete et al. (2011) reported two efflux pumps viz., AcerAB-TolC (Enterobacteriaceae) and MexAB-OprM (Pseudomonasaeruginosa) imparting resistance in Gram-negative bacteria against natural products. AMR is a genetically-modified manifestation, linked to the point mutation in bacterial non-chromosomal DNA. As in case of MRSA, the resistance to methicillin is associated with acquisition of a mobile genetic element, SCCmec, which contains mecA-resistant gene (Okuma et al. 2002). Analytical procedure followed on Escherichia coli showed reversible function of class 1 integron integrase gene machinery under selective pressure (Díaz-Mejía et al. 2008). Similar results were also observed by Hsu et al. (2006) whereby E. coli MDR was found associated with the class 1 integron gene. Detailed mechanism of development of AMR among microbes has been extensively reviewed by Byarugaba (2010).
Developing world: the factory of MDRs
Developing world especially the countries of South East Asia, Western and Central Africa, India and Pakistan are the most vulnerable for various infectious pandemic diseases. Byarugaba (2004) comprehensively reviewed and reported the AMR in developing countries. Several factors are associated with the AMR development including nosocomial infections, unsafe disposal of biomedical waste, inappropriately used antibiotics, self drug abuse, shortfall of antibiotic course and lack of mass awareness of infectious diseases and personal hygiene (Okeke et al. 2005a, b). In addition to these, lack of surveillance data, providing information of microbial infections common to a geographic location and the invasive microbial species have been suggested as the major causes of MDRs development in developing countries (Okeke et al. 2005a, b; Giske and Cornaglia 2010; Kartikeyan et al. 2010; Lalitha et al. 2013). Giske and Cornaglia (2010) emphasized on the surveillance practices especially the monitoring and sampling techniques of invasive microbial isolates. Surveillance of resistance in many developing countries is suboptimal (Okeke et al. 2005b) and unable to present the real picture of infectious diseases and the medication. Recent reports of Lalitha et al. (2013) showed the feasibility of proper surveillance of resistance by carrying experimental surveillance study on the school children in different geographic locations of Indian subcontinent. In India for Salmonellatyphi, MDR has become a norm in strains. This widespread resistant bacterium is associated with contaminated water supply in developing countries and through food products such as contaminated meat in developed countries (Threlfall 2002). Remarkable report of Kumarasamy et al. (2010) provides sufficient evidences in support of the positive role of developing world in the development of ARMs. Resistance to carbapenem conferred by plasmid encoded New Delhi metallo-β-lactamase-1 (blaNDM-1) is a worldwide health problem, especially in UK, (Kumarasamy et al. 2010) having the roots in India and Pakistan. The selective pressure on the bacterial cells is associated with the adaptations causing resistance among microbes for multiple antimicrobials for e.g. genes encoding NDM-1, OXA-23 and OXA-51 enzymes (hydrolyzing specific antibiotics) were observed in three different isolates of Acinetobacter baumannii in India (Kartikeyan et al. 2010). Alterations in gene structure were reported in A. baumannii as a result of selection pressure of antibiotics (Kartikeyan et al. 2010). The literature suggest, substandard surveillance of resistance, non-prescribed antibiotic usage causes huge selection pressure resulting in the development of AMR in developing countries and their suburbs (Byarugaba 2004; Okeke et al. 2005b; Kumarasamy et al. 2010). Figure 1 shows a schematic diagram showing the development of MDR microbe in community.
Fig. 1.
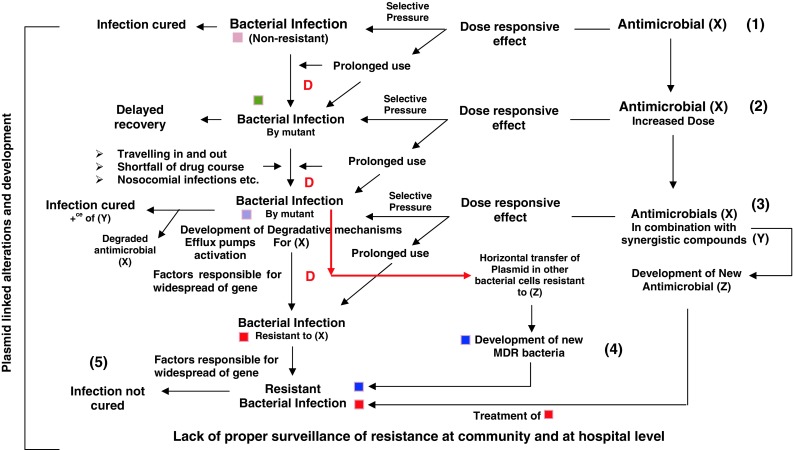
Illustrative sketch of the development of MDR microbes. The sketch is divided into various segments: (1) Bacterial infection was treated with calculated amount of antimicrobial drug (X) followed by complete cure, in the same time prolonged use of drug (X) put selective pressure causing point mutation (D). (2) Second infection (in a community only) was treated with same drug (X) with a higher dose, a delayed response was displayed because of mutant bacterial strain, (3) Third time infection (in a community only) trigger the resistance, in particular microbe for a particular drug (X); therefore, synergistic compounds (Y) were administered along with (X) may be for clinical trials, the successful treatment, leading to the production of new antimicrobial drug (Z), (4) Since the earlier bacteria attained resistance in due course of time for the drug (X) transferred the resistant gene into another strain of same species of bacteria resistant to the drug (Z) which was introduced in this community from the other one, gene cassettes got recombined on the plasmid to confer multi-drug resistant status to the new introduced bacteria. Infection caused by both these bacteria might be having same symptoms which would be treated with the newly developed drug (Z) keeping the resistance against (X) in consideration. (5) Infection could not be cured because the drug was applied to cure the (X) drug-resistant bacteria however; another bacteria having resistance against (Z) remained as such
Plants derived antimicrobial (PDAm): a ray of hope
Antimicrobial resistance is rapidly increasing along with the development of classical antibiotics consequently, there is an urgent need to develop a different drug line to treat and control MDR bacterial infections. Medicinal values of plants were known to earlier traditional medical practitioners (Emeka et al. 2012). PDAm substances are plant-originated secondary metabolites and have great concern because of their antibiotic activity without conferring resistance (Baris et al. 2006; Palaniappan and Holley 2010). PDAms are classified as antimicrobial on the basis of dose ranging from 100 to 1,000 μg ml−1 for the minimum inhibitory concentration (MIC) susceptibility test performed on bacteria (Tegos et al. 2002). Table 2 presents few of the examples of plants and their active antimicrobial compounds. Plants have unlimited ability to produce wide variety of secondary metabolites most of which are aromatic compounds including alkaloids, glycosides, terpenoids, saponins, steroids, flavonoids, tannins, quinones and coumarins (Das et al. 2010) forming the basis of PDAm compounds (Table 3). Target specific plant’s secondary metabolites having potential to treat and control the infections are being screened out globally for e.g. Coumarins having specificity on Staphylococcus aureus and ineffective on Gram-negative bacteria (Lewis and Ausubel 2006). The literature such as Cowan (1999); Lewis and Ausubel (2006) and González-Lomothe et al. (2009) provides comprehensive information on the major secondary metabolites of plant origin. Precise mechanistic approach of PDAm and their activity on microbes has been discussed by Lewis and Ausubel (2006). In general, PDAms (mostly secondary metabolites) are phenol derivatives, sufficiently able to control microbes by reducing pH, increasing membrane permeability, altering efflux pumping. Examples mentioned in Table 2 followed by recent studies of (Machado et al. 2003; Ram et al. 2004; McGaw et al. 2008; Renisheya et al. 2011; Ahmed et al. 2012; Emeka et al. 2012; Upadhyaya 2013) and the references there in, suggest the antimicrobial potential of various local and exotic plant species, although very few reports have suggested the mechanism of their actions. The affectivity of PDAms largely depends upon the extraction methods (Das et al. 2010). In a study carried out by our group, methanolic, ethanolic and water extracts of several plants species viz., Argemone maxicana, Callistomon lanceolatus, Allium sativum, Swietenia mahogany, Citrulus colocynthis, Salvadora persica, Madhuca Indica, Acacia nilotica and Pongamia pinnata were assayed for their antimicrobial activity on most of the common MDRs viz., Staphylococcus aureus, Bacillus cereus, B. pumilus, Klebsiella pneumonia, Salmonella typhi, E. coli exhibiting activity of all the extracts, however; the target specificity of plant extracts could not be established because of uncertain mechanism of plant-derived antimicrobial compounds. A generalized mechanism of PDAms on microbes suggests the effects of efflux pumping on MDRs: increasing permeability and reduce selection pressure (Lewis and Ausubel 2006). Antimicrobial peptides (AMPs) are also produced by plants against the infections also called as defensins. Plant defensins are small basic peptides, having characteristic 3D folding pattern, stabilized by eight disulfide linked cysteines (Thomma et al. 2002). AMPs have antimicrobial properties too (Li et al. 2012) and have been suggested as an alternative approach to improve treatment outcome (Brouwer et al. 2011), for e.g. IbAMP1, a plant originated disulfide linked β-sheet antimicrobial peptide (Wang et al. 2009).
Table 2.
Plant derivatives as antimicrobial for the treatment of microbial infections
| Plants | Plant derivatives | Effective against | References |
|---|---|---|---|
| Medicago sativa | Saponins, canavanine | Enterococcus faecium Staphylococcus aureus | Aliahmadi et al. (2012) |
| Onobrychis sativa | AMPs (antimicrobial peptides) | E. faecium, S. aureus | Aliahmadi et al. (2012) |
| Allium sativum | Organosulfur compounds (phenolic compounds) | Campylobacter jejuni | Lu et al. (2011) |
| Raphanus sativum | RsAFP2 (Antifungal peptide) | Candida albicans | Aerts et al. (2009) |
| Vetiveria zizanioides L. Nash | Vetivone (vetiver oil) | Enterobacter spp. | Srivastava et al. (2007) |
| Chelidonium majus | Glycoprotein | B. cereus, Staphylococcus spp. | Janovska et al. (2003) |
| Sanguisorba officinalis | Alkaloids, antimicrobial peptides | Ps. aeruginosa, E. coli | Janovska et al. (2003) |
| Cinnamomum osmophloeum | Cinnamaldehyde (in essential oil) | Legionella pneumophila | Chang et al. (2008) |
| Ocimum basilicum | Essential oil | Salmonella typhi | Wan et al. (1998) |
| Micromeria nervosa | Ethanolic extract | Proteus vulgaris | Ali-Shtayeh et al. (1997) |
| Rabdosia trichocarpa | Trichorabdal A | Helicobacter pylori | Kadota et al. (1997) |
| Melaleuca alternifolia and Eucalyptus sp. | Essential oil | Staphylococcus spp. and Streptococcus spp. | Warnke et al. (2009) |
| Anthrocephalous cadamba and Pterocarpus santalinus | Ethanolic extract | MDRsM | Dubey et al. (2012) |
| Lantana camara L. | Leaf extract in dichloromethane & methanol | MDRsG + ve and MDRsG−ve | Dubey and Padhy (2013) |
| Butea monosperma Lam. | Ethanolic and hot water extract of leaf | MDRsM | Sahu and Padhy (2013) |
| Jatropha curcas (Linn.) | Ethanolic and methanolic extract | MDRsG + ve + Micrococcus sp. & MDRsG−ve + Shigella sp. + Bacillus sp. | Igbinosa et al. (2009) |
| Ficus exasperate and Nauclea latifolia | Methanolic extract of leaf and stem | E. coli, Shigella dysenteriae, S. typhi, C. albicans, P. aeruginosa | Tekwu et al. (2012) |
| Rhus coriaria | Ethanolic extract | MDR P. aeruginosa | Adwan et al. (2010) |
MDRsM = Staphylococcus aureus + Acinetobacter sp. + Citrobacter freundii + Chromobacterium violaceum + Escherichia coli + Klebsiella sp. + Proteus sp. + Pseudomonas aeruginosa + Salmonella typhi + Vibrio cholera; MDRsG + ve = S. aureus (MRSA) + Streptococcus pyogenes + Enterococcus faecalis (VRE); MDRsG−ve = Acinetobacter baumannii + Citrobacter freundii + Proteus mirabilis + Proteus vulgaris + Pseudomonas aeruginosa
Table 3.
Examples of plant derivatives and their antimicrobial activities
| Plant-derived antimicrobial groups | Structure | Chemical properties | Effective on microbes | References |
|---|---|---|---|---|
| Quinones |
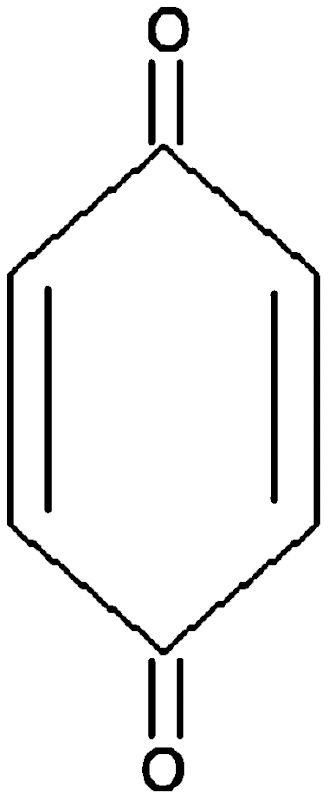
|
Conjugated cyclic-dione structure with molecular formula C6H4O2 e.g. Anthraquinone from Cassia italica | Pseudomonas pseudomallei, Bacillus anthracis, Corynebacterium pseudodiphthericum, Pseudomonas aeruginosa | Kazmi et al. (1994) |
| 6-(4,7 Dihydroxy-heptyl)quinone | Staphylococcus aureus, Bacillus subtilis, Proteus vulgaris | Ignacimuthu et al. (2009) | ||
| Alkaloids |
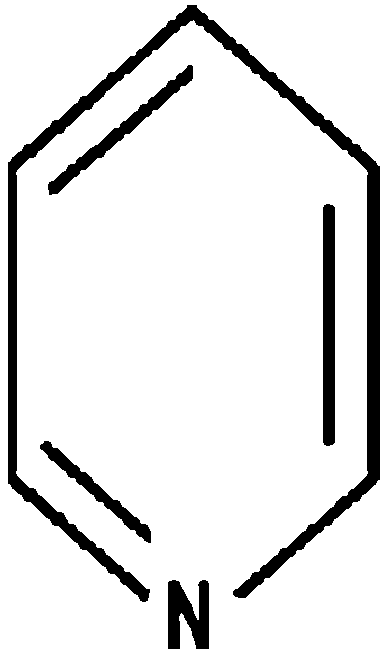
|
Naturally occurring amines having nitrogen in heterocyclic ring of compounds and are the derivative amino acids e.g. glabradine from tubers of Stephania glabra | S. aureus, S. mutans, Microsporum gypseum, M. canis, Trichophyton rubrum | Semwal and Rawat (2009) |
| l-Proline derived Monophyllidin from Zanthoxylum monophyllum | Enterococcus faecalis | Patino and Cuca (2011) | ||
| Lectins and polypeptides | – | Lectins are carbohydrate binding proteins (phytoaglutinin) with MW around 17,000–400,000 | E. coli, P. aeruginosa, Enterococcus hirae, Candida albicans (fungi) | (Zhang and Lewis (1997) |
| Flavones/flavonoids/flavonols |
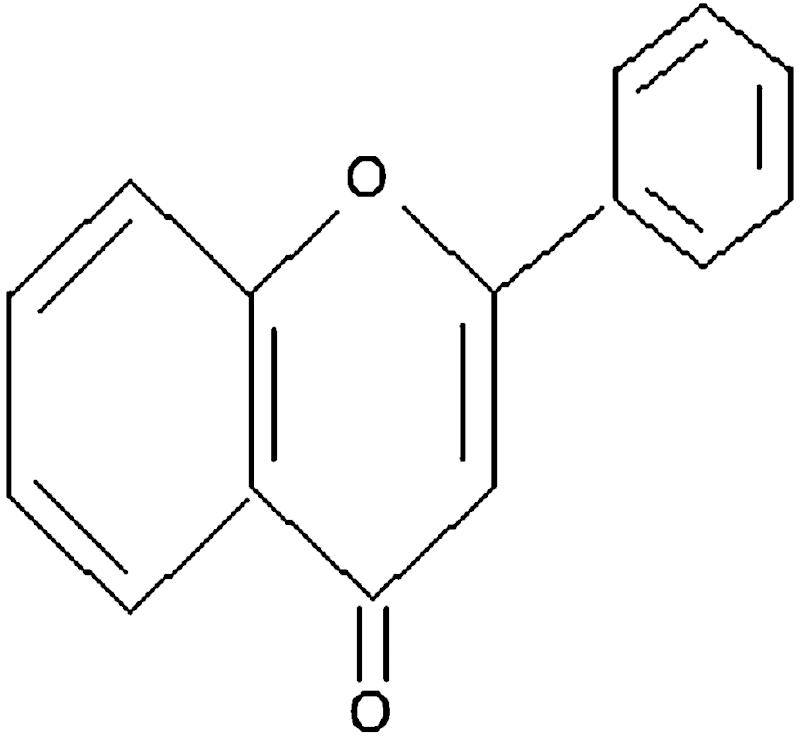
|
Are ubiquitous in plant’s parts, fruits, seeds, flowers and even honey. Flavones are hydroxylated phenolics containing one carbonyl group | MDR Klebsiella pneumoniae, P. aeruginosa, E. coli | Özçelik et al. (2008); Edziri et al. (2012) |
| Coumarins |
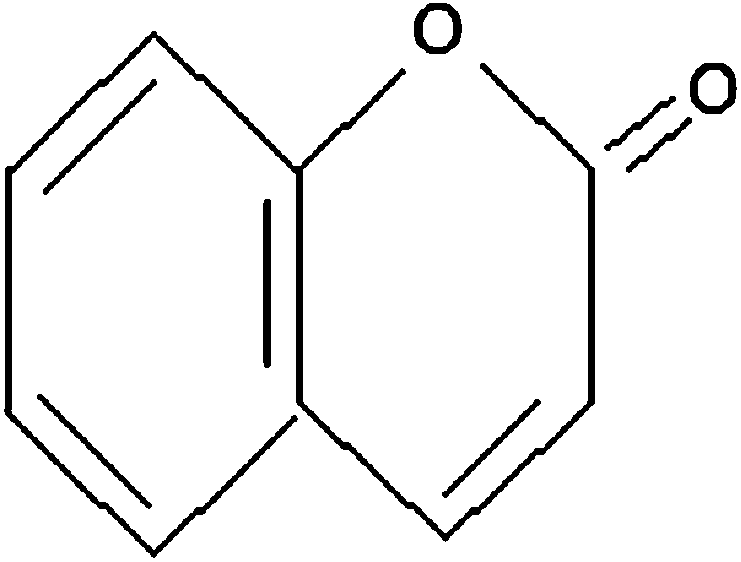
|
Coumarins are phenolic substances made of fused benzene and alpha pyrone ring forming toxic compounds found in plants such as Dipteryx odorata, Anthoxanthum odoratum etc | S. mutans, S. viridans, S. aureus | Widelski et al. (2009); Lewis and Ausubel (2006) |
| Terpenoids and essential oils |
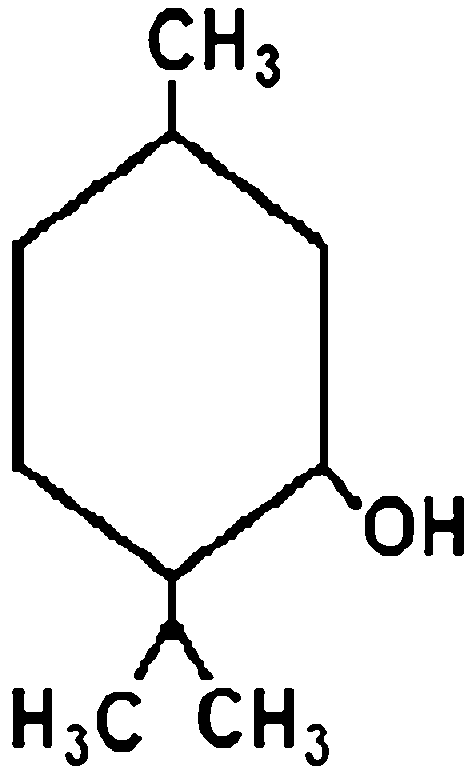
|
Isoprene derivatives having a general formula C10H16 therefore also called as Isoprenoids. Well-known examples include menthol | S. viridans, S. aureus, E. coli, B. subtilis, Shigella sonnei (highly active) P. aeruginosa, E. coli, S. aureus, T. mentagrophytes (low activity) | Banso (2009); Ragasa et al. (2008) |
| Tannins |
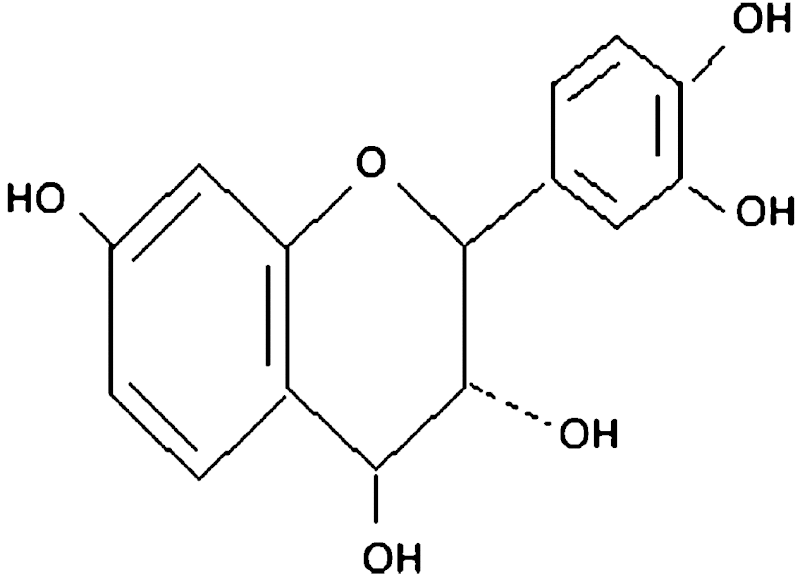
|
Large polyphenolic compound containing sufficient hydroxyls and other suitable groups | S. aureus, S. typhimurium, | Moneim et al. (2007) |
Chemical structure given in front of corresponding group of antimicrobials is not to be considered as generalized one, the references are in correspondence with bacteria
Synergistic actions of PDAms
The AMR is conferred by several factors which have already been reviewed in previous sections. Plasmid encoded resistance facilitate bacterial cells to develop resistance of various degrees. For instance, unlike Gram-positive, MDR Gram-negative bacterial species have developed a sophisticated permeability barrier as outer membrane comprised of hydrophilic lipopolysaccharide restricting the entry of hydrophobic (quinones and alkaloids) and amphipathic antibiotic compounds (Lewis and Ausubel 2006). The biased effect of PDAms on Gram-positive and -negative species has been a key to the discovery of the synergistic compounds of plant origin (Lewis 2001). Plant antimicrobials act well in combinations with other amphipathic compounds. In addition to this, resistance in MDRs conferred by efflux pumping can be treated with the synergistic combinations of antimicrobial with an efflux pump inhibitor (EPI) and altering outer membrane permeability of MDR bacteria providing an effective drug (Savage 2001; Gibbons 2004; Baskaran et al. 2009). Studies of Chusri et al. (2009) reported another example of synergistic effect of plant-derived phenolics such as Ellagic acid (a derivative of Gallic acid) a non-antimicrobial, administered as EPI in combination with classical antibiotic to control Acinetobacter baumannii. Another example belongs to the well-studied plant Berberis fremontii and its amphipathic cation berberine inhibits the NorA MDR pump of Staphylococcusaureus when applied in combination with 5′-MHC (5′-methoxyhydnocarpin, an amphipathic weak acid) a real inhibitor of the pump enhancing the activity of berberine (Stermitz et al. 2000). Similar non-antimicrobial compounds known to enhance effectivity of antimicrobials have been discussed by Lewis (2001). Detailed mechanism of PDAms on MDR S. aureus has been discussed in the review by Gibbons (2004). Wang et al. (2009) defined that the role of AMP plant defensin IbAMP1 isolated from plant Impatiens balsamina have a prime target, intercellular components, forming small channels that permit the transit of ions or protons across the bacterial membrane, the same activity was also observed in the linear analogs of this peptide.
Future studies
Researches on the AMR and alternating drug system are endless and a lot of scope is there in the field of ethno-pharmacology. Scientists are working on the development of safe and effective antimicrobials all over the world. Future studies may involve the development of new plant-derived synergistic compounds capable of enhancing the activity of PDAms. A lot of research potential is also there to answer the questions for e.g. mechanism of resistance in different bacterial species, development of XDRs and their control.
Conclusion
AMR is a worldwide problem. Research literatures suggest that the substandard living in major parts of developing world is one of the major causes of the development of resistance among bacteria. The developed world is also vulnerable of getting widespread infections for e.g. USA is surrounded by the developing countries having high rates of resistance development. Nosocomial, water borne, health care systems and food products especially meats are some of the most common means of widespread of resistant gene globally. Thanks to the modern molecular approaches for making better understanding of the pathways of resistance development and its remedy. Pharmacologists are developing new antibiotic drugs to treat and control various infections, however; the chances of the development of resistance are equal to the emergence of new drugs. In addition, research suggest that the combinations of PDAms and the synergistic compounds work efficiently on resistant strains ensuring no further resistance development. Moreover; concerted efforts have been solicited by the world community because poor countries are worst affected by the antimicrobial resistance and the developed countries are no longer safe (Diáz-Granados et al. 2008). In this regard, PDAms in combination with plant-derived synergistic compounds may be the cost-effective approach to deal with global antimicrobial resistance.
Acknowledgments
The Corresponding and the one of the main contributors of this review article Dr. Jatin K Srivastava is acknowledged to the chairman of Global Group of Institutions Lucknow for providing the necessary facilities during the compilation of this review paper.
Conflict of interest
Authors have no conflict of interest with any of the organization, funding agencies or any person.
Abbreviations
- MDR
Multidrug-resistant
- XDR
Extensively drug-resistant
- AMR
Antimicrobial resistance
- PDAm
Plant-derived antimicrobials
- ARM
Antimicrobial-resistant microbes
- EPI
Efflux pump inhibitor
- AMP
Antimicrobial peptides
References
- Adwan G, Abu-Shanab B, Adwan K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug resistant Pseudomonas aeruginosa strains. Asian Pac J Trop Med. 2010;3(4):266–269. doi: 10.1016/S1995-7645(10)60064-8. [DOI] [Google Scholar]
- Aerts AM, Carmona-Gutierrez D, Lefevre S, Govaert G, François IE, Madeo F, Santos R, Cammue BP, Thevissen K. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009;583(15):2513–2516. doi: 10.1016/j.febslet.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Ahmed AS, Elgorashi EE, Moodley N, McGaw LJ, Naidoo V, Eloff JN. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four South African Bauhinia species used traditionally to treat diarrhea. J Ethnopharmacol. 2012;143(3):826–839. doi: 10.1016/j.jep.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Aliahmadi A, Roghanian R, Emtiazi G, Mirzajani F, Ghassempour A. Identification and primary characterization of a plant antimicrobial peptide with remarkable inhibitory effects against antibiotic resistant bacteria. Afr J Biotechnol. 2012;11(40):9672–9676. [Google Scholar]
- Ali-Shtayeh MS, Al-Nuri MA, Yaghmour RMR, Faidi YR. Antimicrobial activity of Micromerianervosa from the Palestinian area. J Ethnopharmacol. 1997;58:143–147. doi: 10.1016/S0378-8741(97)00088-3. [DOI] [PubMed] [Google Scholar]
- Amábile-Cuevas CF. Gathering of resistance genes in Gram-negative bacteria: an overview. In: Amábile-Cuevas CF, editor. Multidrug resistant bacteria. Wymondham: Horizon Scientific Press; 2003. pp. 9–31. [Google Scholar]
- Amábile-Cuevas CF, et al. Global perspective of antibiotic resistance. In: de-J-Soso A, et al., editors. Antimicrobial resistance in developing countries. New York: Springer; 2010. pp. 3–14. [Google Scholar]
- Banso A. Phytochemical and antibacterial investigation of bark extracts of Acacianilotica. J Med Plants Res. 2009;3(2):82–85. [Google Scholar]
- Baris O, Gulluce M, Sahin F, Ozer H, Kilic HH, Ozkan H, Sokmen M, Ozbek T. Biological activities of the essential oil and methanolic extract of Achillea biebersteinii Afan. (Asteraceae) Turk J Biol. 2006;30:65–73. [Google Scholar]
- Baskaran SA, Kazmer GW, Hinckley L, Andrew JM, Venkitanarayanan K. Antimicrobial effect of plant derived antimicrobials on major bacterial mastitis pathogens in vitro. J Dairy Sci. 2009;92(4):1423–1429. doi: 10.3168/jds.2008-1384. [DOI] [PubMed] [Google Scholar]
- Brouwer CPJM, Rahman M, Welling MM. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptide. 2011;32(9):1953–1963. doi: 10.1016/j.peptides.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Bush K. Antibacterial drug discovery in the 21st century. Clin Microbiol Infect. 2004;10(S4):10–17. doi: 10.1111/j.1465-0691.2004.1005.x. [DOI] [PubMed] [Google Scholar]
- Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Annu Rev Microbiol. 2011;65:455–478. doi: 10.1146/annurev-micro-090110-102911. [DOI] [PubMed] [Google Scholar]
- Byarugaba DK. Antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents. 2004;24(2):105–110. doi: 10.1016/j.ijantimicag.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Byarugaba DK, et al. Mechanism of antimicrobial resistance. In: de-J-Soso A, et al., editors. Antimicrobial resistance in developing countries. New York: Springer; 2010. pp. 15–26. [Google Scholar]
- Campion JJ, McNamara PJ, Evans ME. Evolution of ciprofloxacin-resistant Staphylococcus aureus in in vitro pharmacokinetic environments. Antimicrob Agents Chemother. 2004;48(12):4733–4744. doi: 10.1128/AAC.48.12.4733-4744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli D, Salvia AM, Sami J, Cappuccinelli P, Colombo MM. New cluster of plasmid-located class 1 integrons in Vibriocholerae O1 and a dfrA15 cassette containing integron in Vibrioparahaemolyticus isolated in Angola. Antimicrob Agents Chemother. 2006;50:2493–2499. doi: 10.1128/AAC.01310-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Chang W, Chang S, Cheng S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008;42:278–286. doi: 10.1016/j.watres.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Chusri S, Villanueva I, Voravuthikunchai SP, Davies J. Enhancing antibiotic activity: a strategy to control Acinetobacter infections. J Antimicrob Chemother. 2009;16:1203–1211. doi: 10.1093/jac/dkp381. [DOI] [PubMed] [Google Scholar]
- Coffey TJ, Dowson CG, Daniels M, Zhou J, Martin C, Spratt BG, Musser JM. Horizontal transfer of multiple penicillin-binding protein genes and capsular biosynthetic genes in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991;5(9):2255–2260. doi: 10.1111/j.1365-2958.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J Med Plant Res. 2010;4(2):104–111. [Google Scholar]
- Diáz-Granados CA, Cardo DM, McGowan-Jr JE. Antimicrobial resistance: international control strategies with a focus on limited resource settings. Int J Antimicrob Agents. 2008;32(1):1–9. doi: 10.1016/j.ijantimicag.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Díaz-Mejía JJ, Amábile-Cuevas CF, Rosas I, Souza V. An analysis of the evolutionary relationships of integron integrases with emphasis on the prevalence of class1 integron in Escherichia coli isolates from clinical and environmental origins. Microbiol. 2008;154:94–102. doi: 10.1099/mic.0.2007/008649-0. [DOI] [PubMed] [Google Scholar]
- Dubey D, Padhy RN (2013) Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. JHerb Med (In press). doi:10.1016/j.hermed.2012.12.002
- Dubey D, Sahu MC, Rath S, Paty BP, Debata NK, Padhy RN. Antimicrobial activity of medicinal plants used by aborigines of Kalahandi, Orissa, India against multidrug resistant bacteria. Asian Pac J Trop Biomed. 2012;2(2):S846–S854. doi: 10.1016/S2221-1691(12)60322-0. [DOI] [Google Scholar]
- Edziri H, Mastouri M, Mahjoub MA, Mighri Z, Mahjoub A, Verschaeve L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from Retamaraetam flowers. Molecules. 2012;17:7284–7293. doi: 10.3390/molecules17067284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeka PM, Badger-Emeka LI, Fateru F. In-vitro antimicrobial activities of Acalypha ornata leaf extracts on bacterial and fungal clinical isolates. J Herb Med. 2012;2(4):136–142. doi: 10.1016/j.hermed.2012.09.001. [DOI] [Google Scholar]
- Fonseca EL, dos Santos Freitas FF, Vieira VV, Vicente ACP. New qnr gene cassettes associated with superintegron repeats in Vibriocholerae O1. Emerg Infect Dis. 2008;14:1129–1131. doi: 10.3201/eid1407.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet Infect Dis. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- Ghafur A. The Chennai declaration: an Indian perspective on the antimicrobial resistance challenge. J Global Antimicrob Resist. 2013;1(1):5–6. doi: 10.1016/j.jgar.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Ghafur A, Mathai D, Muruganathan A, Jayalal JA, Kant R, Chaudhary D, Prabhash K, Abraham OC, Gopalakrishnan R, Ramasubramanian V, Shah SN, Pardeshi R, Huilgol A, Kapil A, Gill JPS, Singh S, RIssam HS, Todi S, Hegde BM, Parikh P. The Chennai declaration: recommendations of “A roadmap to tackle the challenge of antimicrobial resistance”—a joint meeting of medical societies of India. Indian J Cancer. 2012;49(4):84–94. doi: 10.4103/0019-509X.104065. [DOI] [PubMed] [Google Scholar]
- Gibbons S. Anti-staphylococcal plant natural products. Nat Prod Rep. 2004;21:263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- Giske CG, Cornaglia G. Supranational surveillance of antimicrobial resistance: the legacy of the last decade and proposals for the future. Drug Resist Updat. 2010;13(4–5):93–98. doi: 10.1016/j.drup.2010.08.002. [DOI] [PubMed] [Google Scholar]
- González-Lomothe R, Mitchell G, Gattuso M, Diarra MS, Malouim F, Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. Int J Mol Sci. 2009;10:3400–3419. doi: 10.3390/ijms10083400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H. The Chennai declaration on antimicrobial resistance in India. Lancet Infect Dis. 2013;13(2):105–106. doi: 10.1016/S1473-3099(12)70346-8. [DOI] [PubMed] [Google Scholar]
- Grohman E, Muth G, Espinosa M. Conjugative plasmid transfer in Gram-positive bacteria. Microbial Mol Biol Rev. 2003;67(2):277–301. doi: 10.1128/MMBR.67.2.277-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock EW. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis. 2005;5(4):209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Katayama Y, Yuzawa H, Ito T. Molecular genetics of methicillin-resistant Staphylococcusaureus. Int J Med Microbiol. 2002;292:67–74. doi: 10.1078/1438-4221-00192. [DOI] [PubMed] [Google Scholar]
- Hsu S, Chiu T, Pang J, Hsuan-Yuan C, Chang G, Tsen H. Characterization of antimicrobial resistance patterns and class 1 integrons among Escherichia coli and Salmonella enterica serovar Choleraesuis strains isolated from humans and swine in Taiwan. Int J Antimicrob Agents. 2006;27(5):383–391. doi: 10.1016/j.ijantimicag.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hsueh P. Antimicrobial drug resistance in Taiwan. Int J Antimicrob Agents. 2008;32(3):S174–S178. doi: 10.1016/S0924-8579(08)70023-2. [DOI] [PubMed] [Google Scholar]
- Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas Linn. Afr J Pharma Phramacol. 2009;3(2):58–62. [Google Scholar]
- Ignacimuthu S, Pavunraj M, Duraipandiyan V, Raja N, Muthu C. Antibacterial activity of a novel quinone from the leaves of Pergulariadaemia (Forsk.), a traditional medicinal plant. Asian J Trad Med. 2009;4(1):36–40. [Google Scholar]
- Jacoby GA, Munoz-Price LS. The new B-lactamases. N Engl J Med. 2005;352:380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- Janovska D, Kubikova K, Kokoska L. Screening for antimicrobial activity of some medicinal plants species of traditional Chinese medicine. Czech J Food Sci. 2003;21(2):107–110. [Google Scholar]
- Kadota S, Basnet P, Ishii E, Tamura T, Namba T. Antibacterial activity of trichorabdal A from Rabdosia trichocarpa against Helicobacter pylori. Zentralbl Bakteriol. 1997;286(1):63–67. doi: 10.1016/S0934-8840(97)80076-X. [DOI] [PubMed] [Google Scholar]
- Kartikeyan K, Thirunarayan MA, Krishnan P. Coexistence of blaOXA-23 with blaNDM1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253–2254. doi: 10.1093/jac/dkq273. [DOI] [PubMed] [Google Scholar]
- Kazmi MH, Malik A, Hameed S, Akhtar N, Noor AS. An anthraquinone derivative from Cassiaitalica. Photochemistry. 1994;36:761–763. doi: 10.1016/S0031-9422(00)89812-X. [DOI] [Google Scholar]
- Kuete V, Alibert-Franco S, Eyong KO, Ngameni B, Folefoc GN, Nguemeving JR, Tangmovo JG, Fatso GW, Komguem J, Ouahouo BMW, Bolla JM, Chevalier J, Ngadjui BT, Nkengfack AE, Pages JM. Antibacterial activity of some natural products against bacteria expressing a multi-drug-resistant phenotype. Int J Antimicrob Agents. 2011;37(2):156–161. doi: 10.1016/j.ijantimicag.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan P, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishna P, Kumar AV, Maharajan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike C, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livemore DM, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan and UK: a molecular, biological and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha MK, David T, Thomas K. Nasopharyngeal swabs of school children, useful in rapid assessment of community antimicrobial resistance patterns in Streptococcus pneumoniae and Haemophilus influenza. J Clin Epidemiol. 2013;66(1):44–51. doi: 10.1016/j.jclinepi.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Lewis K. In search of natural substrates and inhibitors of MDR pumps. J Mol Microbiol. 2001;3:247–254. [PubMed] [Google Scholar]
- Lewis K, Ausubel FM. Prospects for plant–derived antimicrobials. Nat Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiang Q, Zhang Q, Huang Y, Su Z. Overview on the recent study of antimicrobial peptides: origin, functions, relative mechanisms and application. Peptides. 2012;37(2):207–215. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Lu X, Rasco BA, Jabal JM, Aston DE, Lin M, Konkel ME. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacterjejuni by using fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl Environ Microbiol. 2011;77(15):5257–5269. doi: 10.1128/AEM.02845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado TB, Pinto AV, Pinto MCFR, Leal ICR, Silva MG, Amaral ACF, Kuster RM, Netto-dossantos KR. In-vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinone and their analogues, against methicillin resistant Staphylococcus aureus. Int J Antimicrob Agents. 2003;21(3):279–284. doi: 10.1016/S0924-8579(02)00349-7. [DOI] [PubMed] [Google Scholar]
- McGaw LJ, Lall N, Meyer JJM, Eloff JN. The potential of South African plants against Mycobacterium infections. J Ethnopharmacol. 2008;119(3):482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Memish ZA, Venkatesh S, Shibi AM. Impact of travel on international spread of antimicrobial resistance. Int J Antimicrob Agents. 2003;21(2):135–142. doi: 10.1016/S0924-8579(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Moneim A, Suleman E, Issa FM, Elkhalifa EA. Quantitative determination of tannin content in some sorghum cultivars and evaluation of its antimicrobial activity. Res J Microbiol. 2007;2(3):284–288. doi: 10.3923/jm.2007.284.288. [DOI] [Google Scholar]
- Norrby RS, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5(2):115–119. doi: 10.1016/S1473-3099(05)70086-4. [DOI] [PubMed] [Google Scholar]
- Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Klugman KP. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- Okeke IN, Klugman KP, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Laxminarayan R. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis. 2005;5(9):568–580. doi: 10.1016/S1473-3099(05)70217-6. [DOI] [PubMed] [Google Scholar]
- Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O’Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K. Dissemination of new methicillin resistant Staphylococcusaureus clones in the community. J Clin Microbiol. 2002;40(11):4289–4294. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özçelik B, Deliorman OD, Özgen S, Ergun F. Antimicrobial activity of flavonoids against Extended Spectrum β-Lactamase (ESBL) producing Klebsiellapneumoniae. Trop J Pharma Res. 2008;7(4):1151–1157. doi: 10.4314/tjpr.v7i4.14701. [DOI] [Google Scholar]
- Palaniappan K, Holley RA. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol. 2010;140(2–3):164–168. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Patino OJ, Cuca LE. Monophyllidin, a new alkaloid l-Proline derivative from Zanthoxylummonophyllum. Phytochem Let. 2011;4:22–25. doi: 10.1016/j.phytol.2010.10.002. [DOI] [Google Scholar]
- Ragasa CY, Ha HKP, Hasika M, Maridable J, Gaspillo P, Rideout J. Antimicrobial and cytotoxic terpenoids from Cymbopogon citratus Stapf. Phillipin Sci. 2008;45(1):111–122. [Google Scholar]
- Rajpara N, Patel A, Tiwari N, Bahuguna J, Antony A, Choudhury I, Ghosh A, Jain R, Bhardwaj AK. Mechanism of drug resistance in a clinical isolate of Vibrio fluvialis: involvement of multiple plasmids and integrons. Int J Antimicrob Agents. 2009;34:220–225. doi: 10.1016/j.ijantimicag.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Ram AJM, Bhakshu L, Raju RRV. In vitro antimicrobial activity of certain medicinal plants from Eastern Ghats, India, used for skin diseases. J Ethnopharmacol. 2004;90(2–3):353–357. doi: 10.1016/j.jep.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Renisheya JJMT, Johnson M, Mary MU, Arthy A. Antimicrobial activity of ethanolic extracts of selected medicinal plants against human pathogens. Asian Pac J Trop Biomed. 2011;1(1):S76–S78. doi: 10.1016/S2221-1691(11)60128-7. [DOI] [Google Scholar]
- Riordan JT, O’Leary JO, Gustafson JE. Contribution of SigB and SarA to distinct multiple antimicrobial resistance mechanisms of Staphylococcus aureus. Int J Antimicrob Agents. 2006;28(1):54–61. doi: 10.1016/j.ijantimicag.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Sahu MC, Padhy RN. In vitro antimicrobial potency of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asia Pac J Trop Disease. 2013;3(3):217–226. doi: 10.1016/S2222-1808(13)60044-4. [DOI] [Google Scholar]
- Savage PB. Multidrug resistant bacteria: overcoming antibiotic permeability barriers of Gram-negative bacteria. Ann Med. 2001;33:167–171. doi: 10.3109/07853890109002073. [DOI] [PubMed] [Google Scholar]
- Schlegelova J, Vlkova H, Babak V, Holasova M, Jaglic Z. Resistance to erythromycin of Staphylococcus spp. isolates from the food chain. Veterinarni Med. 2008;53(6):307–314. [Google Scholar]
- Semwal DK, Rawat U. Antimicrobial hasubanalactam alkaloid from Stephania glabra. Planta Med. 2009;75(4):378–380. doi: 10.1055/s-0028-1112223. [DOI] [PubMed] [Google Scholar]
- Soge OO, Adeniyi BA, Roberts MC. New antibiotic resistance genes associated with CTX-M plasmids from uropathogenic Nigerian Klebsiellapneumoniae. J Antimicrob Chemother. 2006;58:1048–1053. doi: 10.1093/jac/dkl370. [DOI] [PubMed] [Google Scholar]
- Srivastava J, Chandra H, Singh N. Allelopathic response of Vetiveriazizanioides (L.) Nash on members of the family Enterobacteriaceae and Pseudomonas spp. Environmentalist. 2007;27:253–260. doi: 10.1007/s10669-007-9002-2. [DOI] [Google Scholar]
- Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Appl Biol Sci. 2000;97(4):1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microb Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegos G, Stremitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46(10):3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekwu EM, Pieme AC, Beng VP. Investigations of antimicrobial activity of some Cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethnopharmacol. 2012;142(1):265–273. doi: 10.1016/j.jep.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Thomma BPHT, Cammue BPA, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Thorrold CA, Letsoalo ME, Duse AG, Marais E. Efflux pump activity in fluoroquinolone and tetracycline resistant Salmonella and E. coli implicated in reduced susceptibility to household antimicrobial cleaning agents. Int J Food Microbiol. 2007;113(3):315–320. doi: 10.1016/j.ijfoodmicro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Threlfall EJ. Antimicrobial drug resistance in Salmonella: problems and perspective in food and water-borne infections. FEMS Microbiol Rev. 2002;26(2):141–148. doi: 10.1111/j.1574-6976.2002.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Upadhyaya S. Screening of phytochemicals, nutritional status, antioxidant and antimicrobial activity of Paderia foetida Linn. From different localities of Assam, India. J Pharm Res. 2013;7(1):139–141. [Google Scholar]
- Vila J, Pal T. Update on antimicrobial resistance in low income countries: factors favouring the emergence of resistance. Open Infect Dis J. 2010;4:38–54. [Google Scholar]
- Wan J, Wilcock A, Coventry MJ. The effect of essential oil of basil on the growth of Aeromonashydrophila and Pseudomonasfluorescens. J Appl Microbiol. 1998;84:152–158. doi: 10.1046/j.1365-2672.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Bang JK, Kim HJ, Kim JK, Kim Y, Shin SY. Antimicrobial specificity and mechanism of action of disulfide—removed linear analogs of the plant derived cys-rich antimicrobial peptides Ib-AMP1. Peptides. 2009;30(12):2144–2149. doi: 10.1016/j.peptides.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Warnke PH, Becker ST, Podschun R, Sivanathan S, Springer IN, Russo PAI, Wiltfang J, Fickenscher H, Sherry E. The battle against multi resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital acquired infections. J Cranio Maxillofacial Surg. 2009;37(7):392–397. doi: 10.1016/j.jcms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) (2011) Combat antimicrobial resistance. http://www.who.int/world-health-day/2011
- Widelski J, Popova M, Graikou K, Glowniak K, Chinou I. Coumarins from Angelica lucida L.—antibacterial activities. Molecule. 2009;14:2729–2734. doi: 10.3390/molecules14082729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GD. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev. 2005;57(10):1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lewis K. Febatins: new antimicrobial plant peptides. FEMS Microbiol Lett. 1997;149:59–64. doi: 10.1111/j.1574-6968.1997.tb10308.x. [DOI] [PubMed] [Google Scholar]


