Abstract
Rabbit immunoglobulin (Ig)M and IgG antibodies to Brucella abortus and Bordetella pertussis were isolated as purified products and their specific secondary biological activities were compared. IgM antibodies were found to be more active than IgG proteins in inducing agglutination and sensitization of B. abortus for the complement-dependent bactericidal effect and in inhibiting B. pertussis-induced lymphocytosis in the mouse. IgM and IgG antibodies were found to be equally effective in inducing agglutination of B. pertussis suspended in a colloidal solution. These data parallel previous work to indicate that IgM antibodies to bacterial surface antigens are more efficient than IgG molecules in initiating biological processes concerned with the inactivation of these pathogens.
Full text
PDF


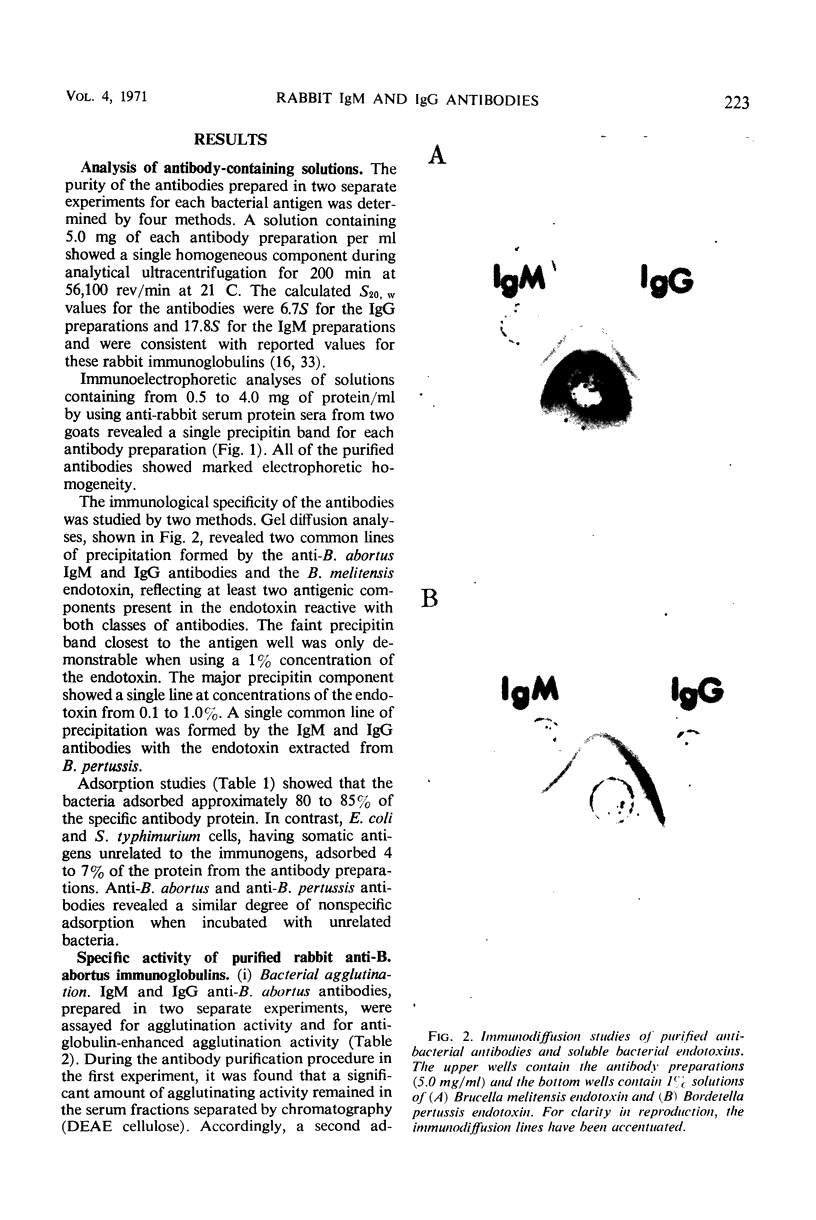



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemeier W. A., 3rd, Robbins J. B., Smith R. T. Quantitative studies of the immunoglobulin sequence in the response of the rabbit to a somatic antigen. J Exp Med. 1966 Sep 1;124(3):443–460. doi: 10.1084/jem.124.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH K. J., KOURILSKY F. M., OVARY Z., BENACERRAF B. PROPERTIES OF GUINEA PIG 7S ANTIBODIES. IV. ANTIBODY RESPONSE TO E. COLI BACTERIA. Proc Soc Exp Biol Med. 1963 Oct;114:52–56. doi: 10.3181/00379727-114-28583. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Complement fixation on cell surfaces by 19S and 7S antibodies. Science. 1965 Oct 22;150(3695):505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- COHEN S., FREEMAN T. Metabolic heterogeneity of human gamma-globulin. Biochem J. 1960 Sep;76:475–487. doi: 10.1042/bj0760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daguillard F., Edsall G. The agglutinating and bactericidal activity of IgM and IgG antibodies to the 9 and 12 factors of Salmonella typhi O 901. J Immunol. 1968 May;100(5):1112–1120. [PubMed] [Google Scholar]
- GELZER J., KABAT E. A. SPECIFIC FRACTIONATION OF HUMAN ANTIDEXTRAN ANTIBODIES. II. ASSAY OF HUMAN ANTIDEXTRAN SERA AND SPECIFICALLY FRACTIONATED PURIFIED ANTIBODIES BY MICROCOMPLEMENT FIXATION AND COMPLEMENT FIXATION INHIBITION TECHNIQUES. J Exp Med. 1964 Jan 1;119:983–995. doi: 10.1084/jem.119.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. C., Robbins J. B. Horse anti-pneumococcal immunoglobulins. II. Specific mouse protective activity. Proc Soc Exp Biol Med. 1966 Oct;123(1):105–108. doi: 10.3181/00379727-123-31414. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Kaplan M. E., Kabat E. A. Studies on human antibodies. IV. Purification and properties of anti-A and anti-B obtained by absorption and elution from insoluble blood group substances. J Exp Med. 1966 Jun 1;123(6):1061–1081. doi: 10.1084/jem.123.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Early antibody response in mice to either infection or immunization with Salmonella typhimurium. J Bacteriol. 1967 Mar;93(3):773–778. doi: 10.1128/jb.93.3.773-778.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm M. E., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. II. gamma-M-immunoglobulin. Biochemistry. 1966 Jan;5(1):267–276. doi: 10.1021/bi00865a035. [DOI] [PubMed] [Google Scholar]
- MORSE S. I. STUDIES ON THE LYMPHOCYTOSIS INDUCED IN MICE BY BORDETELLA PERTUSSIS. J Exp Med. 1965 Jan 1;121:49–68. doi: 10.1084/jem.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHEL L. H., TREFFERS H. P. Quantitative studies on the bactericidal actions of serum and complement. I. A rapid photometric growth assay for bactericidal activity. J Immunol. 1956 Jan;76(1):1–10. [PubMed] [Google Scholar]
- Morse S. I., Riester S. K. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. I. Radioautographic analysis of the circulating cells in mice undergoing pertussis-induced hyperleukocytosis. J Exp Med. 1967 Mar 1;125(3):401–408. doi: 10.1084/jem.125.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Biologic properties of 19 S and 7 S mouse isoantibodies directed against isoantigens of the H-2 system. J Immunol. 1966 Mar;96(3):430–439. [PubMed] [Google Scholar]
- NUSSENZWEIG R. S., MERRYMAN C., BENACERRAF B. ELECTROPHORETIC SEPARATION AND PROPERTIES OF MOUSE ANTIHAPTEN ANTIBODIES INVOLVED IN PASSIVE CUTANEOUS ANAPHYLAXIS AND PASSIVE HEMOLYSIS. J Exp Med. 1964 Aug 1;120:315–328. doi: 10.1084/jem.120.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R. W., Ishizaka K. Human diphtheria antitoxin in immunoglobulin classes IgG and IgA. J Immunol. 1967 Jul;99(1):40–48. [PubMed] [Google Scholar]
- Nussenzweig V., Benacerraf B. Antihapten antibody specificity and L chain type. J Exp Med. 1967 Oct 1;126(4):727–743. doi: 10.1084/jem.126.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVARY Z., BENACERRAF B., BLOCH K. J. Properties of guinea pig 7S antibodies. II. Identification of antibodies involved in passive cutaneous and systemic anaphylaxis. J Exp Med. 1963 Jun 1;117:951–964. doi: 10.1084/jem.117.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVARY Z., FUDENBERG H., KUNKEL H. G. Anuphylactic reactions in the skin of the guinea pig with high and low molecular weight antibodies and gamma globulins. J Exp Med. 1960 Nov 1;112:953–961. doi: 10.1084/jem.112.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue K., Tanigaki N., Yagi Y., Pressman D. IgM and IgG anti-hapten antibody: hemolytic, hemagglutinating and precipitating activity. Proc Soc Exp Biol Med. 1965 Nov;120(2):340–346. doi: 10.3181/00379727-120-30531. [DOI] [PubMed] [Google Scholar]
- POLLACK W., HAGER H. J., RECKEL R., TOREN D. A., SINGHER H. A STUDY OF THE FORCES INVOLVED IN THE SECOND STAGE OF HEMAGGLUTINATION. Transfusion. 1965 Mar-Apr;5:158–183. doi: 10.1111/j.1537-2995.1965.tb01152.x. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Number of molecules of antibody required to promote phagocytosis of one bacterium. Nature. 1966 Apr 30;210(5035):496–498. doi: 10.1038/210496a0. [DOI] [PubMed] [Google Scholar]
- SCHLOSSMAN S. F., KABAT E. A. Specific fractionation of a population of antidextran molecules with combining sites of various sizes. J Exp Med. 1962 Oct 1;116:535–552. doi: 10.1084/jem.116.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINK W. W., McCULLOUGH N. B., HUTCHINGS L. M., MINGLE C. K. A standardized antigen and agglutination technic for human brucellosis. Am J Clin Pathol. 1954 Apr;24(4):496–498. doi: 10.1093/ajcp/24.4_ts.496. [DOI] [PubMed] [Google Scholar]
- Small P. A., Jr, Lamm M. E. Polypeptide chain structure of rabbit immunoglobulins. I. gamma-G-immunoglobulin. Biochemistry. 1966 Jan;5(1):259–267. doi: 10.1021/bi00865a034. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALMAGE D. W. Antibodies in the rabbit with different rates of metabolic decay. J Infect Dis. 1956 Jul-Aug;99(1):21–33. doi: 10.1093/infdis/99.1.21. [DOI] [PubMed] [Google Scholar]
- TALMAGE D. W., FRETER G. G., TALIAFERRO W. H. Two antibodies of related specificity but different hemolytic efficiency separated by centrifugation. J Infect Dis. 1956 May-Jun;98(3):300–305. doi: 10.1093/infdis/98.3.300. [DOI] [PubMed] [Google Scholar]