Abstract
Objective
To evaluate mucosal healing after 24-month low-dose azathioprine (AZA) treatment in Chinese patients with moderate small bowel Crohn's disease (CD).
Methods
Patients with lesions mainly located at the ileum were screened by baseline multislice computed tomography enterography and anal-route double-balloon enteroscopy (DBE). They were naive to any immunomodulators and biological agents before the enrollment. Lesions from 150 cm of the terminal ileum proximal to ileocecal valve were assessed by DBE with the simple endoscopic score for CD (SES-CD) after 12 and 24 months of low-dose AZA treatment, respectively.
Results
The average maximal tolerance dose of AZA was 61.8 ± 17.2 mg/day. The total rates of complete, near-complete, partial and no mucosal healing in 36 patients were 19.4%, 5.6%, 27.8% and 47.2% at month 12 and 30.6%, 25.0%, 33.3% and 11.1% at month 24, respectively. The baseline SES-CD (odds ratio [OR] 2.71, 95% confidence interval [CI] 1.11–6.63, P = 0.029) and duration of disease (OR 1.27, 95% CI 1.10–1.47, P = 0.001) were two relevant factors associated with the mucosal healing of patients with small bowel CD.
Conclusion
A 24-month low-dose AZA regimen as maintenance treatment in moderate small bowel CD could achieve a higher mucosal healing rate than that of 12-month treatment in Chinese patients, especially in those with duration of disease less than 12 months and a baseline SES-CD of 5 or 6.
Keywords: azathioprine, Crohn's disease, double-balloon enteroscopy, mucosal healing, small intestine
Introduction
Mucosal healing is an important indicator to evaluate the efficacy of treatment for Crohn's disease (CD). It is also a predictor of the delayed onset of complications and a decreased need for surgery.1 Therefore, mucosal healing is presently utilized as a therapeutic end-point in many clinical trials and is increasingly applied in daily practice as well.2–4 However, nearly 30% of CD patients have lesions predominantly in the small bowel,5 where mucosal healing cannot be efficiently assessed by conventional ileocolonscopy due to the limitations of this procedure. Double-balloon enteroscopy (DBE) is a revolutionary technique that could be successfully introduced into the deep part of the small bowel, making it possible not only to diagnose diseases but also to evaluate disease activity and therapeutic effects of the drugs after treatment.6,7
Azathioprine (AZA), as one of the most common immunomodulator drugs for CD, is suitable primarily for maintaining remission and preventing recurrence. There is cumulative evidence showing that therapies with AZA and infliximab (IFX) can achieve long-term mucosal healing of ulcers in the colon and terminal ileum.3,4 However, the results of these studies cannot be simply translated and applied to predict the clinical outcomes of small bowel CD. The recommended dose of AZA is 2.0–3.0 mg/kg/day in many European and American reports.2–4,8–10 Although most Chinese patients may obtain good therapeutic outcomes with the abovementioned dose, their prevalence and severity of adverse events are higher compared with the Western patients.11 In our previous study a dose step-up strategy was carried out by gradually increasing the dose of AZA from 25 mg/day to the maximal tolerance dose (MTD) under a close monitoring of the patients' laboratory results. The average MTD of AZA in 178 CD patients was 1.24 ± 0.16 mg/kg/day,12 showing that most Chinese CD patients had a good tolerance of low-dose AZA treatment (1.0–1.5 mg/kg/day) and could achieve medium-term and long-term clinical efficacy under the recommended dose.
In this prospective study we aimed to evaluate mucosal healing in CD patients having lesions predominantly involving the ileum who were treated with low-dose AZA by DBE through the anal route, and to investigate the clinical outcomes after a 24-month low-dose AZA treatment and the factors associated with the therapeutic efficacy of AZA.
Patients and Methods
Patients
Patients with suspected small bowel CD aged from 18 to 65 years were screened. The severity and extent of the lesions were evaluated by multislice computed tomography enterography (MSCTE) and DBE via the anal route. Patients with active disease (a Crohn's disease activity index [CDAI] score of ≥220 and <450) mainly located at the ileum, including single luminal narrowing or short-segment stenosis were eligible for the study. They were naive to any immunomodulators such as AZA, 6-mercaptopurine (6-MP) or methotrexate and biological agents. Exclusion criteria were: (i) no response to steroids treatment for one month; (ii) severe side effects with AZA; (iii) uncontrolled disease activity after treated with AZA for 6 months; (iv) total small bowel obstruction; (v) positive T-SPOT.TB test or evidence of active tuberculosis; (vi) a history of tumor; (vii) pregnancy or lactation; (viii) chronic hepatic or renal dysfunction.
Study design
This study was prospectively conducted from January 2007 to June 2010. DBE was performed prior to the study as a baseline examination and was repeated at approximately 12 and 24 months after AZA therapy, respectively. The primary outcome was defined as mucosal healing, including the amelioration of ulcers and single luminal narrowing or short-segment stenosis at month 24. Low-dose AZA treatment was maintained even after complete mucosal healing was achieved within 12 months. The DBE follow-up in the last patient was carried out in July 2012. The study protocol was approved by the Institutional Ethics Committee and informed consent was obtained from each patient.
All the enrolled patients were subjected to corticosteroid induction therapy. The initial dose (0.75–1.0 mg/kg/day) of prednisolone was prescribed for 2–4 weeks, followed by tapering by 2.5 mg per 1–2 weeks until its discontinuation. When the dose of prednisolone began to taper, AZA (Imuran, Prometheus Laboratories, San Diego, CA, USA) was introduced at a dose of 25 mg/day for 2 weeks and was then increased by 25 mg at a 2-week interval unless the patient's peripheral white blood cell count was decreased or other adverse events (such as obvious abnormality of liver function, alopecia and menolipsis) occurred.
Low-dose prednisolone (<0.5 mg/kg/day) was repeated for flare-up in patients with clinical remission or in response to 6-month AZA therapy when its dose was gradually tapered as described above. In all, 16 patients received total parenteral nutrition for 2–4 weeks for symptom relief prior to the initiation of the study drug. During the 24 months of AZA therapy, 5-aminosalicyclic acid (5-ASA; via tablets or a suppository) and/or enteral nutrition was used as supportive supplementation in several patients intermittently.
All patients were assessed at the Outpatient Center twice a month during the first 3 months of treatment and at monthly or bimonthly visits afterwards. At each visit the CDAI score was calculated and the patients were asked to complete a questionnaire containing items on their overall well-being and laboratory examinations were routinely checked up, including blood tests, C-reactive protein (CRP), liver and renal functions. In addition, drug-related adverse events, comorbidities and any disease-related management issues were recorded.
Evaluation of clinical efficacy
Clinical remission was defined as a decreased CDAI score >150 but <220. Clinical response was defined as a decrease from baseline CDAI score of >70–100.13 A flare-up was defined as any changes in the disease course that required unscheduled visit or admission to hospital and adjustment of medication. Relapse was defined as the recurrence of the symptoms, resulting in an increase in the CDAI score of >100. The clinical efficacy of AZA was evaluated at month 6.
Evaluation of endoscopic mucosal healing
The findings of DBE were assessed and scored by two endoscopists using the simple endoscopic score for CD (SES-CD system).14 In this study the lesions for endoscopic evaluation were mainly selected from 150 cm of the terminal ileum proximal to the ileocecal valve with DBE via anal route. At least three typical lesions were assessed and recorded, which were correspondingly compared after AZA therapy.
The criteria for the evaluation of mucosal healing included the four categories described by D'Haens et al.8 The improvement of luminal narrowing after AZA therapy was also evaluated. Complete mucosal healing was defined as the disappearance of all lesions at the follow-up enteroscopy in patients with ulcers at baseline DBE (SES-CD = 0). Near-complete healing was defined as a marked endoscopic improvement but aphthous ulcers (<0.5 cm) or erosions in the absence of stenosis and the affected segment was less than 50% (SES-CD = 3). Partial mucosal healing was defined as <50% affected areas and the size of the biggest ulcer of <2 cm, but considerable numbers of ulcers still persisted and single luminal narrowing could be observed but was passable by DBE (SES-CD = 4–5). Unchanged or worse condition was defined as lesions that were similar to or more severe than the baseline findings and unimproved short-segment stenosis (SES-CD ≥ 6).
Statistical analysis
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean ± standard deviation (SD) and were compared by anova. Univariate and multivariate logistic regression analysis were performed to evaluate the association between endoscopic mucosal healing (0, complete and near-complete healing; 1, partial and no healing) and factors, such as age (<40 and ≥40 years), gender, smoking (yes/no), duration of disease (<1 and ≥1 year) and baseline SES-CD. P ≤ 0.05 was regarded as statistical significant.
Results
A total of 45 patients with moderate small bowel CD confirmed by MSCTE and DBE screening were recruited in our study. Seven patients were excluded before the first DBE follow-up due to severe adverse events to AZA (n = 6) and uncontrolled disease activity after treated with AZA for 6 months (n = 1). Another two patients were withdrawn because no mucosal healing was achieved at month 12. Finally, 36 patients (25 men and 11 women) completed the trial (Fig. 1). Their characteristics and clinical data are shown in Table 1. The mean age of the patients was 31.6 ± 8.6 years (range 17–45 years). The mean duration of disease was 18.9 ± 11.0 months (range 5–38 months). The average baseline score of SES-CD was 6.3 ± 1.1 (range 5–9). There were 7 patients with a baseline SES-CD of 5, 17 patients with a baseline SES-CD of 6 and 12 patients with a baseline SES-CD of 7–9. The baseline CDAI was 305 ± 42 and CRP was 26 ± 5.1 mg/L.
Figure 1.
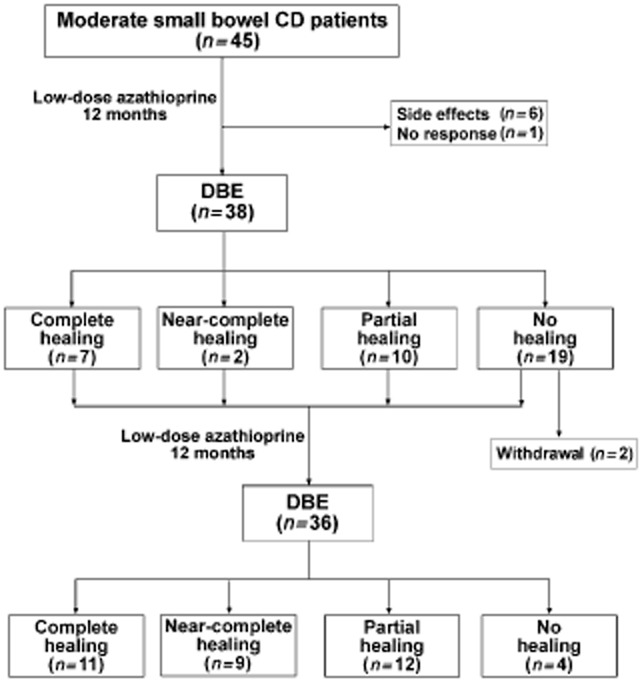
Flowchart of baseline screening of Crohn's disease (CD) patients by multislice computed tomography enterography and anal-route double-balloon enteroscopy (DBE) and evaluation of mucosal healing after 12 months and 24 months of low-dose azathioprine treatment, respectively.
Table 1.
Baseline characteristics of patients (N = 36)
| Gender, n (male/female) | 25/11 |
| Age at diagnosis, years (mean ± SD [range]) | 31.6 ± 8.6 (17–45) |
| Smokers, n (%) | 9 (25.0) |
| Perianal lesion, n (%) | 7 (19.4) |
| Stenosis, n (%) | 12 (33.3) |
| Disease duration, months (mean ± SD [range]) | 18.9 ± 11.0 (5–38) |
| SES-CD at inclusion, n | |
| 5 | 7 |
| 6 | 17 |
| 7–9 | 12 |
| CDAI at inclusion | 305 ± 42 |
| C-reactive protein, mg/L (mean ± SD) | 26 ± 5.1 |
CDAI, Crohn's disease activity index; SD, standard deviation; SES-CD, simple endoscopic score for Crohn's disease.
In this study the average MTD of AZA in the remission period was 61.8 ± 17.2 mg/day. After having been treated with AZA for 2–3 months, the participants' CRP returned to normal levels. At month 6, 26 patients (72.2%) achieved clinical remission and the other 10 (27.8%) had a clinical response.
At month 12, complete mucosal healing was observed in 7 patients (19.4%), near-complete healing in 2 (5.6%), partial healing in 10 (27.8%) and no healing in 17 (47.2%) (Fig. 2). All patients with a baseline SES-CD of 5 (n = 7) had complete mucosal healing (Fig. 3a). However, in patients with a baseline SES-CD of 6, the proportion of patients with complete, near-complete, partial healing and no change was 0, 11.8% (2/17), 47.0% (8/17) and 41.2% (7/17), respectively (Fig. 2b). No patient with a baseline SES-CD of 7–9 achieved complete or near-complete mucosal healing at month 12, and the proportion of the patients with partial healing and no change was 16.7% (2/12) and 83.3% (10/12), respectively (Fig. 2c).
Figure 2.
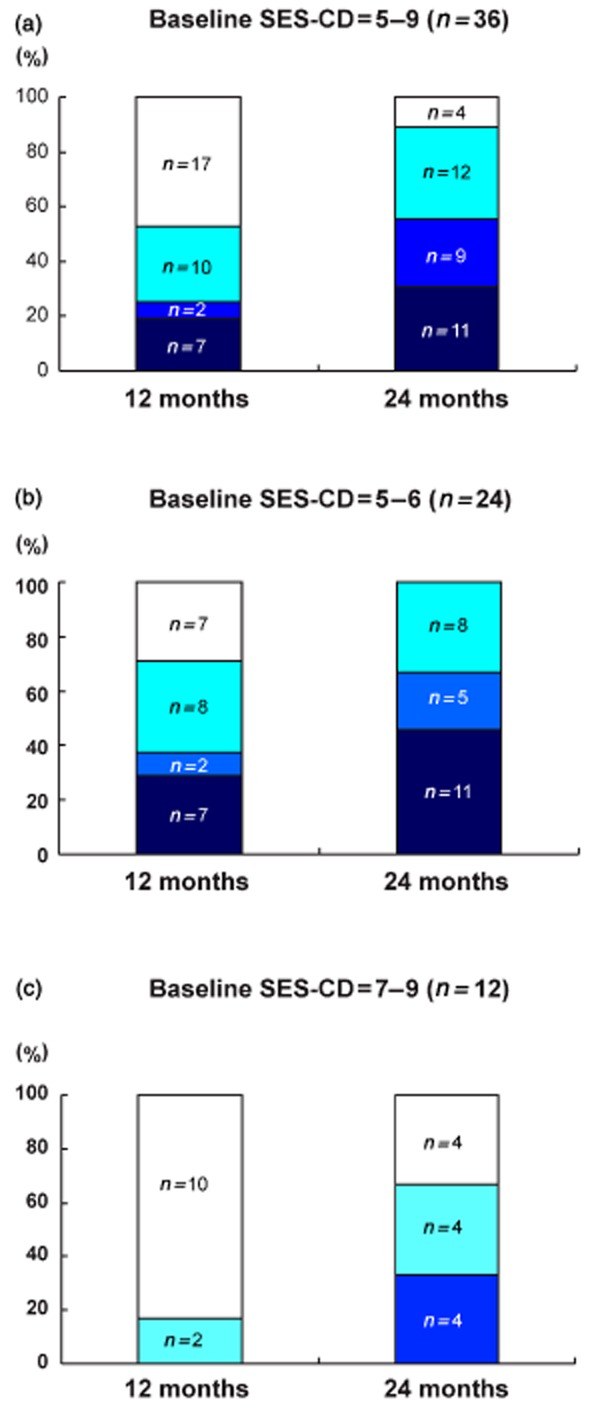
Effect of low-dose azathioprine on mucosal healing in patients with moderate small bowel Crohn's disease at month 12 and 24, respectively. (a) The rates of complete, near-complete, partial and no mucosal healing for all included patients (n = 36). (b) Corresponding rates for patients with a baseline simple endoscopic score for Crohn's disease (SES-CD) of 5–6 (n = 24). (c) Corresponding rates for patients with baseline SES-CD of 7–9 (n = 12).  , complete healing;
, complete healing;  , near-complete healing;
, near-complete healing;  , partial healing;
, partial healing;  , no healing.
, no healing.
Figure 3.
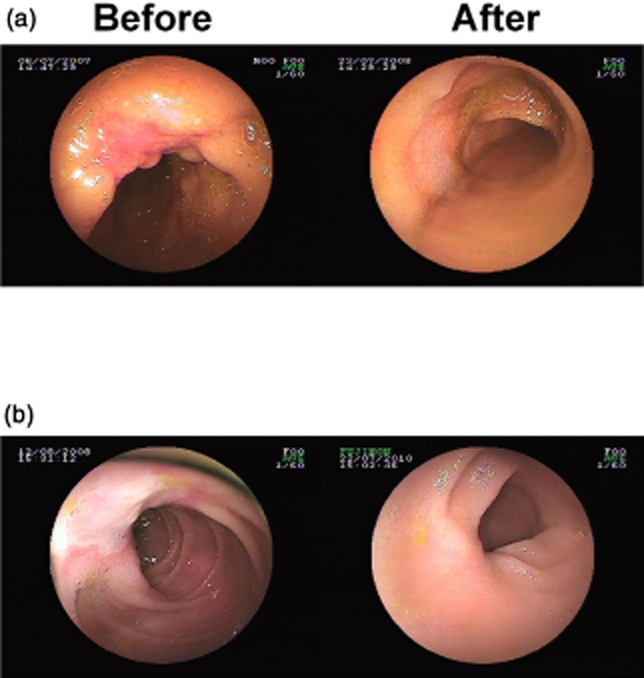
Double-balloon enteroscopy showing complete mucosal healing achieved after low-dose azathioprine treatment in two patients. (a) A patient with a baseline simple endoscopic score for Crohn's disease (SES-CD) of 5 after 12 months of treatment. (b) Another patient with a baseline SES-CD of 6 after 24 months of treatment.
The rate of complete/near-complete mucosal healing at month 24 was much higher than that at month 12. Of the 36 patients, complete healing was observed in 11 (30.6%), near-complete healing in 9 (25.0%), partial healing in 12 (33.3%) and no healing in 4 (11.1%). None of the patients with baseline SES-CD of 5 (n = 7) had a recurrence at month 24. Four patients with a baseline SES-CD of 6 achieved complete healing (Fig. 3b). The corresponding proportions of patients with a baseline SES-CD of 5–6 who had complete, near-complete and partial healing were 45.8% (11/24), 20.9% (5/24) and 33.3% (8/24), respectively (Fig. 2b). No patients with a baseline SES-CD of 7–9 achieved complete healing after 24 months of treatment. Near-complete healing, partial healing and no change were found in 4, 4 and 4 patients, respectively (Fig. 2c). The univariate logistic regression analysis showed that complete or near-complete healing of the ileum mucosa was associated with the baseline SES-CD (odds ratio [OR] 2.71, 95% confidence interval [CI] 1.11–6.63, P = 0.029).
The mean duration of the disease differed significantly among patients with the baseline SES-CD of 5 (9.1 ± 2.3 months), SES-CD of 6 (17.9 ± 9.5 months) and SES-CD of 7–9 (27.4 ± 8.4 months) (P < 0.05). According to the disease duration, the 36 patients were divided into two groups. Those whose disease duration was <12 months were assigned to group A (n = 12) and the others were assigned to group B (≥12 months, n = 24). Group A had a good response to low-dose AZA treatment; after 24 months of AZA therapy, 9 patients (75.0%) achieved complete mucosal healing and the other 3 (25.0%) achieved near-complete healing. However, the result was poor for patients in group B. The proportion of patients having complete and near-complete healing was only 33.3% (8/24), while those of patients with partial healing and no change were 50.0% (12/24) and 16.7% (4/24), respectively. Multivariate logistic regression analysis indicated that the only factor associated with the complete or near-complete healing of the ileum mucosa was disease duration (OR 1.27, 95% CI 1.10–1.47, P = 0.001).
There were six patients with large ulcers (>2 cm), but the affected segment was <50% without stenosis (baseline SES-CD of 6). After 24 months of treatment, near-complete healing was observed in two patients and partial healing in four patients. Among patients with single luminal narrowing or short-segment stenosis (baseline SES-CD of 7–9), near-complete healing was achieved in 2 of the 12 patients (the single luminal narrowing disappeared after treatment) and partial healing was obtained in 6 of the 12 patients. The remaining 4 patients with large ulcers (>2 cm) and/or short segment stenosis originally were judged as not having healed (the baseline scores of their SES-CD decreased from 8–9 to 6–7). In these patients, although the ulcers became smaller, the narrowing of the affected segment remained unchanged after 24 months of AZA therapy. Only 2 of 16 patients with partial or no healing developed disease relapse, and the symptoms were alleviated with the repeated administration of low-dose prednisone.
Discussion
Currently, AZA is still widely applied for the treatment of active and moderate CD to achieve or maintain remission, especially in developing countries, although its therapeutic effect appears slowly. This prospective study was performed in Chinese patients who were naive to immunomodulators and biological agents before their enrollment into the study with moderate CD predominantly in the ileum. After 6-month low-dose AZA treatment, 72.2% (26/36) of the patients achieved clinical remission and the remainder had a clinical response. However, the proportions of patients with complete, near-complete, partial and no healing at month 24 were 30.6%, 25.0%, 33.3% and 11.1%, respectively, showing that the mucosal healing in small bowel CD observed by DBE follow-up took a much longer duration of time than clinical remission (even when the symptoms were relieved and CRP returned to normal levels).
In our study, the optimal dose of AZA for the maintenance of remission was determined individually, and we further investigated the therapeutic healing effect in patients with lesions mainly located at the ileum at month 12 and 24. The results showed that patients receiving 24-month treatment achieved a much higher rate of complete healing than 12-month treatment, especially for those with disease duration < 1 year and baseline SES-CD ≤ 6. These data suggest that the healing effect of low-dose AZA is substantially evident if it is initiated in the early stage of the disease for active and moderate small bowel CD patients with baseline SES-CD of 5 or 6.
In our preliminary study, 86 patients diagnosed as CD by DBE from April 2003 to January 2006 were enrolled. Many of the patients suffered adverse events although they obtained good therapeutic effects with the recommended dose of AZA (2.0–3.0 mg/kg/day) in European and American reports. The total withdrawal rate due to adverse events was 22.1% (19/86). The overall AZA-induced adverse events included leukocytopenia, hepatic dysfunction, fatigue, alopecia and acute pancreatitis. Afterwards, a dose step-up strategy was carried out by gradually increasing the dose of AZA from 25 mg/day to the MTD under a close monitoring of laboratory results and clinical response. For most Chinese CD patients, the average MTD of AZA was 1.24 ± 0.16 mg/kg/day, which also could achieve clinical efficacy.12 Recently, Murakami et al.15 reported the average dose of AZA for Japanese patients with CD was 54.2 ± 18.0 mg/day, which was very similar to that in the present study (61.8 ± 17.2 mg/day). In Murakami et al.'s study,15 patients receiving AZA were individuals with mild to moderate relapse. Most of the patients (84.9%, 45/53) had colitis and ileocolitis. Their CDAI before treatment was relatively lower (245.0 ± 60.4), but the duration of their disease was longer (11.8 ± 7.6 years) than that of the patients in our study. In their study, only 30.2% (16/53) of patients underwent follow-up colonoscopy after AZA therapy, among whom only three patients had small bowel disease.
At present, ileocolonscopy is still the most frequently used modality for evaluating the mucosal healing of lesions involving the terminal ileum or colon.1–4,8,9 However, it can only reach up to 20–30 cm of the terminal ileum, which limits its value in the assessment of lesions deep in the small bowel. The clinical scenario and outcome of small bowel CD may possess its own characteristics and might not be covered by results from studies on colonic CD. Capsule endoscopy can go through and scan the entire small bowel but it is not suitable for detecting target or corresponding lesions due to its technical limitations. The main advantages of DBE are that it can be steered and it is precise. It can not only find corresponding ulcers in a specified area but also observe the lesion surface directly. Therefore, DBE can effectively and precisely evaluate the healing of ulcers in patients with small bowel CD, and could thus serve as the standard tool for the evaluation of mucosal healing in the future. A study by Mensink et al.7 has demonstrated that DBE is feasible and safe for assessing small bowel disease activity and degrees of disease improvement. In our study the lesions were selected from the ileocecal valve to 150 cm of the terminal ileum. This segment is the most frequently affected area of small bowel CD and can easily and safely be reached by DBE via the anal route.
Until the time of writing, no validated evaluation system of mucosa healing, especially for small bowel CD, has been reported. The SES-CD was applied in our study for two reasons. First, the size of ulcers and the extent of ulcerated surface are the best reproducible endoscopic parameters in the SES-CD system.14 We chose at least three most typical and severe ulcers for endoscopic evaluation and the rest of the ulcers might have similar tendencies of healing simultaneously. Second, SES-CD includes the classification of different kinds of luminal stenosis.14 There were 12 patients with single luminal narrowing or short-segment stenosis in our study. The results suggest that 24-month low-dose of AZA therapy can maintain sustained clinical remission for these patients, but its effect on mucosal healing is limited.
At week 26 (month 6) in Colombel et al.'s trial,2 mucosal healing occurred in 43.9% (47/107) of patients receiving combination therapy (IFX and AZA compared with IFX monotherapy (30.1%, 28/93) and AZA monotherapy (16.5%, 18/109). Moreover, the step-up/top-down trial demonstrated that at week 104 (month 24), early combined IFX induction and AZA maintenance (the top-down regimen) results in a higher proportion of complete mucosal healing than the conventional steroid–AZA regimen (73.1% [19/26] vs 30.4% [7/23], P = 0.028).3 In our study, after 24-month low-dose AZA treatment, none of small bowel CD patients with a baseline score of SES-CD of 7–9 achieved complete healing. If these patients had received combination therapy (the top-down regimen) at an earlier stage, a better clinical improvement and quicker mucosal healing would have been expected.
Three limitations might be present in our study. First, thiopurine methyltransferase (TPMT) activity was not measured prior to the initiation of AZA treatment. The concentration of AZA would have changed from a low dose to a high dose in some patients in combination with 5-ASA due to its interference with the function of TPMT. However, the dose of AZA was gradually increased from 25 mg/day to MTD under a close monitoring unless adverse events occurred. Moreover, the differences in AZA metabolism among different ethnic groups should be considered. Second, enteral nutrition was used as a supportive treatment in some patients. However, it takes more than 6 months to achieve mucosal healing in active CD patients, even with IFX treatment. Enteral nutrition might help to achieve clinical remission in 8 weeks with an improvement in the patients' quality of life but not mucosal healing.16 Based on this evidence, the effect of the intermittent use of enteral nutrition on mucosal healing does not play a major role in mucosal healing and can be ignored in the present study. Third, AZA dose of 2.0–3.0 mg/kg/day is recommended for Caucasian patients with CD. As reported, the dose of AZA used for Asian patients with ulcerative colitis is lower (1.0 mg/kg/day).17,18 According to the Chinese Consensus for the Diagnosis and Treatment of Inflammatory Bowel Disease published in 2012, no definite dose of AZA has been suggested. Thus, we did not set a control group with the dose of 2.0–3.0 mg/kg/day because we cannot predict whether most Chinese small bowel CD patients could complete a 24-month treatment when such a dose is used.
To our knowledge, this is the first study to evaluate the effect of low-dose AZA on mucosal healing in Chinese patients with moderate small bowel CD by DBE. The optimal time point for the first follow-up by DBE is, according to our experiences, at least 12 months after treatment. Low-dose AZA can be recommended as an effective and safe regimen for Asian patients with moderate small bowel CD. Moreover, our results indicated that the determination of medication largely depends on the patients' baseline SES-CD. The stratification of patients with small bowel CD is a prerequisite for individualized regimens for optimizing clinical outcomes. For patients with a baseline SES-CD of 5–6, early treatment with low-dose AZA is strongly recommended. For patients with a baseline SES-CD of ≥7 but in the absence of stenosis or in those who cannot tolerate AZA therapy, early treatment with IFX alone or in combination with a lower dose of immunomodulator therapy (IFX and AZA) is preferable. For patients with a baseline SES-CD of ≥7 and with single luminal narrowing or short-segment stenosis, low-dose AZA can be continued for more than 24 months if a timely positive response is achieved. Otherwise, surgical intervention is advised if relapses occur frequently.
Acknowledgments
This study was partially supported by the Natural Science Foundation of China (No. 30872973) and the Research Foundation of Shanghai Key Medical Subjects (No. S30204-k03).
References
- 1.Frøslie KF, Jahnsen J, Moum BA, Vatn MH IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 3.D'Haens G, Baert F, van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 4.Baert F, Moortgat L, Van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. quiz e10–1. [DOI] [PubMed] [Google Scholar]
- 5.Papadakis KA, Tabibzadeh S. Diagnosis and misdiagnosis of inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:433–449. doi: 10.1016/s1052-5157(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 6.Sunada K, Yamamoto H, Yano T, Sugano K. Advances in the diagnosis and treatment of small bowel lesions with Crohn's disease using double-balloon endoscopy. Therap Adv Gastroenterol. 2009;2:357–366. doi: 10.1177/1756283X09343542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mensink PB, Aktas H, Zelinkova Z, West RL, Kuipers EJ, van der Woude CJ. Impact of double-balloon enteroscopy findings on the management of Crohn's disease. Scand J Gastroenterol. 2010;45:483–489. doi: 10.3109/00365520903563774. [DOI] [PubMed] [Google Scholar]
- 8.D'Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn's (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50:667–671. doi: 10.1016/s0016-5107(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 9.Mantzaris GJ, Christidou A, Sfakianakis M, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn's disease. Inflamm Bowel Dis. 2009;15:375–382. doi: 10.1002/ibd.20777. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Ding H, Qian JM, Shan KS. Adverse effects of azathioprine in the treatment of inflammatory bowel disease. Chin J Clin Gastroenterology. 2011;23:40–42. (in Chinese) [Google Scholar]
- 12.Zhou J, Zhong J, Wang ZT, Yuan JL, Yu XJ, Fan R. The efficacy of medium- and long-term immunosuppressive therapy in Crohn's disease. Chin J Dig. 2012;32:312–315. (in Chinese) [Google Scholar]
- 13.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–530. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 14.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 15.Murakami Y, Matsui T, Hirai F, et al. Efficacy of azathioprine in mild or moderate relapse in Crohn's disease: clinical and endoscopic evaluation. Dig Endosc. 2010;22:25–32. doi: 10.1111/j.1443-1661.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 16.Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, et al. Improvement in quality of life of children with acute Crohn's disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther. 2004;20:167–172. doi: 10.1111/j.1365-2036.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 17.Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T. Low-dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol. 2003;38:740–746. doi: 10.1007/s00535-003-1139-2. [DOI] [PubMed] [Google Scholar]
- 18.Ooi CJ, Fock KM, Makharia GK, et al. Asia Pacific Association of Gastroenterology Working Group on Inflammatory Bowel Disease. The Asia–Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010;25:453–468. doi: 10.1111/j.1440-1746.2010.06241.x. [DOI] [PubMed] [Google Scholar]


