TO THE EDITOR:
Patients who develop IgE to the oligosaccharide galactose-alpha-1, 3-galactose (alpha-gal) report delayed allergic reactions after eating beef, pork or lamb. To date, there have been no published reports indicating whether IgE to alpha-gal is associated with a risk of anaphylaxis at the time of engraftment of a bovine or porcine valve. This case series documents the clinical courses of three patients with elevated IgE to alpha-gal who required porcine or bovine valve replacement. Two patients experienced perioperative or postoperative hypersensitivity reactions, but all three are tolerating valve replacement.
CASE 1**
A 53-year-old male with acute bacterial endocarditis requiring mitral valve replacement (MVR) also reported a three-year history of diffuse urticaria and flushing that occurred two hours after ingesting beef or pork. He also reported a history of tick bites that took several months to resolve.
Given the requirement for lifelong anticoagulation with a mechanical valve, this patient elected to have MVR with a porcine valve that had undergone alpha amino oleic acid treatment (to decrease valve calcification) and physiologic fixation process (to preserve leaflet structure) prior to implantation (Figure IA).
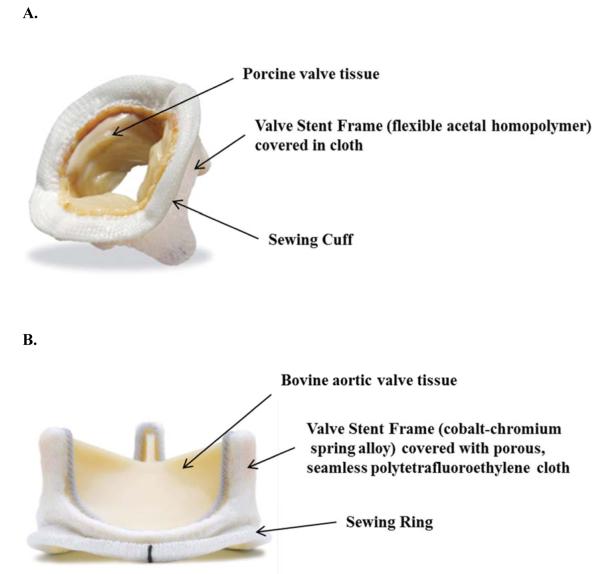
Figure I.
A. Medtronic Mosaic tissue (porcine) valve. B. Carpentier-Edwards PERIMOUNT Magna Ease tissue (bovine) aortic valve.
For further details of the preparation of these valves, see: www.medtronic.com and www.edwards.com
On the morning of postoperative day 1, the patient developed extensive urticaria on his chest and lower extremities that resolved when treated with diphenhydramine and famotidine. Although he tolerated ingestion of beef without development of symptoms for the following 6 months, he currently develops extensive urticaria after eating beef.
CASE 2
A 73-year-old male with severe calcific aortic stenosis also reported a five-year history of delayed reactions (5-6 hours) after consuming mammalian meat, including three syncopal episodes with urticaria that necessitated emergency treatment. He had an extensive history of tick bites. On a diet excluding red meat, he had had no further reactions.
He underwent aortic valve replacement (AVR) with a bovine valve that was treated with a ThermaFix process designed to prevent calcification (Figure IB). At the end of the operation, he developed a diffuse maculopapular rash, hypoxemia, and wheezing. The patient responded well to epinephrine, dexamethasone, famotidine, and fluids. Bronchoscopy did not reveal airway edema. He was extubated successfully later that day and maintained on cetirizine, famotidine, and prednisone. Tryptase level was found to be elevated at 24.7 ng/ml during the anaphylactic reaction; baseline tryptase performed a few months later while he was asymptomatic was 6.35 ng/ml. He was discharged on post-operative day 5. During a one-year follow up after surgery, he has only developed allergic symptoms after consumption of mammalian meat.
CASE 3
A 75-year-old male with history of aortic insufficiency and ascending aortic aneurysm (AAA) experienced anaphylaxis during induction of anesthesia in preparation for AVR and AAA repair at an outside facility. Several anesthetic agents had been administered over the thirty minutes prior to the reaction, but, in addition, his central line was flushed with heparin immediately before the reaction. He developed hypotension; an erythematous rash on his chest, arms, and scalp; and angioedema of his head and tongue. He received intravenous antihistamine, steroid, and multiple vasopressor medications, and required intubation and ICU care for three days.
Heparin-induced thrombocytopenia (Type II) was excluded because of the absence of thrombocytopenia or thromboembolic complications.
He had experienced urticaria four months earlier after undergoing diagnostic cardiac catheterization, although he had previously undergone multiple procedures also involving the use of sedatives, anesthetic agents, and heparin-- without reaction. AVR was performed using a processed bovine valve (Figure IB), and an alternate anticoagulant (bivalirudin-- a synthetic, direct thrombin inhibitor) was used.
Before surgery, even though his heparin-specific IgE level was found to be negative, we pursued further evaluation of his reaction as it might relate to heparin. Non-synthetic, pharmaceutical-grade heparin, a polysaccharide derived from bovine lung and porcine intestinal mucosa1, has been shown to contain structurally-related impurities that can vary from lot to lot.2 Because alpha-gal is also a carbohydrate and is also found in bovine and porcine tissue, specific IgE to this oligosaccharide was checked and found to be significantly positive, although this patient had no history of urticaria or angioedema that could be associated with ingestion of mammalian meat.
His post-operative course proceeded without development of urticaria, angioedema, or anaphylaxis. At phone follow-up 6 months later, he reported that he was rehabilitating well and consuming an unrestricted diet inclusive of mammalian meat without any symptoms.
DISCUSSION
Serum IgE antibody specific for alpha-gal has been confirmed as a cause of delayed anaphylaxis and/or generalized urticaria 3- 6 hours after the ingestion of mammalian meat.3 Sensitization can occur via tick bites, specifically from Amblyomma americanum (the lone star tick), or via chigger bites.
Most immunocompetent humans have alpha-gal-specific serum IgG and IgM antibodies.4 Hyperacute rejection of porcine organs by primates is triggered by the binding of these “xenoreactive natural antibodies” to endothelium. Similarly, in two of these IgE-positive cases, implantation of bioprostheses appears to trigger a relatively immediate onset of symptoms; this is likely due to the rapid release of known remaining alpha-gal antigen in the valve tissue after processing. Minimal release of antigen thereafter is likely the reason for lack of ongoing symptoms. Konakci et al. demonstrated that patients whose valves were replaced with valves from other mammals developed a significant increase of anti-alpha-gal-IgM antibodies compared to patients who received mechanical valve replacements.5
Conventional biological valves of xenogenic origin, as were used in these cases, are treated with and preserved in glutaraldehyde to reduce antigenicity of native tissue and to ensure sterility and improve mechanical strength of the implant. However, replacement is often necessary due to calcification and subsequently decreased durability. Decellularized valves, shown to have no detectable expression of alpha-gal6, have been reported to be less immunogenic and more durable.7, 8 Bloch et al. found a considerable anti-alpha-gal antibody response to glutaraldehyde-treated porcine and bovine valves, but no response to decellularized valves, suggesting that glutaraldehyde treatment is not sufficient to eliminate immune response to the alpha-gal epitope completely.7 Therefore, it is possible that decellularized valves should be considered for use in patients with IgE to alpha-gal.
These case studies, given the difference in clinical courses including lack of reactivity of Case 3, are not sufficient to identify risk factors or predict outcome for such perioperative anaphylaxis episodes. In addition, although we chose to discuss heparin in this report since it is a polysaccharide derived from porcine and bovine sources, we currently have no evidence that heparin reactions are related to IgE to alpha-gal. These studies do emphasize that patients with IgE to alpha-gal should be evaluated and carefully observed for signs of perioperative and postoperative anaphylaxis and that the possible risk should be considered.
Clinical Implications.
We report three cases of porcine/ bovine valve replacement in patients with IgE antibodies to alpha-gal; two patients had perioperative reactions.
At present, there is no way to predict the severity of reactions following valve replacement in patients with mammalian meat allergy.
Table I.
Total IgE and Specific IgE to alpha-gal and Various Mammalian Meats1
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
|
| |||
| Total IgE (IU/mL) | 403 | 1601 | 364 |
|
| |||
| Allergen | Specific IgE (ku/L) | ||
|
| |||
| Alpha-gal | 65 | 75.3 | 78.4 |
| Beef | 10 | 4.96 | 23.0 |
| Pork | 4.43 | 4.68 | 23.6 |
| Lamb | 5.09 | 2.02 | 5.96 |
| Heparin2 | Not done | Not done | <0.35 |
Assays are carried out with the specific ImmunoCAP using an ImmunoCAP 250 machine from ThermoFisher Scientific.
The assay was prepared for this study using biotinylated heparin (EMD Millipore, Darmstadt, Germany) on an ImmunoCAP with streptavidin on the solid phase.
Footnotes
Case 1 was presented at the 2012 Annual Meeting of the American College of Asthma, Allergy & Immunology.9
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gatti G, Casu B, Hamer GK, Perlin AS. Studies on the Conformation of Heparin by lH and 13C NMR Spectroscopy. Macromolecules. 1979;12(5):1001–1007. [Google Scholar]
- 2.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, et al. Contaminated Heparin Associated with Adverse Clinical Events and Activation of the Contact System. N Engl J Med. 2008;358(23):2457–67. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commins SP, Platts-Mills TA. Allergenicity of carbohydrates and their role in anaphylactic events. Curr Allergy Asthma Rep. 2010;10(1):29–33. doi: 10.1007/s11882-009-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galili U. The alpha-gal epitope and the anti-gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 5.Konakci KZ, Bohle B, Blumer R, Hoetznecker W, Roth G, Moser B, et al. Alpha-gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35(1):17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 6.Simon P, Kasimir MT, Rieder P, Seebacher G, Wolner E, Weigel G. Presence and Elimination of the Xenoantigen Gal (alpha1, 3) Gal in Tissue-Engineered Heart Valves. Tissue Eng. 2005;11(7-8):1274–80. doi: 10.1089/ten.2005.11.1274. [DOI] [PubMed] [Google Scholar]
- 7.Bloch O, Golde P, Dohmen PM, Posner S, Konertz W, Erdbrugger W. Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves. Tissue Eng Part A. 2011;17(19-20):2399–405. doi: 10.1089/ten.TEA.2011.0046. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves AC, Griffiths LG, Anthony RV, Orton EC. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis. 2005;14(2):212–7. [PubMed] [Google Scholar]
- 9.Mozzicato S, Posthumus J, Platts-Mills T, Commins S. Porcine Valve Replacement in a Patient with Positive Titers to Alpha-Gal. Abstract, Ann Allergy Asthma Immunol. 2012;109(5, Suppl.1):A74. [Google Scholar]