Abstract
The purpose of this exploratory study was to examine the interaction of 5-HTTLPR and DRD4 exon III polymorphisms with gender in non-treatment seeking alcohol dependent (AD) individuals while alternately taking ondansetron and sertraline. Evidence suggests that alcohol dependence may be influenced by a genetic interaction that may be gender specific with temporal changes making pharmacological treatment with serotonergic drugs complex. The main trial was a within-subject double-blind placebo-controlled human laboratory study with 77 non-treatment-seeking AD individuals randomized (55 completed, 49 complete data) to receive 200mg/day of sertraline or 0.5mg/day of ondansetron for 3-weeks followed by an alcohol self-administration experiment (ASAE), then placebo for three weeks followed by a second ASAE, then receive the alternate drug, in a counterbalanced order, for three weeks followed by a third ASAE. Results for men were not significant. Women with the LL 5-HTTLPR genotype receiving ondansetron and SS/SL 5-HTTLPR genotypes receiving sertraline (matched), drank significantly fewer drinks per drinking day (DDD) during the 7-days prior to the first and third ASAEs than women receiving the mismatched medication (i.e. sertraline to LL and ondansetron to SS/SL). In a three-way interaction, 5-HTTLPR alleles by DRD4 alleles by medications, women with the LL genotype who received ondansetron and had DRD4 ≥7 exon III repeats drank significantly fewer DDD as did SS/SL women who received sertraline but conversely had DRD4 <7-repeats in the 7-day period leading up to the first and third ASAEs. Consistent with these data was a significant reduction of milliliters consumed ad lib during these same ASAEs. These exploratory findings add possible support to gender and genetic differences among AD individuals in response to serotonergic pharmacotherapies. Future trials should be powerful enough to take into account that endophenotypes and a targeting of serotonergic interactions may be essential to successfully treat alcohol dependence.
Keywords: Alcohol, Alcoholism, Gender, Ondansetron, Sertraline, 5'-HTTLPR, DRD4, Polymorphism, Endophenotype
Introduction
Alcohol influences the release of several key neurotransmitters including dopamine (DA) and serotonin (5-HT), which, in turn, may mediate alcohol-related reward processing. For example, DA is an important neurotransmitter to facilitate motivation, reward and reinforcement in alcohol dependent patients and is interconnected with 5-HT (Koob, 2011; Tupala and Tiihonen, 2004). An increase in 5-HT3 receptors results in an increase in DA release at the nucleus accumbens via the mesolimbic pathway (De Deurwaerdere et al., 1998). This pathway is strongly associated with susceptibility to alcoholism (Volkow et al., 2002), the development of craving and loss of control (Robinson and Berridge, 1993) and the acquisition of excessive motivational properties by alcohol related cues (DiChiara, 1995). Indeed, the action of a serotonergic non-dopaminergic medication such as ondansetron to reduce drinking may in fact be mediated by DA (De Deurwaerdere et al., 1998). As a result, treatment of alcohol dependence with pharmacotherapies that affect these neurotransmitters has strong clinical appeal. Yet, pharmacotherapy results particularly with serotonergic medications that ultimately affect both 5-HT and DA remain inconsistent (Garbutt et al., 1999; Kranzler and McKay, 2012). To address these inconsistencies, polymorphisms and gene × gene interactions are of growing significance in the context of personalized medicine research associated with alcoholism that may also affect medication response (Heilig et al., 2011).
One hypothesis suggests that alcoholic individuals with a strong biological predisposition have a dysregulation of serotonergic function primarily associated with 5-HT transporter (5’-HTTLPR) function (Johnson, 2000). The foundation of this hypothesis examines the polymorphic differences associated with 5-HTTLPR expression initially proposed as biallelic with three possible allele combinations: homozygous LL, SS and heterozygous SL (Heils et al., 1997; Johnson, 2000). The efficacy of ondansetron, a 5-HT3 antagonist and anti-emetic, is proposed to be the result of modulating 5-HT3 function in alcoholics with LL 5-HTTLPR genotype rather than in alcoholics with the SS/SL genotypes and reduction in pre-cortical DA release manifested as a decrease in alcohol’s rewarding effects (Johnson, 2000).
A complementary mechanism to the 5-HT3 pathway in this hypothesis is also proposed for the predominant SS/SL variants that have similar rates of serotonin transport. Treatment with a selective serotonin reuptake inhibitor (SSRI) such as sertraline facilitates 5-HT transmission and inhibition of DA (Pettinati et al., 2000). This putatively results in a diminution of reward during acute alcohol consumption. The differential response to sertraline and ondansetron therefore may be in part due to possessing one of these 5-HTTLPR alleles (Johnson, 2000).
We previously reported the results of a within-subject double-blind placebo-controlled counter-balanced human laboratory pilot study that matched and mismatched 5-HTTLPR genotypes, with alternate administration of either ondansetron or sertraline to 15 non-treatment seeking AD individuals (Kenna et al., 2009). In this within-group design, we reported that at the first alcohol self-administration experiment (ASAE), ondansetron compared to sertraline significantly improved drinking outcomes for the LL genotype only for the ASAE on volume of alcohol consumed and for drinks per drinking day (DDD) during the 7 days prior to the first ASAE. By contrast there was no support that sertraline reduced alcohol use in individuals who had SS/SL alleles (Kenna et al., 2009).
More recent clinical trials continue to refine and delineate these and other polymorphic combinations providing strong support for the role on pharmacogenetics in the response to ondansetron (e.g. Johnson et al., 2011; Johnson et al., 2013) and sertraline (Kranzler et al., 2011) in AD individuals. For example, Johnson et al. (2013) reported that participants receiving ondansetron compared to placebo carrying one or more of genotypes rs1150226-AG and rs1176713-GG in HTR3A and rs17614942-AC in HTR3B demonstrated a significant overall mean difference in DDD, percentage of heavy drinking days, and days abstinent. Furthermore, combining HTR3A/HTR3B and SLC6A4-LL/TT genotypes increased the target cohort to 34% from a previously reported approximately 20% (Johnson et al., 2011). Kranzler and colleagues (2011) reported results demonstrating that the moderating effect of age of onset on the response to sertraline was conditional on genotype. In LALA homozygotes, the effects of medication group varied by age of onset as late onset alcoholics reported fewer drinking and heavy drinking days when treated with sertraline, compared to early onset alcoholics receiving placebo who reported significantly fewer drinking and heavy drinking days.
A 48-base pair variable number tandem repeat (VNTR) polymorphism in exon III located on the gene encodes the DA receptor D4 (DRD4). For most populations, several length variants exist however common length variants consist of 2, 4 and 7 repeat (Van Tol et al., 1992). G-protein-linked D4 receptor activation attenuates signaling by inhibiting adenylyl cyclase coupling. This inhibition is blunted by the presence of the DRD4 7-repeat allele resulting in decreased receptor sensitivity and is associated with increased measures of novelty seeking and addiction phenotypes (Asghari et al 1995; Ebstein et al. 1998;Ebstein, 2006; McGeary et al., 2007; Oak et al., 2000).
To further account for the complex mechanisms associated with AD, gene by gene interactions are of growing importance in the context of endophenotype research (Ray et al., 2010). For example, there is consistent evidence of an interaction between DA and 5-HT related polymorphisms on impulsivity in infants (Auerbach et al., 2001) and temperament in adults (Varga et al., 2012). Additionally, alcohol use during adolescence may increase the risk for establishing a substance use disorder in adulthood and alcohol stimulates polymorphisms from both the DA and 5-HT systems but differently by gender (Skowronek et al., 2006). However, no exploratory study has examined this gene × gene interaction as a function of gender in an adult AD population receiving serotonergic medications. Such data may help to provide further guidance for serotonergic treatment matching using ondansetron and sertraline. More specifically, we sought to investigate an interaction between gender, the 5-HTTLPR and DRD4 alleles, and alternating pharmacotherapy consisting of ondansetron and sertraline in non-treatment seeking alcoholics.
In this exploratory analysis, based on results previously reported by Skowronek and colleagues (2006) with adolescents, we hypothesized that men with the DRD4 ≥ 7 repeats would be associated with the most drinking and women taking ondansetron with the 5-HTTLPR LL but with the DRD4 < 7 repeats would have the greatest response to ondansetron and reduction of drinking. As for sertraline we examined if sertraline had any efficacy based on the interaction of these polymorphisms, but consistent with the “subtype” hypothesis proposed by Johnson (2000) one might expect that sertraline would demonstrate the most efficacy to reduce drinking in participants with the SS/SL 5-HTTLPR genotypes. Though exploratory, this analysis was performed before the data for the main trial were analyzed (Kenna et al., in press) based on our interest and above hypothesis from several studies published after the main study was initiated.
MATERIALS AND METHODS
Participants
The present sample was recruited with local advertisements in the Providence, RI area. The study was conducted at the Brown University Center for Alcohol and Addiction Studies, approved by the Brown University Institutional Review Board and listed on clinicaltrials.gov (NCT01113164). Written informed consent was obtained from each human subject and that the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 1983. Participants were diagnosed as AD as assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders -IV- Text Revision (SCID-I/P; First et al., 2002) were drinking ≥ 35 standard drinks/week for men or ≥ 28 standard drinks/week for women, and were not seeking treatment for AD.
Study design
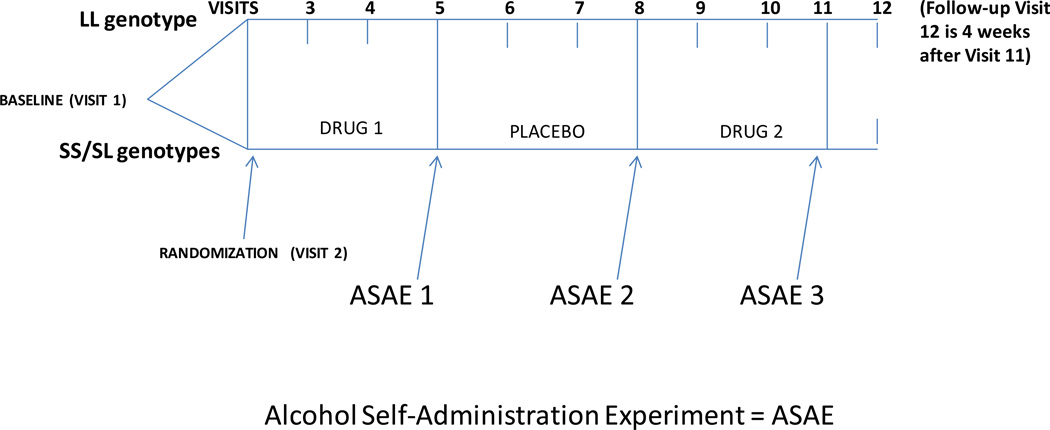
The 14-week experimental study design was a double-blind placebo-controlled mixed two-factor design in which the LL vs. SS/SL genotypes were crossed with the medication condition (within subjects factor). If screened eligible (at Visit 1), participants were randomized at Visit 2 and received three weeks of one active medication, followed by three weeks of placebo, followed by three weeks of the other active medication. An alcohol self-administration experiment (ASAE) was conducted at the end of each of the three medication phases (see Figure 1). Participants were also asked to return for a final visit (12) four weeks after their final ASAE. The order of the active medications was controlled through urn randomization with gender and baseline DDD as the two urn variables, so that half the subjects received 100 mg twice a day of sertraline for the first active medication phase, and the other half received 0.25 mg twice a day of ondansetron for the first medication phase. The main results of this study are published in Kenna et al. (2014).
Figure 1.
Study Design
Procedure
Details of this procedure are provided in a previous publication (Kenna et al., 2009). In brief, the procedure involves administration of a priming drink which must be consumed. The volume of alcohol for all drinks including the priming drink was adjusted for gender, body mass index and age (Watson, 1989). Subsequently, individuals were offered two trays of 4 drinks, each tray followed by a 45 minute drinking period. As an alternative reinforcement, participants received $3.00 for each drink they decided not to drink.
The main dependent measures for this analysis was alcohol consumption as measured by the Timeline Followback (TLFB; Sobell and Sobell, 2000) during the 7-day period leading up to each ASAE, and total milliliters (mls) consumed during each ASAE. The study was conducted in consecutive phases with volunteers in both arms taking the same number of doses a day throughout the study: (1) a one-week screening period; (2) a 21-day treatment period consisting of a 9-day titration up period for those receiving sertraline and a minimum of 12-days (± 3 days) at the target dose; (3) an ASAE on the last day of treatment (± 3 days) at the target dose; (4) a 21-day placebo controlled period; (5) a second ASAE on the last day of placebo (± 3 days); (6) a 9-day titration up period for those receiving sertraline last; (7) a minimum of 12-days (± 3 days) at the target dose; (8) an ASAE on the last day of treatment at the target dose (± 3 days). Sertraline was started at 50 mg for three days and increased 50mg every three days to a maximum dose of 200mg until the day that the ASAE was conducted. Then, sertraline was titrated down over 7 days. Whether ondansetron was administered first or last, there was no titration required for the dose of 0.25mg twice a day (total 0.5mg/day) given for 21 days. Participants remained eligible and included in the analyses as long as they exceeded thresholds of 100mg per day of sertraline and/or 0.25mg of ondansetron (i.e. 50% of the maximum target dose). All participants received interviews with the same trained staff who focused on drug side effects and adherence.
Screening assessments such as the physical, the SCID I/P, family history of alcoholism, genotyping and age of onset of alcoholism were performed at the start of the study. Clinical and psychological assessments were conducted at baseline, weekly throughout the study, and during a 1-month follow-up visit. Alcohol consumption using the TLFB particularly in the 7-day period prior to each ASAE and medication assessment measures was performed throughout the study and at follow-up. Pregnancy tests were performed at screening and immediately before each ASAE in women of childbearing potential. The inclusion and exclusion criteria for this study are the same as were reported previously for our pilot study [15].
Genotyping
We described the genotyping used in this main study in a previous publication of the pilot data (Kenna et al., 2009). It is important to note however that while we were aware of the importance that the 5’HTTLPR that was discovered to be triallelic (i.e. LALA etc; Hu et al., 2005; Hu et al., 2006) due to one cell missing participants, planned analyses using this polymorphism were not feasible. Genotype frequencies were in Hardy–Weinberg equilibrium in both genes.
Statistical Analysis
Analyses were conducted on baseline characteristics to describe the 77 persons urn randomized in the main trial and 49 participants in this exploratory analysis. The skewness and kurtosis of the two outcome measures were examined, to verify that the distributions approximated a normal distribution. Preliminary hierarchical linear modeling (HLM) compared medication matches (LL and ondansetron, SS/SL and sertraline) versus mismatches (SS/SL and ondansetron, LL and sertraline) for the two active medication periods for the two dependent outcome measures (of alcohol consumed during the first and third ASAEs and DDD seven days prior to these two ASAEs). We considered this particular period was consistent with the notion that each medication would be at its relative maximum pharmacodynamic effect. Baseline DDD was entered as a covariate in all HLMs. Next, two HLMs (one for each DV) were conducted adding the LL versus SS/SL variable and a LL versus SS/SL by medication (match vs mismatch) interaction term. Third, two HLMs were conducted adding DRD4 status and three 2-way and one 3-way interactions. Fourth, since there is evidence that the DRD4 exon III moderation effect may vary by gender (Ray et al., 2009; Skowronek et al., 2006) we conducted, two more HLMS with all interactions (baseline DDD was entered as a covariate but was not incorporated in the interaction terms). Finally, given the complexity of interpreting 4-way interactions, we reran the third set of HLMs separately for each gender. Statistical analyses were conducted using SPSS version 20.
RESULTS
Demographics
A total of 117 participants were screened and 77 participants were urn randomized into the study and received study medication. Of the participants randomized, 55 completed the ASAEs during the two active medication phases and we had complete data for 49 participants (19 women and 30 men) in this sample who are the focus of this exploratory research. Demographics for the main trial and this exploratory analysis are shown in Table 1. Those participants receiving ondansetron first and those participants receiving sertraline first, were equivalent on gender [45% women and 50% women, respectively, X2(1,N = 49) = 0.13, p = .72]. Likewise, baseline DDD was equivalent across the two groups [ondansetron first: 11.94 DDD (SD = 7.84), sertraline first: 13.92 DDD (SD = 7.04), t(47) = 0.93, p = .36]. There were slightly more SS/SL participants (55%) than LL participants (45%). Forty one percent of the sample received a hypothetical matching medication first (ondansetron + LL or sertraline + SS/SL alleles) and 59% of the sample received a hypothetical mismatched medication first (ondansetron + SS/SL or sertraline + LL alleles). Fifty seven percent of the sample had < 7 DRD4 repeats (range 2 – 10 repeats).
Table 1.
Demographics of study participants
| Main Trial (N=77) |
Exploratory Study (n=49) |
|
|---|---|---|
| Age (years) | ||
| (M) | 43.4 | 43.0 |
| (SD) | 10.4 | 11.2 |
| Gender (%) | ||
| Women | 35 | 39 |
| Men | 65 | 61 |
| Ethnicity (%) | ||
| African-American | 20.8 | 28.6 |
| Hispanic | 5.2 | 6.1 |
| Asian | 0 | 0 |
| Caucasian | 64.9 | 53.1 |
| American Indian, Hawaiian or Alaskan |
1.3 | 2.0 |
| Multiethnic | 7.8 | 10.2 |
| AUDIT Score (baseline) | ||
| (M) | 16.2 | 16.0 |
| (SD) | 7.3 | 7.8 |
| Drinks per Drinking Day (DDD; 28-day baseline) |
||
| (M) | 12.9 | 12.3 |
| (SD) | 7.16 | 7.0 |
Preliminary qualitative analyses and subsequent HLM analyses suggested a carryover effect for the placebo condition, as was suggested by the results of our earlier pilot study (Kenna et al., 2009). More specifically, (1) overall drinking was lower during the placebo period than the first active medication period; (2) the ranks of the four groups (medication X LL vs SS/SL) were similar from the first active medication period to the placebo period (if there was no carryover effect, some crossover would be expected); (3) when all three periods were entered in HLM the medication match versus mismatch term, was weaker than when only the first and third period were included. We therefore focus on data from the two active medication conditions. The mean number of DDD during the 7-days prior to the ASAE was 10.8 (SD = 6.8). The mean number of milliliters (mls) consumed post priming drink during the ASAE was 209.0 mls (SD = 320.0). Skewness and kurtosis for these two variables were greater than −2.0 and less than 2.0.
Genotype effects
HLMs evaluating the medication match versus mismatch term with baseline DDD were not significant for DDD during the week prior [F(1,81.7) = 0.33, p = 0.57] nor for mls of alcohol consumed after the priming drink during the ASAEs [F(1,69.5) = 1.09, p = .30]. When L/L versus S/S or S/L was added to the HLMs, along with a gene X medication match vs mismatch term, the interaction term was not significant for DDD [F(1,80.9) = 0.40, p = .53] and not significant for mls consumed [F(1,68.5) = 0.25, p = .62]. Next, DRD4 status was added to the HLMs creating a 3-way interaction, which again was not significant for DDD [F(2,80.5) = 0.86, p = .43] and not significant for alcohol consumed during the ASAE [F(2,69.0) = 2.35, p = .10]. When gender was added to the HLMs, there was a significant 4-way interaction [DDD: F(3,78.3) = 9.04, p = .004; ASAE: F(3,44.1) = 3.93, p = .05]. However, given the difficulties in interpreting 4-way interactions, and the limited cell-sizes in this analyses we next conducted HLMs looking at the previously examined 3-way interaction for each gender separately. Table 2 shows the breakdown of cell sizes for the three way interaction. In this approach, the 3-way interaction was significant in both analyses for women [DDD: F(1,16.3) = 9.09, p = .002; ASAE: F(1,33.2) = 11.88, p = .002. Analyses comparing 5HTT matches versus mismatches for men on DDD prior to the ASAE and mls during the ASAE were not significant.
Table 2.
Cell participants in analysis by gender and 5-HTTLPR and DRD4 polymorphisms
| Gender | Females (n=19) | Males (n=30) | ||||||
|---|---|---|---|---|---|---|---|---|
| 5’HTTLPR | LL | SS or SL | LL | SS or SL | ||||
| DRD4 | < 7 DRD4 ≥7 | < 7 DRD4 ≥7 | < 7 DRD4 ≥7 | < 7 DRD4 ≥7 | ||||
|
Number in cell TOTAL (n = 49) |
6 | 4 | 4 | 5 | 9 | 4 | 9 | 8 |
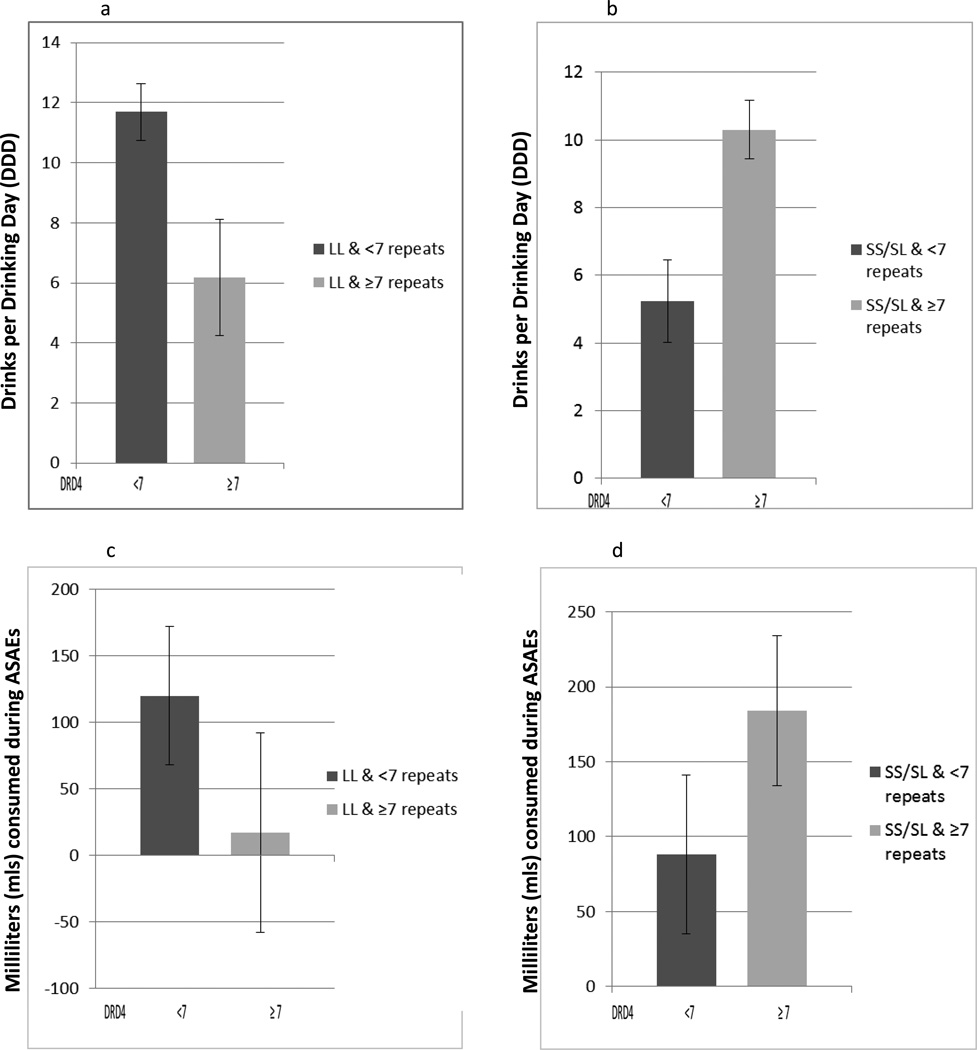
In Figure 2a (for ondansetron) and 2b (for sertraline) half of the 2 × 2 × 2 interaction is plotted in each figure for DDD in the 7- day period leading up to the 1st and 3rd ASAEs for women alone; i.e., only the medication matches are shown (in Figure 2a ondansetron matched to LL alleles and in Figure 2b sertraline matched to SS or SL alleles). The baseline for the ondansetron responders was 23.41 DDD. Thus the data shown in Figure 2a (the group with DRD4 ≥7 repeats) represents a drop from 23.41 DDD to 6.18 DDD (or a 74% reduction). Likewise, sertraline responders (Figure 2b., the group with < 7 repeats), DDD decreased from 15.51 DDD to 5.23 DDD (or a 66% reduction). The HLM mixed-model analysis in women (medication match vs. medication mismatch × LL vs SS/SL × DRD4 <7 vs ≥ 7 repeats) was significant (p=0.002). Women receiving ondansetron with LL 5-HTTLPR alleles but with the DRD4 <7 repeats reported greater DDD [estimated mean (M)=11.69, standard error (SE)=.94] than women with DRD4 ≥7 repeats [M=6.18, SE=1.93]. Women receiving sertraline with SS/SL 5-HTTLPR alleles and DRD4 <7 reported fewer DDD [M=5.23, SE=1.21] than women with DRD4 ≥7 repeats [M=10.3 SE=8.67]. Figure 2c and 2d also show a significant 3-way interaction (p = 0.002] across ASAEs for women. The result across the first and third ASAE was consistent with drinking in the 7-days prior to the ASAEs. Women receiving ondansetron (Figure 2c) with L/L 5-HTTLPR alleles and DRD4 <7 drank more mls during the ASAE [M= 120.0, SE=.52] than women with DRD4 ≥7 repeats [M= 17.0, SE= .75]. Women receiving sertraline (Figure 2d) with SS/SL 5-HTTLPR alleles and DRD4 <7 drank fewer mls during the ASAE [M= 88.0, SE=.53] than women with DRD4 ≥7 repeats [M= 184.0, SE= .50].
Figure 2.
a Ondansetron matched with 5-HTTLPR LL vs. DRD4 <7 or ≥ 7 repeat alleles for women; Drinks per drinking day in 7-day period prior to first and third Alcohol Self-Administration Experiments; 3-way interaction, [F (1,16.3)=9.09, p=0.002]; Estimated Means and Standard Errors.
b Sertraline matched with 5-HTTLPR SS/SL vs. DRD4 <7 or ≥ 7 repeat alleles for women; Drinks per drinking day in 7-day period prior to first and third Alcohol Self-Administration Experiments; 3-way interaction, [F (1,16.3)=9.09, p=0.002]; Estimated Means and Standard Errors.
c Ondansetron matched with 5-HTTLPR LL vs. DRD4 <7 or ≥ 7 repeat alleles for women; milliliters consumed during first and third Alcohol Self-Administration Experiments; 3-way interaction, F(1,33.2)=11.88, p = 0.002; Estimated Means and Standard Errors.
d Sertraline matched with 5-HTTLPR SS/SL vs. DRD4 <7 or ≥ 7 repeat alleles for women; milliliters consumed during first and third Alcohol Self-Administration Experiments; 3-way interaction, F(1,33.2)=11.88, p = 0.002; Estimated Means and Standard Errors.
Lower order interactions and terms in these two analyses for women were also analyzed. The LL status X medication interaction was significant for both dependent measures [DDD: F(1,15.6) = 25.7, p < .001; ASAE: F(1,15.7) = 11.53, p = .004; cell means for both measures suggested a medication matching effect for SS/SL and sertraline, but not LL and ondansetron]. A DRD4 X medication interaction was significant for the ASAE [F(1,18) = 9.41, p = .007] but not DDD. A DRD4 by LL status interaction was not significant for either DV. LL status predicted DDD [F(1,15.6) = 14.3, p = .002, higher for SS/SL] and alcohol consumed during the ASAE [F(1,15.7) = 26.95, p < .001, again higher for SS/SL]. DRD4 status predicted alcohol consumed during the ASAE [F(1,16.3) = 12.8, p = .002, lower drinking for 7 or more repeats] but not DDD. Baseline drinking predicted DDD [F(1,17.7) = 54.5, p < .001] but not alcohol consumed during the ASAE.
DISCUSSION
In this exploratory study we provide evidence for a 3-way gene × gene × medication interaction for women only. This finding, albeit preliminary, may help provide the basis to further explore the ambiguous nature of serotonergic pharmacotherapy for alcoholism literature to date. Our study confirmed a gender effect demonstrated by an interaction between the 5-HTTLPR the DRD4 and serotonergic medications but was not consistent with our a priori hypothesis. Specifically women with the LL 5-HTTLPR and DRD4≥7-repeat allele receiving ondansetron and women with the SS/SL 5-HTTLPR but the DRD4 <7-repeat allele receiving sertraline, had a significant reduction in drinking, both in the naturalistic (assessed via the TLFB) and the ‘bar-like’ (assessed via the ASAE) human laboratory settings. Speculatively, given that the majority of alcohol studies consist mostly of men, having homogeneous subgroups like women respond or not respond to treatment could be one potential source of systematic error that results in the inconclusive main effects reported using serotonergic drugs. These results suggest the DRD4 allele should be considered particularly in context with the 5-HTTLPR alleles and gender in alcoholism research and potential influence on serotonergic medications.
Data suggest that 5-HT has a regulatory role over DA and serotonergic dysfunction may thus alter DA function and DA mediated behavior (Johnson 2000; Olijslagers et al., 2006; Quist and Kennedy 2001). However, while the 5-HTTLPR is well researched its role in psychiatric medicine is still not fully understood as the data are not consistent (for review, see: Kenna et al., 2012). Dopamine and 5-HT are both linked to variations in personality traits as well as to psychiatric disorders (Holmboe et al., 2011). For example, in a study of temperament (Auerbach et al., 2001) and response to novelty in infants (Lakatos et al., 2003), the shortest duration of looking and greater anxiety to a stranger’s initiation of contact, respectively, were both associated with the DRD4 7-repeat allele and SS 5-HTTLPR genotypes. By contrast, in a sample of 90 infants, those carrying the DRD4 7-repeat allele had a higher level of negative affect, and infants with both the DRD4 VNTR 7-repeat allele and the highest expressing 5-HTTLPR homozygous triallele LALA, had the highest level of negative affect (Holmboe et al., 2011). Furthermore, changes in negative affect have been demonstrated to be moderated by gender and 5-HTTLPR genotype (Brummett et al., 2008). In alcoholics, impulsivity, novelty seeking (Evren et al., 2012) and negative affect (Heilig et al., 2010) are linked to increased risk for craving and relapse to drinking. While personality traits were not a focus of this study, this line of research does suggest that DRD4 and 5-HTTLPR polymorphisms could predispose some individuals to alcoholism and may react differently to pharmacotherapy.
The number of tandem repeats may express functional differences in D4 receptors, and influence craving after alcohol consumption when exposed to alcohol associated stimuli (Oak et al., 2000). Hutchison and colleagues (2002) reported that individuals with the DRD4 7-repeat allele demonstrated greater craving after alcohol consumption than after a control beverage and greater craving when exposed to alcohol cues suggesting that these individuals may be particularly sensitive to the phasic effects of D4 stimulation triggered by exposure to a priming dose of alcohol or alcohol cues. A subsequent study replicated the finding that individuals with the 7-repeat allele experienced greater craving after alcohol consumption and also showed that olanzapine attenuated craving and reduced alcohol consumption (Hutchison et al., 2003). As such, it is possible to hypothesize that the significant effect of ondansetron in reducing alcohol use in women with DRD4≥7-repeat allele may be due to the ability of the medication to reduce craving triggered by cues such as the priming dose of alcohol used in the ASAE.
Alternatively the DRD4 <7-repeat allele has also been shown to be a risk factor for alcoholism (Du et al., 2010). In a sample of adolescents the influence of both the DRD4 exon III and the 5-HTTLPR polymorphisms, and an interaction with alcohol and nicotine experimentation was reported by gender. The DRD4 7-repeat allele alone was associated with greater drinking and smoking involvement in boys. In girls however, a significant DRD4 × 5-HTTLPR interaction was reported, as girls who had the LL 5-HTTLPR alleles, but without the DRD4 7-repeat genotype, reported the highest smoking and drinking activity (Skowronek et al., 2006). The LL 5-HTTLPR genotype combined with the DRD4 <7-repeats, is proposed to cause an imbalance between DA and 5-HT systems resulting in greater substance use (Skowronek et al., 2006). Contrary to our prediction however, this particular group of women had the least efficacious response to ondansetron.
One possible explanation why men and women as well as adolescents and adults may differ on genetic associations could be related to changes in gene expression as a consequence of neuroendocrine influences that change developmentally over time (Edelman et al., 2012; Munafò et al., 2005). Additionally, there are strong and consistent differences in sex and stress hormones between men and women that affect or are affected by alcohol consumption (Kenna et al., 2012; Mendelson and Mello, 1988). Therefore it is not uncommon to see subtype and gender differences in response to treatment for AD with sertraline and ondansetron, and suggest that there is enough extant evidence that these differences are at least partially moderated by genes (Kranzler et al., 2012; Pettinati et al., 2004; Roache et al., 2008).
There are limitations to this research that should be noted. First, the small sizes of the cells contribute to instability of the results and lack of statistical power limit our ability to consider the full importance of this exploratory analysis within the context of endophenotype response to medications. However, we do note that while there are substantial differences in assessing alcohol use naturalistically to that under the conditions of a bar-laboratory, our results measured during the ASAEs were consistent with self-reported drinking by female participants. Second is that since this study was conceived, several other important polymorphisms and associations have been further researched (e.g. Johnson et al., 2011; Johnson et al., 2013; McGeary et al., 2007; Ray et al., 2009; Skowronik et al., 2006; Varga et al., 2012). Moreover, we attempted to examine our results in light of the importance of the LALA 5-HTTLPR genotype however our cell sizes were too small. Finally, the likelihood of population stratification as a confound was considered as there are suspected differences in alcohol consumption and ethnic differences based on 5-HTTLPR, DRD4 polymorphic variants (e.g. Chang et al., 1997; Lusher et al., 2001; Praschak-Rieder et al., 2007; Vaughn et al., 2009). As a result it is possible that the relationship between 5-HTTLPR, DRD4 polymorphisms and alcoholism is real, but only in some populations and not others (Cardon and Palmer, 2003). While the overall sample in this study was relatively small and the majority of participants were of the same race (Caucasian), the most effective strategy to limit population stratification is considered to carefully match cases with controls (Cordell and Clayton, 2005). While formal tests of population stratification using genomic control were not conducted, given that the current design used cases as their own controls, limits, but of course does not eliminate, the potential for confounding. Therefore there is much more that could be done to perform this type of genotype research in much larger studies.
In conclusion, the implications of our research are consistent with previous alcohol research suggesting that individuals with specific characteristics may possibly demonstrate a particular pharmacotherapy response. Additionally it must be remembered that the participants in this study were not seeking treatment for their alcohol use yet still recorded reductions in drinking. While we recognize the potential importance of treatment matching, we are mindful that the study wasn’t powered for this hypothesis and the small sample size used in this analysis that may contribute to the instability of the results.
Evidence from clinical trials suggests that serotonergic medications interact pharmacogenetically with genes. However, recent clinical trials with ondansetron (Johnson et al., 2011) and sertraline (Kranzler et al., 2011) were not designed to investigate the possible moderating effect of the DRD4 alleles on the 5-HTTLPR alleles and treatment response. While we recognize that our results do little to facilitate the kind of prominent light needed to clarify the targeted clinical use of ondansetron or sertraline in a treatment-seeking population, nonetheless, this exploratory study suggests a possible gender specific medication × gene × gene interaction in the effects of serotonergic pharmacotherapies in AD that warrants further investigation.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant R01-AA016079 (PI: Kenna), Shared Equipment grants (ShEEP) from the Medical Research Service Department of Veteran Affairs, and 1S10RR023457-01A1 (PI: McGeary). The authors declare that they have no conflict of interest or financial relationship with the funding agency (NIAAA) and that the study was performed according to the approval of the Brown University Institutional Review Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. J. Child Psychol. Psychiatry. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Muller CL, Collins AL, Boyle SH, Kuhn CM, Siegler IC, Williams RB, Ashley-Koch A. 5-HTTLPR and gender moderate changes in negative affect responses to tryptophan infusion. Behav Genet. 2008;38:476–483. doi: 10.1007/s10519-008-9219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Chang FM, Ko HC, Lu RB, Pakstis AJ, Kidd KK. The dopamine D4 receptor gene (DRD4) is not associated with alcoholism in three Taiwanese populations: six polymorphisms tested separately and as haplotypes. Biol Psychiatr. 1997;41:394–405. doi: 10.1016/S0006-3223(96)00248-X. [DOI] [PubMed] [Google Scholar]
- Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366:1121–1131. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P, Stinus L, Spampinato U. Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J. Neurosci. 1998;18:6528–6538. doi: 10.1523/JNEUROSCI.18-16-06528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Du Y, Yang M, Yeh HW, Wan YJ. The association of exon 3 VNTR polymorphism of the dopamine receptor D4 (DRD4) gene with alcoholism in Mexican Americans. Psychiatry Res. 2010;177:358–360. doi: 10.1016/j.psychres.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol. Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH. Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Mol. Psychiatry. 1998;3:238–246. doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Edelman S, Shalev I, Uzefovsky F, Israel S, Knafo A, Kremer I, Mankuta D, Kaitz M, Ebstein RP. Epigenetic and genetic factors predict women’s salivary cortisol following a threat to the social self. PLoS One. 2012;7:e48597. doi: 10.1371/journal.pone.0048597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren C, Durkaya M, Evren B, Dalbudak E, Cetin R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug Alcohol Rev. 2012;31:81–90. doi: 10.1111/j.1465-3362.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. November 2002. [Google Scholar]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: A Review of the Evidence. J. Am. Med. Assn. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat. Rev. Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism--basic research and clinical implications. J. Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RM, Sasvari-Szekely M, Johnson MH. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes Brain Behav. 2011;10:513–522. doi: 10.1111/j.1601-183X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the LR to alcohol and alcoholism risk. Alcohol. Clin. Exp. Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary JE, Smolen A, Bryan A, Swift RM. The DRD4 VNTR Polymorphism Moderates Craving After Alcohol Consumption. Health Psychol. 2002;21:139–146. [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, Paris L. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsych. 2003;28:1882–1888. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept--an hypothesis. Alcohol Clin. Exp. Res. 2000;24:1597–1601. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am. J. Psychiatry. 2011;168:265–275. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of Genotype Combinations That Can Predict the Outcome of the Treatment of Alcohol Dependence Using the 5-HT3 Antagonist Ondansetron. Am. J. Psychiatry. 2013;170:1020–1031. doi: 10.1176/appi.ajp.2013.12091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Hanna-Roder N, Leggio L, Zywiak WH, Clifford J, Edwards S, Kenna JA, Shoaff J, Swift RM. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: review of psychopathology and pharmacotherapy. Pharmacogenomics Pers. Med. 2012;5:19–35. doi: 10.2147/PGPM.S23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol. Rev. 2012;22:211–228. doi: 10.1007/s11065-012-9209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Zywiak WH, McGeary JE, Leggio L, McGeary C, Wang S, Grenga A, Swift RM. A within-group design of nontreatment seeking 5-HTTLPR genotyped alcohol-dependent subjects receiving ondansetron and sertraline. Alcohol. Clin. Exp. Res. 2009;33:315–323. doi: 10.1111/j.1530-0277.2008.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Zywiak WH, Swift RM, McGeary JE, Clifford JS, Shoaff JR, Vuittonet C, Fricchione S, Brickley M, Beaucage K, Haass-Koffler C, Leggio L. Ondansetron reduces naturalistic drinking in non-treatment seeking alcohol dependent subjects with LL 5’-HTTLPR alleles: a lab study. Alcohol. Clin. Exp. Res. 2014 doi: 10.1111/acer.12410. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit Disorder. Curr. Top. Behav. Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Pettinati H, Oncken C. Double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J. Clin. Psychopharmacol. 2011;31:22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Armeli S, Tennen H. Comparison of alcoholism subtypes as moderators of the response to sertraline treatment. Alcohol. Clin. Exp. Res. 2012;36:509–16. doi: 10.1111/j.1530-0277.2011.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, McKay JR. Personalized treatment of alcohol dependence. Curr. Psychiatry Rep. 2012;14:486–493. doi: 10.1007/s11920-012-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, Toth I, Sasvari-Szekely M, and Gervai J. Association of D4 dopamine receptor gene and serotonin transporter promoter polymorphisms with infants’ response to novelty. Mol. Psychiatry. 2003;8:90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- Lusher JM Chandler C, Ball D. Dopamine D4 receptor gene (DRD4) is associated with Novelty Seeking (NS) and substance abuse: the saga continues. Mol Psychiatry. 200(5):497–499. doi: 10.1038/sj.mp.4000918. 6. [DOI] [PubMed] [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol. Biochem. Behav. 2009;93:222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary JE, Esposito-Smythers C, Spirito A, Monti PM. Associations of the dopamine D4 receptor gene VNTR polymorphism with drug use in adolescent psychiatric inpatients. Pharmacol. Biochem. Behav. 2007;86:401–406. doi: 10.1016/j.pbb.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Chronic alcohol effects on anterior pituitary and ovarian hormones in healthy women. J. Pharmacol. Exp. Ther. 1988;245:407–412. [PubMed] [Google Scholar]
- Munafò MR, Lingford-Hughes AR, Johnstone EC, Walton RT. Association between the serotonin transporter gene and alcohol consumption in social drinkers. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;135B:10–14. doi: 10.1002/ajmg.b.30162. [DOI] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur. J. Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Olijslagers JE, Werkman TR, McCreary AC, Kruse CG, Wadman WJ. Modulation of midbrain dopamine neurotransmission by serotonin, a versatile interaction between neurotransmitters and significance for antipsychotic drug action. Curr. Neuropharmacol. 2006;4:59–68. doi: 10.2174/157015906775203020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist JF, Kennedy JL. Genetics of childhood disorders: XXIII. ADHD, Part 7: The serotonin system. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:253–256. doi: 10.1097/00004583-200102000-00022. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in Type A alcohol dependence. Am. J. Addict. 2004;13:236–247. doi: 10.1080/10550490490459906. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence. Interactive effects of medication and alcoholic subtype. Alcohol Clin. Exp. Res. 2000;24:1041–1049. [PubMed] [Google Scholar]
- Ray LA, Bryan A, Mackillop J, McGeary J, Hesterberg K, Hutchison KE. The Dopamine D4 Receptor (DRD4) Gene Exon III Polymorphism, Problematic Alcohol Use, and Novelty Seeking: Direct and Mediated Genetic Effects. Addict. Biol. 2009;14:238–244. doi: 10.1111/j.1369-1600.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Subst. Use Misuse. 2010;45:1742–1765. doi: 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roache JD. Commentary on comparison of alcoholism subtypes as moderators of the response to sertraline treatment. Alcohol. Clin. Exp. Res. 2012;36:561–563. doi: 10.1111/j.1530-0277.2012.01781.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Skowronek MH, Laucht M, Hohm E, Becker K, Schmidt MH. Interaction between the dopamine D4 receptor and the serotonin transporter promoter polymorphisms in alcohol and tobacco use among 15-year-olds. Neurogenetics. 2006;7:239–246. doi: 10.1007/s10048-006-0050-4. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Handbook of Psychiatric Measures. Wash DC: American Psychological Association; 2000. Alcohol Timeline Followback (TLFB) pp. 477–479. [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Varga G, Szekely A, Antal P, Sarkozy P, Nemoda Z, Demetrovics Z, Sasvari-Szekely M. Additive effects of serotonergic and dopaminergic polymorphisms on trait impulsivity. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2012;159B:281–288. doi: 10.1002/ajmg.b.32025. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow K, Batt R, editors. Human metabolism of alcohol (Vol. 1): Pharmacokinetics, medicolegal aspects, and general interest. Boca Raton, FL: CRC Press; 1989. pp. 41–58. [Google Scholar]