Abstract
BACKGROUND
Although exhaled breath condensate (EBC) pH has been identified as an “emerging” biomarker of interest for asthma clinical trials, the clinical determinants of EBC pH remain poorly understood. Other studies have associated acid refluxeinduced respiratory symptoms, for example, cough, with transient acidification of EBC.
OBJECTIVE
We sought to determine the clinical and physiologic correlates of EBC acidification in a highly characterized sample of children with poorly controlled asthma. We hypothesized that (1) children with asymptomatic gastroesophageal reflux determined by 24-hour esophageal pH monitoring would have a lower EBC pH than children without gastroesophageal reflux, (2) treatment with lansoprazole would alter EBC pH in those children, and (3) EBC acidification would be associated with increased asthma symptoms, poorer asthma control and quality of life, and increased formation of breath nitrogen oxides (NOx).
METHODS
A total of 110 children, age range 6 to 17 years, with poor asthma control and esophageal pH data enrolled in the Study of Acid Reflux in Children with Asthma (NCT00442013) were included. Children submitted EBC samples for pH and NOx measurement at randomization and at study weeks 8, 16, and 24.
RESULTS
Serial EBC pH measurements failed to distinguish asymptomatic gastroesophageal reflux and was not associated with breath NOx formation. EBC pH also did not discriminate asthma characteristics such as medication and health care utilization, pulmonary function, and asthma control and quality of life both at baseline and across the study period.
CONCLUSION
Despite the relative ease of EBC collection, EBC pH as a biomarker does not provide useful information of children with asthma who were enrolled in asthma clinical trials.
Keywords: Asthma, Children, Gastroesophageal reflux, Exhaled breath condensate, Airway inflammation, Airway acidification, Asthma biomarker, Asthma clinical trial
Exhaled breath condensate (EBC) contains volatile and droplet species derived from the respiratory surface liquid, which may identify underlying biochemical and inflammatory disturbances in the airway tree. Although a number of methodologic limitations persist with the analysis of EBC, including unanswered questions about the dilution factor and the distal airway contribution to the sample,1 biomarkers measured in EBC have been shown to discriminate between health and a variety of airway pathologies.2,3 The noninvasive nature of EBC collection, therefore, has generated significant interest of patients with asthma wherein bronchoscopy cannot be routinely conducted. Indeed, a recent National Institutes of Health—sponsored workshop identified EBC biomarkers as “emerging” outcomes of interest for asthma clinical trials and encouraged the use of EBC biomarkers for the characterization of participants with asthma and prospective efficacy and/or effectiveness analyses.4
Although a variety of EBC biomarkers have been investigated in asthma over the past decade, much of the work to date has focused on EBC acidity. Early studies noted that EBC pH values were lower in patients with asthma versus healthy controls at baseline5-8 and decreased further during acute exacerbations.9-11 In these same studies, EBC pH values also normalized with corticosteroid treatment, which suggests that EBC acidity may be a marker of asthma control.9-11 By contrast, in more recent analyses, EBC pH failed to differentiate a large cohort of children with asthma versus controls12 or a large sample of adults and children with severe versus nonsevere asthma.13 Furthermore, no associations between EBC pH and lung function, airway reactivity, or airway cellular profiles in induced sputum were noted.12-14 However, in other studies, acid reflux—induced respiratory symptoms, for example, cough, have been associated with transient acidification of EBC.15-17 In recognizing that the clinical determinants of EBC breath pH remain poorly understood and in further recognizing that the utility of EBC pH monitoring may only apply to selected asthma phenotypes, the purpose of this study was to determine the clinical and physiologic correlates of low EBC pH in a highly characterized sample of children with poorly controlled asthma. We hypothesized that children with asthma and with asymptomatic gastroesophageal reflux as determined by 24-hour esophageal pH monitoring would have a lower EBC pH than children without gastroesophageal reflux, and, further, that treatment with lansoprazole would alter EBC pH in those children. We further hypothesized that breath acidification would be associated with increased formation of breath nitrogen oxides (NOx), increased asthma symptoms, and poorer asthma control and quality of life.
METHODS
This ancillary study included children 6 to 17 years of age with poor asthma control who were enrolled in the Study of Acid Reflux in Children with Asthma trial (NCT00442013).18 Informed consent was obtained for the study, and safety was overseen by a data and safety monitoring board.18 Briefly, the Study of Acid Reflux in Children with Asthma trial was a randomized, double-blinded, placebo-controlled study of 300 children with persistent asthma to determine whether lansoprazole therapy improved poor asthma control. Criteria for enrollment included the following: (1) physician diagnosis of asthma confirmed with either bronchodilator reversibility or airway hyperresponsiveness in response to methacholine or exercise, and (2) stable inhaled corticosteroid therapy for at least 8 weeks. Exclusion criteria included the following: (1) severe persistent asthma evidenced by a FEV1 of less than 60% of predicted norms19; (2) symptoms clearly attributable to gastroesophageal reflux, which required treatment at the time of enrollment; or (3) less than 80% completion of symptom and medication diaries during the screening period. Poor asthma control at the end of the 4-week screening period was defined as either (1) use of short-acting β-agonists twice per week or more on average; (2) nocturnal awakening with asthma symptoms more than once per week on average; (3) 2 or more emergency department visits, unscheduled physician visits, prednisone courses, or hospitalizations for asthma in the past 12 months; or (4) an Asthma Control Questionnaire score of ≥1.25.20 Asthma control throughout the study also was determined by participant diaries in which episodes of poor asthma control (EPAC) were recorded. We defined 2 types of EPACs derived from the daily diaries. The first EPAC was a decrease ≥30% in morning peak flow rate from personal best (assessed during run-in) for 2 consecutive days, or addition of an oral corticosteroid to treat asthma symptoms, or unscheduled contact with a health care provider for asthma symptoms. The second EPAC also included increased use of short-acting β-agonists from baseline (4 or more additional puffs of rescue medication or 2 or more additional nebulizer treatments in 1 day). The primary outcome measurement for the study was a change in the Asthma Control Questionnaire score, with a 0.5-unit change from baseline (randomization) considered clinically meaningful.21 Other secondary outcome measurements included changes in Asthma Symptom Utility Index scores22 and changes in Asthma Quality of Life Questionnaire scores23 between the baseline (randomization) visit and study completion.
Children submitted EBC samples at the randomization visit (week 0) and at study weeks 8, 16, and 24 according to recommended procedures.24 Children were instructed to take tidal breaths for 10 minutes into a plastic tube that contained a 1-way exhalation valve and chilled aluminum condensation tube (RTube; Respiratory Research Inc, Charlottesville, Va). Samples were frozen at –70° C and were transported every other month on dry ice to Emory University for analysis. EBC pH was measured to 2 decimal places before and after de-aeration with argon gas for 8 minutes, as previously described,25 by inserting a glass pipet into 250 mL of the EBC sample. This method allows for constant turbulence of the EBC sample and maximal exposure to carbon dioxide—free gas, which results in stabilization of pH for the purpose of measurement.10,26 The pH was determined by using a standard pH meter and probe (Orion 525 and Orion 98-03; Thermo Electron Corp, Beverly, Mass) calibrated in standard pH solutions (pH 4.0, 7.0, and 10.0) before each use. EBC acidification was defined as a de-aerated EBC pH value <7.4, consistent with previous observations in healthy participants without asthma and in participants with poorly controlled asthma after 48 hours of systemic corticosteroid treatment.10 EBC NOx concentrations (reflected by total nitrite plus nitrate concentrations) were quantified as previously described27 by using a colorimetric assay (Cayman Chemical, Ann Arbor, Mich) with a lower detection limit of 0.1 μM analyzed at a wavelength of 540 nm. To minimize false nitrate and nitrate readings during the assay, samples were analyzed in duplicate immediately after thawing.
Esophageal pH studies were performed in a subset of children whose parents provided informed consent for the procedure before randomization. Esophageal pH studies were performed according to standards established by the North American Society for Pediatric Gastroenterology and Nutrition.28 Children were monitored for 16 to 24 hours, during which eating, drinking, and activities were reported. Thresholds for gastroesophageal reflux were defined according to age as either an esophageal pH ≤4 for ≥6% of the time for children 6 to 11 years old or an esophageal pH ≤4 for ≥4% of the time for children 12 to 17 years old.
Statistical analyses were done by using SAS version 9.2 (SAS Institute Inc, Cary, NC). A comparison of characteristics of participants with and without gastroesophageal reflux or with high versus low EBC pH were made by using χ2 tests for categorical variables and by using Wilcoxon rank sum tests for continuous variables. Generalized linear models were used to determine the association between EBC pH and variables of interest. The associations of baseline EBC pH on change in asthma questionnaires (see supplementary tables in this article's Online Repository at www.jaci-inpractice.org) were estimated from longitudinal models by using generalized estimating equations.29
RESULTS
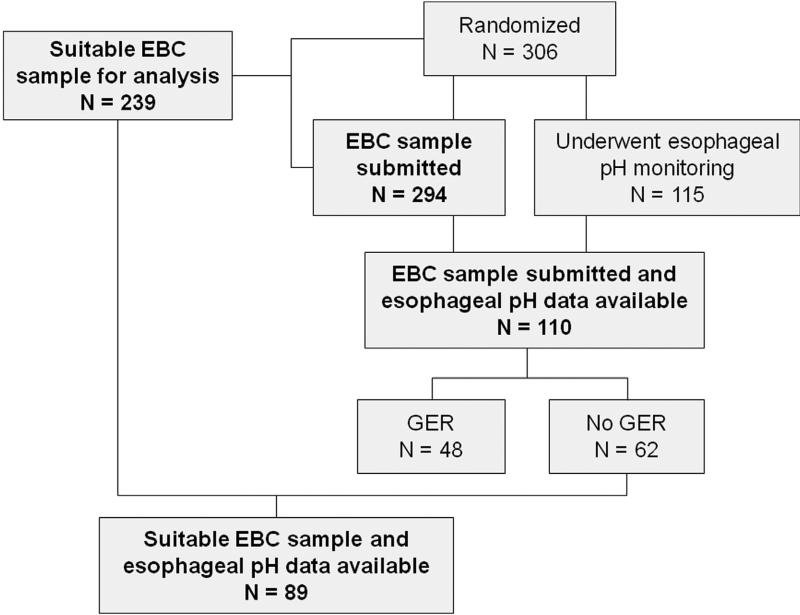
Baseline (randomization) EBC samples were submitted for 294 of the 306 children enrolled in the trial, and 239 children had suitable samples for analysis (Figure 1). Among these, 110 children had 24-hour esophageal pH monitoring studies of suitable quality for interpretation. Features of these children were similar to those of the children enrolled in the parent clinical trial (see Table E1 in this article's Online Repository at www.jaci-inpractice.org). Forty-eight of these children (44%) had asymptomatic gastroesophageal reflux upon analysis, but there were no differences between children with and those without gastroesophageal reflux with regard to age, body mass index (BMI) percentile, and asthma medical history (Table I). However, children with gastroesophageal reflux were more likely to be white. Treatment assignment to lansoprazole or placebo as well as other key asthma characterization variables such as asthma control, asthma-related quality of life, symptoms, and spirometry values were not significantly different between those with or those without gastroesophageal reflux.
FIGURE 1.
Participant inclusion in the ancillary study. GER, Gastroesophageal reflux.
TABLE I.
Characteristics of the participants, with stratification for GER*
| Total sample (n = 110) | GER (n = 48) | No GER (n = 62) | P value† | |
|---|---|---|---|---|
| Age (y), mean ± SD | 12 ± 3 | 12 ± 3 | 11 ± 3 | .31 |
| Race, number of patients (%) | .04 | |||
| White | 47 (43) | 27 (56) | 20 (32) | |
| Black | 55 (50) | 19 (40) | 36 (58) | |
| Other | 8(7) | 2(4) | 6(10) | |
| Sex, number of patients (%) | <.01 | |||
| Boys | 68 (62) | 37 (77) | 31 (50) | |
| Girls | 42 (38) | 11 (23) | 31 (50) | |
| Second-hand smoke exposure, number of patients (%) | 17 (15) | 4(8) | 13 (21) | .11 |
| BMI percentile, mean ± SD | 73 ± 29 | 79 ± 25 | 68 ± 30 | .11 |
| Obese (BMI > 95th percentile), mean ± SD | 38 (35) | 20 (42) | 18 (29) | .48 |
| Self-reported comorbid conditions, number of patients (%) | ||||
| GER | 2(2) | 1 (2) | 1 (2) | >.99 |
| Atopic dermatitis | 53 (48) | 25 (52) | 28 (45) | .47 |
| Chronic sinusitis | 43 (39) | 20 (42) | 23 (37) | .63 |
| Food allergy | 28 (25) | 11 (23) | 17 (27) | .59 |
| Atopic dermatitis | 45 (41) | 18 (38) | 27 (44) | .52 |
| Long-term asthma controller medications, number of patients (%) | ||||
| ICS | 38 (35) | 15 (31) | 23 (37) | .52 |
| ICS + long-acting β-agonist | 72 (65) | 33 (69) | 39 (63) | .52 |
| Montelukast | 61 (55) | 22 (46) | 39 (63) | .07 |
| Urgent care visit for asthma (previous year), number of patients (%) | 80 (73) | 33 (69) | 47 (76) | .41 |
| ACQ score, mean ± SD‡ | 1.2 ± 0.8 | 1.3 ± 0.7 | 1.2 ± 0.8 | .56 |
| AQLQ score, mean ± SD§ | 5.3 ± 1.3 | 5.4 ± 1.2 | 5.3 ±1.4 | .78 |
| ASUI score, mean ± SD∥ | 0.81 ± 0.15 | 0.80 ± 0.15 | 0.81 ± 0.16 | .45 |
| Baseline pulmonary function, mean ± SD | ||||
| FVC (% predicted) | 102 ± 14 | 102 ± 15 | 104 ± 13 | .77 |
| FEV1 (% predicted) | 92 ± 16 | 91 ± 16 | 93 ± 15 | .52 |
| Postbronchodilator pulmonary function, mean ± SD | ||||
| FVC (% predicted) | 103 ± 13 | 103 ±13 | 104 ± 13 | >.99 |
| FEV1 (% predicted) | 99 ± 14 | 97 ± 14 | 101 ± 14 | .22 |
| Methacholine PC20 (mg/mL), mean ± SD | 1.1 ± 5.0 | 0.8 ± 6.0 | 1.0 ± 6.0 | .55 |
ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; ASUI, Asthma Symptom Utility Index; BMI, body mass index; FVC, forced vital capacity; GER, gastroesophageal reflux; ICS, inhaled corticosteroid; PC20, provocative concentration of methacholine resulting in a 20% decline in FEV1.
Data were collected at the randomization visit.
For GER vs no GER.
Scores range from 0 to 6, with lower scores indicating better asthma control and 0.5 as the minimal clinically important difference.
Scores range from 1 to 7, with higher scores indicating better quality of life and 0.5 as the minimal clinically important difference.
Scores range from 0 to i, with higher scores indicating less severe asthma.
EBC characteristics
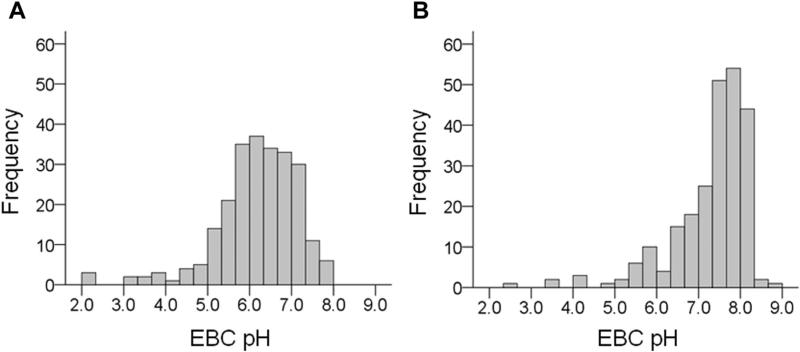
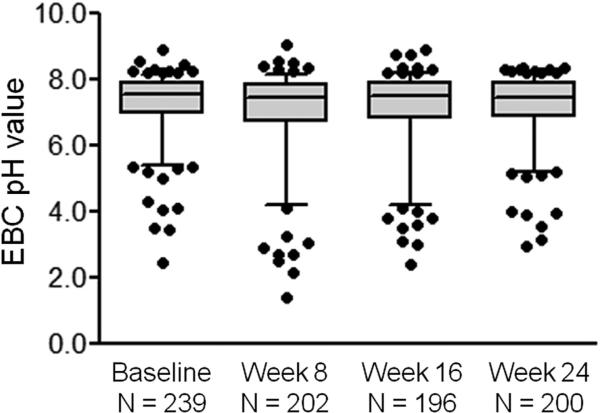
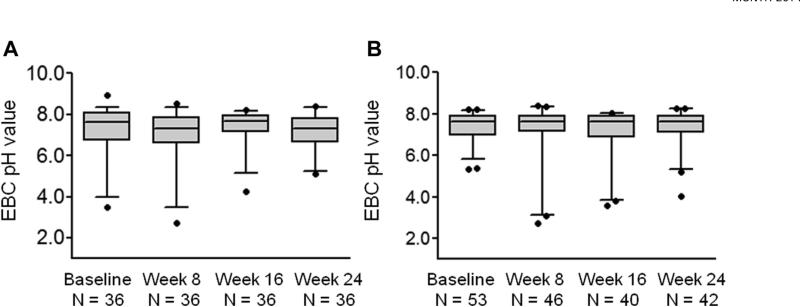
Baseline de-aerated EBC pH values obtained at the randomization visit ranged from 2.46 to 8.92 across all study participants and were skewed toward values higher than 7.40 (median, 7.57; range, 2.46-8.92) (Figure 2). Overall, EBC pH values were relatively stable across the study period. Median EBC pH values in all study participants were 7.44 (range, 1.41-9.06) at week 8, 7.51 (range, 2.39-8.90) at week 16, and 7.43 (range, 2.94-8.37) at week 24 (Figure 3). However, there was some variation in EBC pH values for individual participants (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). No differences in EBC pH were noted between children with and those without gastroesophageal reflux at baseline (pH, median [inter-quartile range], 7.63 [6.71-8.05] vs 7.63 [6.95-7.92], respectively; P = .87) or at study weeks 8, 16, or 24 (Figure 4). Similarly, no differences in EBC pH were observed with lansoprazole treatment across the study period (Figure 5). CIs for mean differences in EBC pH values are shown in Table E2 (in this article's Online Repository at www.jaci-inpractice.org). Samples that were analyzed before the de-aeration procedure showed findings similar to the de-aerated samples and also did not achieve statistical significance (data not shown).
FIGURE 2.
Distribution of baseline EBC pH values (A) before and (B) after de-aeration across all study participants. De-aeration did not significantly alter the EBC pH distribution.
FIGURE 3.
De-aerated EBC pH values across the study period. Horizontal lines represent the median, and whiskers represent the 5th to 95th percentile. EBC pH values were not significantly different between time points.
FIGURE 4.
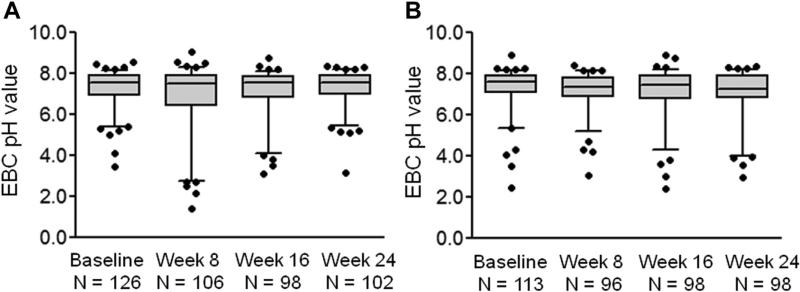
De-aerated EBC pH values across the study period in children (A) with and (B) without gastroesophageal reflux confirmed by 24-hour esophageal pH monitoring. Horizontal lines represent the median, and whiskers represent the 5th to 95th percentile. No significant differences in EBC pH were noted at any time point between children with and without gastroesophageal reflux.
FIGURE 5.
De-aerated EBC pH values across the study period in children treated with (A) placebo and (B) lansoprazole. Horizontal lines represent the median, and whiskers represent the 5th to 95th percentile. No significant differences in EBC pH were noted at any time point between children treated with placebo and children treated with lansoprazole.
Association between EBC pH and NOx concentrations
In recognizing that oxidative stress is present in childhood asthma27,30 and that airway acidification may promote the formation of reactive species,10,31 we explored the association between EBC NOx concentrations and pH. At baseline (randomization) visit, EBC NOx concentrations were skewed to the right and ranged from 0 to 27.06 μM (mean, 3.16 mM; median, 1.92 μM [95% CI, 2.69-3.64 μM]) (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org). There were no significant associations between EBC pH and NOx concentrations (see Figure E3 in this article's Online Repository at www.jaci-inpractice.org). NOx concentrations did not differ across the study period and also did not differ according to gastroesophageal reflux status at baseline (see Figures E4 and E5 in this article's Online Repository at www.jaci-inpractice.org).
Association between EBC pH and asthma control variables
We also explored the association between EBC pH and asthma characteristics regardless of gastroesophageal reflux status. By using a cut point of 7.4 for “high” versus “low” pH, as previously reported,25 no significant differences in baseline asthma characteristics, including asthma symptoms, asthma control, and asthma quality of life were observed between the groups (Table II). Similarly, pH category at baseline did not predict EPAC rates (data not shown). Although children with “low” de-aerated EBC pH <7.4 tended to have a slightly larger BMI percentile, the prevalence of obesity, defined as a BMI above the 95th percentile, did not differ between the groups (Table II). Furthermore, EBC pH values at the baseline (randomization) visit failed to predict changes in Asthma Control Questionnaire, Asthma Quality of Life Questionnaire, or Asthma Symptom Utility Index scores over the study period, even after controlling for the potentially confounding effects of treatment assignment, gastroesophageal reflux, race, and BMI percentile (see Tables E3,E4, and E5 in this article's Online Repository at www.jaci-inpractice.org). When analyses were repeated by using non—de-aerated EBC pH values, no significant findings were noted (data not shown).
TABLE II.
Characteristics of the participants according to “high” (pH ≥ 7.4) vs “low” (pH < 7.4) de-aerated pH*
| EBC pH < 7.4 (n = 90) | EBC pH ≥ (n = 149) | P value | |
|---|---|---|---|
| Age (y), mean ± SD | 11 ± 3 | 12 ± 3 | .20 |
| Race, number of patients (%) | .67 | ||
| White | 32 (36) | 57 (38) | |
| Black | 49 (54) | 73 (49) | |
| Other | 9 (10) | 19 (13) | |
| Sex, frequency (%) | .41 | ||
| Boys | 52 (58) | 94 (63) | |
| Girls | 38 (42) | 55 (37) | |
| Second-hand smoke exposure, number of patients (%) | 9 (10) | 27 (18) | .09 |
| BMI percentile, mean ± SD | 78 ± 25 | 70 ± 30 | .03 |
| Obese (BMI > 95th percentile), number of patients (%) | 33 (37) | 47 (32) | .48 |
| Self-reported comorbid conditions, number of patients (%) | |||
| Gastroesophageal reflux, number of patients (%) | 1 (1) | 2 (1) | .88 |
| Atopic dermatitis | 33 (37) | 67 (45) | .21 |
| Chronic sinusitis | 34 (38) | 42 (28) | .12 |
| Food allergy | 22 (24) | 44 (30) | .39 |
| Atopic dermatitis | 52 (38) | 91 (61) | .61 |
| Long-term asthma controller medications, number of patients (%) | |||
| ICS | 43 (48) | 54 (36) | .08 |
| ICS + long-acting β-agonist | 47 (52) | 95 (64) | .08 |
| Montelukast | 55 (61) | 83 (56) | .41 |
| Urgent care visit for asthma (previous year), number of patients (%) | 71 (79) | 104 (70) | .12 |
| ACQ score, mean ± SD† | 1.2 ± 0.8 | 1.1 ± 0.8 | .45 |
| AQLQ score, mean ± SD‡ | 5.3 ± 1.2 | 5.5 ± 1.2 | .29 |
| ASUI score, mean ± SD§ | 0.8 ± 0.2 | 0.8 ± 0.2 | .20 |
| Baseline pulmonary function, mean ± SD | |||
| FVC (% predicted) | 102 ± 14 | 101 ± 14 | .55 |
| FEV1 (% predicted) | 94 ± 16 | 92 ± 16 | .32 |
| Postbronchodilator pulmonary function, mean ± SD | |||
| FVC (% predicted) | 104 ± 14 | 103 ± 15 | .47 |
| FEV1 (% predicted) | 102 ± 15 | 99 ± 15 | .22 |
| Methacholine PC20 (mg/mL), mean ± SD | 1.1 ± 6.0 | 1.0 ± 5.0 | .80 |
ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; ASUI, Asthma Symptom Utility Index; FVC, forced vital capacity; ICS, inhaled corticosteroid; PC20, provocative concentration of methacholine resulting in a 20% decline in FEV1.
Data were collected at the randomization visit.
Scores range from 0 to 6, with lower scores indicating better asthma control and 0.5 as the minimal clinically important difference.
Scores range from 1 to 7, with higher scores indicating better quality of life and 0.5 as the minimal clinically important difference.
Scores range from 0 to 1, with higher scores indicating less severe asthma.
CONCLUSION
A recent National Institutes of Health—sponsored workshop recently identified EBC constituents and EBC pH in particular as “emerging” biomarkers of interest for asthma characterization and prospective efficacy and/or effectiveness analyses in asthma clinical trials.4 The recommendations of that workshop committee were based on the assumption that EBC pH reflects a biochemical disturbance in general inflammatory disease,25 which may have clinical utility in the context of asthma. As such, the workshop committee concluded that “careful use of EBC pH may allow identification of acute acid reflux events.”4 However, the results from the present study do not support the use of EBC pH measurements in this context and in asthma clinical trials in general. Although other reports have demonstrated breath acid-ification in patients with chronic coughing32 and in those with asthma and with gastroesophageal reflux disease,17 serial measurements of EBC pH in the present study failed to distinguish asymptomatic gastroesophageal reflux in this highly characterized sample of children with poorly controlled asthma. Contrary to our hypothesis, EBC pH also failed to discriminate relevant asthma characteristics, such as medication and health care utilization, pulmonary function, and asthma symptoms, control, and quality of life both at baseline, and across the study period. Thus, despite the relative ease of EBC collection, we concluded that measurements of EBC pH as a characterization variable or predictive biomarker in clinical trials does not provide useful information in children with asthma.
To our knowledge, this is the second-largest study of EBC pH in an asthma population to date13 and the largest study of EBC pH in school-age children with asthma. Moreover, although Liu et al13 previously reported associations between EBC pH and a number of variables, such as BMI, airway neutrophil counts, and prebronchodilator FEV1 in a heterogeneous asthma sample, that study was primarily limited to adults with a different phenotypic pattern of disease (ie, greater airflow limitation, increased neutrophilia, and decreased allergic sensitization) compared with children.33,34 However, Liu et al13 failed to demonstrate differences in EBC pH between patients with severe versus patients with nonsevere asthma. Given the magnitude of symptoms that differentiate severe from nonsevere asthma, those findings are in keeping with the present observation that EBC pH did not discriminate cross-sectional or longitudinal changes in asthma symptoms, control, or quality of life. The relative homogeneity of children enrolled in the present study may also have limited our ability to detect significant associations with baseline asthma characteristics or outcome variables. Indeed, a recent study of high-risk preschool children with respiratory disease noted significantly lower EBC pH values in children with atopy and with wheezy bronchitis compared with children without atopy and healthy controls.35 By contrast, within the population of children with atopy and with wheezy bronchitis, no differences between children with acute versus recurrent disease were noted,35 which suggests that EBC pH measurements may be of limited value within an asthma or at-risk population of children. However, a large unselected birth cohort of 630 children also found no differences between children with asthma and healthy controls at 8 years of age.12 Furthermore, no associations between EBC pH and lung function, airway hyperresponsiveness, and airway inflammation were noted in that same study,12 which also raises questions about the utility of EBC pH monitoring in the epidemiologic setting.
This study has a number of strengths, including the large sample size and the comprehensive phenotypic characterization of enrolled children. The fact that the parent clinical trial failed to demonstrate improvements in asthma control with lansoprazole therapy despite adequate power lends further support to the clinical meaningfulness of our results. However, given the ancillary nature of this study, power considerations do remain, and it is possible that our sample size was not sufficient to detect statistical differences in EBC pH values. Indeed, the widths of our observed CIs for EBC pH were relatively large, and precision would be improved with a larger sample size. Other limitations also are present. With regard to the capacity of EBC pH values to discriminate gastroesophageal reflux, this study may be limited by the exclusion of subjects with clinically symptomatic gastroesophageal reflux at enrollment. EBC pH may identify children with symptomatic gastroesophageal reflux regardless of the level of asthma control. In addition, although gastroesophageal reflux was determined by 24-hour pH monitoring, EBC samples were not collected at the time of the procedure. Thus, it may be that breath acidification is a transient event that was undetected by our methods. Another potential limitation is that EBC pH might fall during acute exacerbations associated with viral infections. Because the participants were studied in their usual state of asthma control rather than during severe exacerbations or acute illnesses, we cannot exclude the possibility that EBC pH might fall during those episodes. Although seasonality also could exert a theoretical effect on longitudinal EBC sampling, our EBC pH measurements were relatively stable and other studies have likewise demonstrated good repeatability of EBC in patients with asthma over 1 year in the absence of smoking.36 Furthermore, children in the present study completed diary cards, and there was no predictive value of EBC pH for EPACs. Another limitation could be related to the multicenter study design. However, samples were collected according to standardized operating procedures, which ensured consistency of EBC collection and shipping across the study sites. Indeed, other researchers have shown that changes in airway caliber, minute ventilation, and breathing pattern do not significantly affect the composition of airway constituents.37 Furthermore, all EBC pH values were measured during the first freeze-thaw cycle immediately after receipt. Our EBC pH values also are similar to those that have been previously reported in patients with poorly controlled asthma.13
In summary, the results from this study demonstrated that EBC pH is not associated with asymptomatic gastroesophageal reflux or characteristics of asthma in school-age children. Although other EBC constituents, such as cysteinyl leukotrienes, may prove to be of value in asthma therapeutic monitoring, we concluded that measurements of EBC pH have limited predictive utility in the long-term assessment of childhood asthma.
What is already known about this topic? Exhaled breath condensate (EBC) pH has been identified as an emerging biomarker of interest for asthma clinical trials and may allow for identification of acute acid reflux events.
What does this article add to our knowledge? The results of this study demonstrated that EBC pH is not associated with asymptomatic gastroesophageal reflux or characteristics of asthma in school-age children.
How does this study impact current management guidelines? Although other EBC constituents may prove to be of value in asthma therapeutic monitoring, we concluded that measurements of EBC pH have limited predictive utility in the long-term assessment of childhood asthma.
Acknowledgments
This study was funded by the National Institutes of Health, National Heart, Lung and Blood Institute U01HL080433 and U01HL1080450.
A. M. Fitzpatrick has received consultancy fees from MedImmune, Merck, GlaxoSmithKline, Boehringer Ingelheim, and Genentech. J. T. Holbrook has received research support from the National Heart, Lung, and Blood Institute (NHLBI). R. A. Wise is on the GlaxoSmithKline Clinical Endpoint Committee, the BIPI Data Safety Monitoring Board/Steering Committee, the Mylan Mortality Review Committee, the AstraZeneca Scientific Advisory Board, the Spiration Steering Committee, the Sunovion Scientific Advisory Board, the Roche/Genentech DSMB, Forest Laboratories Steering Committee, the Janssen and Intermune DSMB, the MedImmune Endpoint Committee, and the Merck and Pulmonx DSMB; and has received research support from BIPI, Merck, GlaxoSmithKline, Pearl Therapeutics, and Forest Laboratories. W. G. Teague has received research support and travel support from National Institutes of Health/NHLBI; has received consultancy fees from GlaxoSmithKline and Boehringer Ingelheim; has received lecture fees from Merck and Genentech/Roche; and has received payment for development of educational Web site materials from Not One More Life.
Abbreviations used
- ACQ
Asthma Control Questionnaire
- AQLQ
Asthma Quality of Life Questionnaire
- ASUI
Asthma Symptom Utility Index
- BMI
Body mass index
- EBC
Exhaled breath condensate
- EPAC
Episode of poor asthma control
- GER
Gastroesophageal reflux
- ICS
Inhaled corticosteroid
- NOx
Nitrogen oxides
- SARCA
Study of Acid Reflux in Children with Asthma
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Effros RM, Casaburi R, Su J, Dunning M, Torday J, Biller J, et al. The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am J Respir Crit Care Med. 2006;173:386–92. doi: 10.1164/rccm.200507-1059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popov TA. Human exhaled breath analysis. Ann Allergy Asthma Immunol. 2011;106:451–6. doi: 10.1016/j.anai.2011.02.016. quiz 7. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmeyer F, Raulf-Heimsoth M, Bruning T. Exhaled breath condensate and airway inflammation. Curr Opin Allergy Clin Immunol. 2009;9:16–22. doi: 10.1097/ACI.0b013e32831d8144. [DOI] [PubMed] [Google Scholar]
- 4.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129:S9–23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpagnano GE, Barnes PJ, Francis J, Wilson N, Bush A, Kharitonov SA. Breath condensate pH in children with cystic fibrosis and asthma: a new noninvasive marker of airway inflammation? Chest. 2004;125:2005–10. doi: 10.1378/chest.125.6.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ojoo JC, Mulrennan SA, Kastelik JA, Morice AH, Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60:22–6. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364–70. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 8.Carraro S, Folesani G, Corradi M, Zanconato S, Gaston B, Baraldi E. Acid-base equilibrium in exhaled breath condensate of allergic asthmatic children. Allergy. 2005;60:476–81. doi: 10.1111/j.1398-9995.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 9.Antus B, Barta I, Kullmann T, Lazar Z, Valyon M, Horvath I, et al. Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease: a longitudinal study. Am J Respir Crit Care Med. 2010;182:1492–7. doi: 10.1164/rccm.201003-0451OC. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 11.Brunetti L, Francavilla R, Tesse R, Strippoli A, Polimeno L, Loforese A, et al. Exhaled breath condensate pH measurement in children with asthma, allergic rhinitis and atopic dermatitis. Pediatr Allergy Immunol. 2006;17:422–7. doi: 10.1111/j.1399-3038.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou NC, Lowe LA, Murray CS, Woodcock A, Simpson A, Custovic A. Exhaled breath condensate pH and childhood asthma: unselected birth cohort study. Am J Respir Crit Care Med. 2006;174:254–9. doi: 10.1164/rccm.200601-140OC. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Teague WG, Erzurum S, Fitzpatrick A, Mantri S, Dweik RA, et al. Determinants of exhaled breath condensate pH in a large population with asthma. Chest. 2011;139:328–36. doi: 10.1378/chest.10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseliou E, Bessa V, Hillas G, Delimpoura V, Papadaki G, Roussos C, et al. Exhaled nitric oxide and exhaled breath condensate pH in severe refractory asthma. Chest. 2010;138:107–13. doi: 10.1378/chest.09-1257. [DOI] [PubMed] [Google Scholar]
- 15.Hunt J, Yu Y, Burns J, Gaston B, Ngamtrakulpanit L, Bunyan D, et al. Identification of acid reflux cough using serial assays of exhaled breath condensate pH. Cough. 2006;2:3. doi: 10.1186/1745-9974-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu Y, Dobashi K, Mori M. Exhaled breath marker in asthma patients with gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;41:147–53. doi: 10.3164/jcbn.2007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu Y, Dobashi K, Zhao JJ, Kawata T, Ono A, Yanagitani N, et al. Proton pump inhibitor improves breath marker in moderate asthma with gastroesophageal reflux disease. Respiration. 2007;74:558–64. doi: 10.1159/000101437. [DOI] [PubMed] [Google Scholar]
- 18.Writing Committee for the American Lung Association Asthma Clinical Research Center. Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307:373–81. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–6. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 22.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 24.Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–48. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 25.Paget-Brown AO, Ngamtrakulpanit L, Smith A, Bunyan D, Hom S, Nguyen A, et al. Normative data for pH of exhaled breath condensate. Chest. 2006;129:426–30. doi: 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan J, Ngamtrakulpanit L, Pajewski TN, Turner R, Nguyen TA, Smith A, et al. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J. 2003;22:889–94. doi: 10.1183/09031936.03.00038803. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AM, Brown LA, Holguin F, Teague WG. National Institutes of Health/National Heart L, Blood Institute Severe Asthma Research P. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol. 2009;124:990–996. e1–9. doi: 10.1016/j.jaci.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colletti RB, Christie DL, Orenstein SR. Statement of the North American Society for Pediatric Gastroenterology and Nutrition (NASPGN). Indications for pediatric esophageal pH monitoring. J Pediatr Gastroenterol Nutr. 1995;21:253–62. doi: 10.1097/00005176-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 30.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Severe Asthma Research Program. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaston B, Kelly R, Urban P, Liu L, Henderson EM, Doctor A, et al. Buffering airway acid decreases exhaled nitric oxide in asthma. J Allergy Clin Immunol. 2006;118:817–22. doi: 10.1016/j.jaci.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 32.Niimi A, Nguyen LT, Usmani O, Mann B, Chung KF. Reduced pH and chloride levels in exhaled breath condensate of patients with chronic cough. Thorax. 2004;59:608–12. doi: 10.1136/thx.2003.012906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389. e1–13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Jagwitz M, Pessler F, Akmatov M, Li J, Range U, Vogelberg C. Reduced breath condensate pH in asymptomatic children with prior wheezing as a risk factor for asthma. J Allergy Clin Immunol. 2011;128:50–5. doi: 10.1016/j.jaci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Accordino R, Visentin A, Bordin A, Ferrazzoni S, Marian E, Rizzato F, et al. Long-term repeatability of exhaled breath condensate pH in asthma. Respir Med. 2008;102:377–81. doi: 10.1016/j.rmed.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Debley JS, Ohanian AS, Spiekerman CF, Aitken ML, Hallstrand TS. Effects of bronchoconstriction, minute ventilation, and deep inspiration on the composition of exhaled breath condensate. Chest. 2011;139:16–22. doi: 10.1378/chest.10-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]