Figure 7. Membrane Extraction of Poly-ubiquitinated Proteins by Cdc48p.
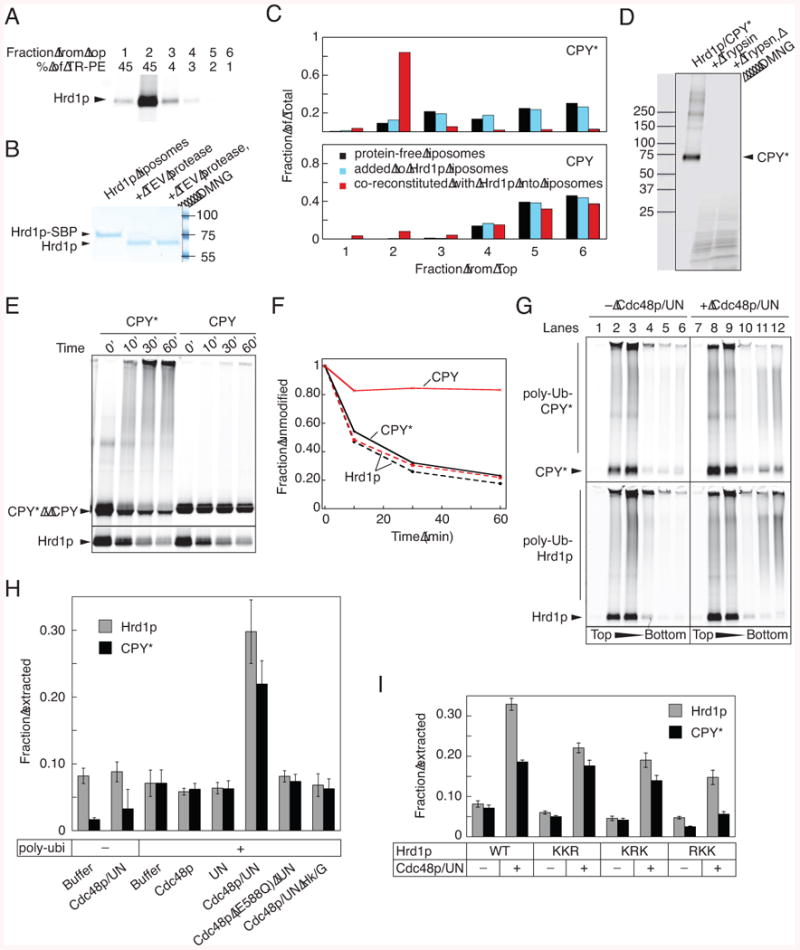
(A) Proteoliposomes containing fluorescently labeled Hrd1p-SBP and Texas-red labeled phosphatidyl ethanolamine (TR-PE) were subjected to flotation in a Nycodenz gradient. Fractions were collected from the top, and analyzed by SDS-PAGE and fluorescence scanning of the gel. The lipid content of the fractions was determined by absorbance at 590 nm.
(B) Proteoliposomes containing Hrd1p-SBP with a TEV-cleavage site at the C-terminus were treated with TEV protease in the absence or presence of DMNG. The samples were analyzed by SDS-PAGE and staining with Coomassie blue.
(C) Fluorescently labeled CPY* was incubated with either protein-free liposomes or proteoliposomes containing Hrd1p, or it was co-reconstituted with Hrd1p into vesicles (black, light blue and red bars, respectively). The samples were subjected to flotation in a Nycodenz gradient and fractions were analyzed by SDS-PAGE and fluorescence scanning. Wild type CPY was used as a control.
(D) Proteoliposomes containing Hrd1p and labeled CPY* were incubated with trypsin in the absence or presence of DMNG. The samples were analyzed by SDS-PAGE and fluorescence scanning.
(E) Proteoliposomes were generated by co-reconstitution of Hrd1p with either CPY* or wild type CPY. Hrd1p and substrate were labeled with different fluorophores. The vesicles were incubated with the ubiquitination machinery for different time periods, and samples were analyzed by SDS-PAGE and fluorescence scanning. For Hrd1p, the gel was cropped to only show the disappearance of unmodified protein.
(F) The disappearance of unmodified protein in (D) was quantitated. Solid and broken lines show the modification of substrate and Hrd1p, respectively.
(G) Fluorescently labeled Hrd1p and CPY* were co-reconstituted into proteoliposomes. The vesicles were incubated with the ubiquitination machinery, followed by incubation in the absence or presence of the Cdc48p complex (Cdc48p/UN). The vesicles were floated in a Nycodenz gradient and fractions analyzed by SDS-PAGE and fluorescence scanning.
(H) Experiments as in (G) were quantified by determining the total fluorescence in the bottom two fractions (material released from the vesicles) as a fraction of the total fluorescence in the gradient (mean and standard deviation of at least three experiments). Where indicated, ATP was depleted with hexokinase/glucose (Hk/G) or an ATPase-deficient Cdc48p mutant (Cdc48p E588Q) was used.
(I) As in (G), but with Hrd1p mutants carrying Lys to Arg mutations in three different regions. Quantification of three experiments was done as in (H).
See also Figure S7.
