Abstract
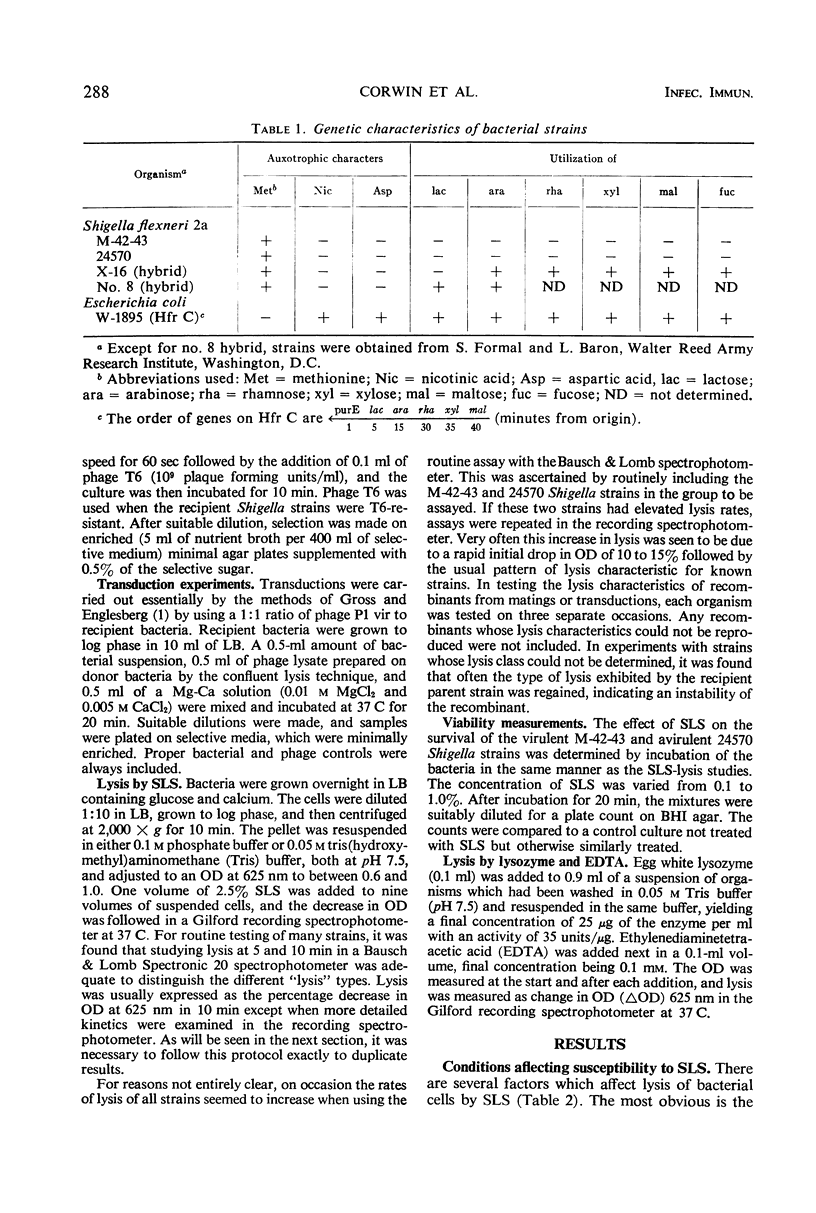
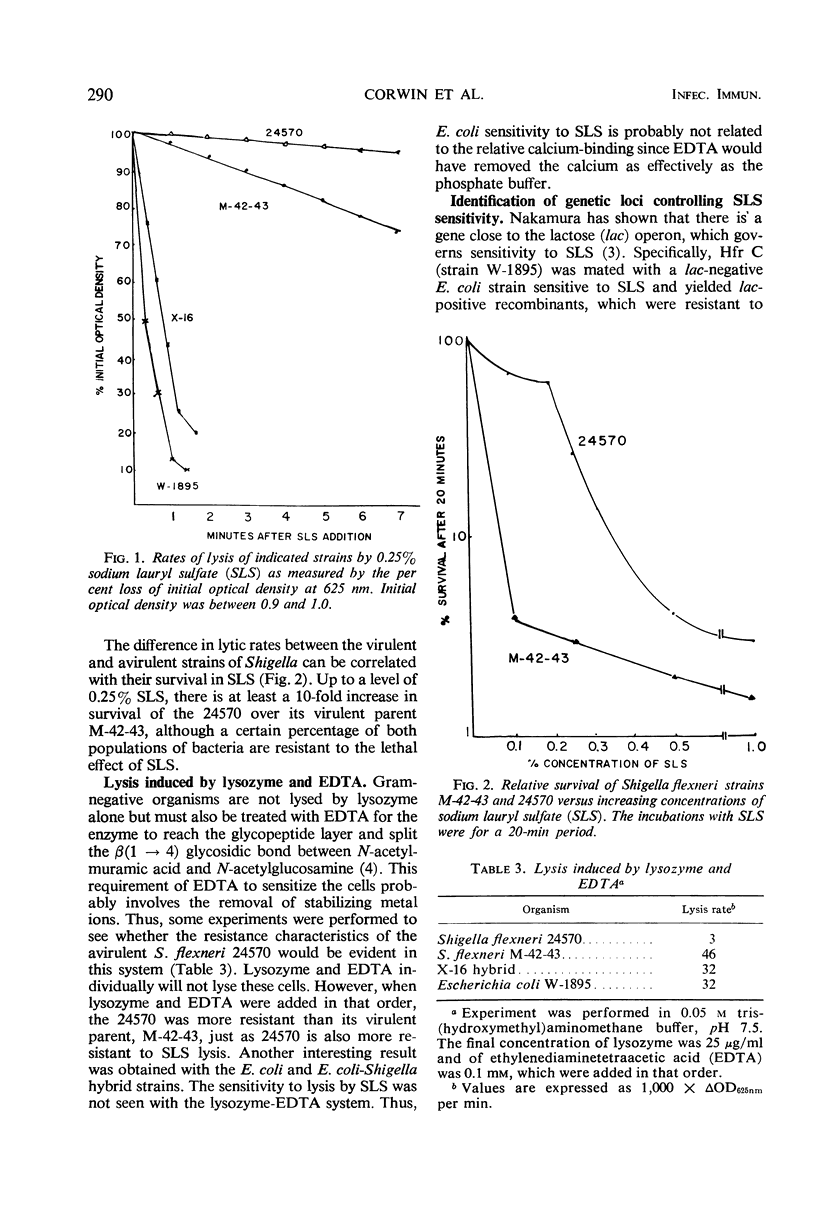
The sensitivity to sodium lauryl sulfate (SLS) of Shigella flexneri and Escherichia coli is determined by at least three genes. One site is located near the lactose operon, and two loci are cotransducible with the arabinose operon. Calcium ions protect against SLS lysis. One gene is concerned with the relative ability of the bacterium to retain calcium against such chelating agents as ethylenediaminetetraacetic acid or phosphate buffer. This was first observed in a mutation from virulence to avirulence in S. flexneri with a concomitant loss of ability to penetrate the intestinal epithelium. The avirulent strain is far less sensitive to lysis by SLS in the presence of phosphate buffer than its virulent parent. The avirulent strain is also less sensitive to lysozyme and ethylenediaminetetraacetic acid. E. coli K-12 is much more sensitive to SLS than both of these Shigella strains. An E. coli-S. flexneri hybrid, which is unable to survive well in the gut and thus only produces an abortive infection, has inherited this extreme sensitivity to SLS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., LEUTGEB W. Autolytic enzymes as a source of error in the preparation and study of gram-negative cell walls. J Gen Microbiol. 1963 Jan;30:127–130. doi: 10.1099/00221287-30-1-127. [DOI] [PubMed] [Google Scholar]



