Abstract
Introduction
Racial differences in bone structure likely have roots in childhood as bone size develops predominantly during growth. This study aimed to compare cortical bone health within the tibial diaphysis of black and white children in the early stages of puberty, and explore the contributions of biochemical variables in explaining racial variation in cortical bone properties.
Methods
A cross-sectional study was performed comparing peripheral quantitative computed tomography-derived cortical bone measures of the tibial diaphysis and biochemical variables in 314 participants (n=155 males; n=164 blacks) in the early stages of puberty.
Results
Blacks had greater cortical volumetric bone mineral density, mass and size compared to whites (all p<0.01), contributing to blacks having 17.0% greater tibial strength (polar strength-strain index [SSIP]) (p<0.001). Turnover markers indicated blacks had higher bone formation (osteocalcin [OC] and bone specific alkaline phosphatase) and lower bone resorption (N-terminal telopeptide) than whites (all p<0.01). Blacks also had lower 25-hydroxyvitamin D [25(OH)D], and higher 1,25-dihydroxyvitamin D [1,25(OH)2D] and parathyroid hormone (PTH) (all p<0.05). There were no correlations between tibial bone properties, and 25(OH)D and PTH in whites (all p≥0.10); however, SSIP was negatively and positively correlated with 25(OH)D and PTH in blacks, respectively (all p≤0.02). Variation in bone cross-sectional area and SSIP attributable to race was partially explained by tibial length, 25(OH)D/PTH and OC.
Conclusions
Divergence in tibial cortical bone properties between blacks and whites is established by the early stages of puberty with the enhanced cortical bone properties in black children possibly being explained by higher PTH and OC.
Keywords: peripheral quantitative computed tomography; 25 hydroxy vitamin D; 1,25 dihydroxy vitamin D; PTH; bone turnover; race
Introduction
Osteoporotic fracture rates differ according to race with blacks having up to half the rate of whites (1, 2). The reduced fracture rate in blacks has been attributed to their superior bone mass which develops as a result of higher peak bone mass in early adulthood (3, 4) and potentially lower rates of bone loss with aging (5, 6). However, bone mass is not the sole determinant of bone strength. Bone strength, and consequent fracture risk, is also influenced by how bone tissue is distributed or structured (7). At cortical rich sites blacks have greater bone size than whites (8, 9). As bone strength is proportional to how far cortical bone material is distributed from mechanical axes, larger bone size in blacks may also contribute to their reduced risk of osteoporotic fracture.
Cortical bone structure develops primarily as a result of the tissue-level activity of modeling, which involves temporally and spatially independent formation and resorption on periosteal and endosteal surfaces. As modeling principally occurs during growth, it is likely that any racial differences in cortical bone structure have their roots in childhood. Recent studies utilizing peripheral quantitative computed tomography (pQCT) explored racial differences in cortical bone properties at the tibial diaphysis in children and adolescents (10-14). The consensus is that blacks have greater bone cross-sectional size and estimated strength compared to whites during growth, even after adjustment for racial differences in bone length and muscle size.
A limitation of previous studies exploring racial differences in tibial cortical bone properties during growth is that biochemical factors associated with bone metabolism were not investigated. Black children reportedly have lower serum 25(OH)D concentrations (15-20), higher serum 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations (16, 18, 21), the same (9, 16, 18, 21, 22) or higher (15) serum parathyroid hormone [PTH] concentrations, and equivalent levels of bone turnover markers (16, 18, 21-23) compared to white children. How racial differences in biochemical variables relate to cortical bone properties have not been studied, but may contribute to explaining the difference in cortical bone properties in black and white children.
The aims of the current study were to explore: 1) differences in pQCT-derived cortical bone density, structure, and estimated strength at the tibial diaphysis in black and white children in the early stages of puberty; 2) the relationship between cortical bone properties, and serum 25(OH)D and PTH concentrations within each race, and; 3) the relative contributions of biochemical variables in explaining racial variation in cortical bone properties.
Methods
Study design and subjects
Baseline (cross-sectional) data were analyzed from subjects participating in a randomized controlled trial investigating vitamin D supplementation in children in the early stages of puberty. Study sites were in Georgia (University of Georgia [UGA]; latitude 34°N) and Indiana (Purdue University [PU] and Indiana University [IU]; latitude 40°N). The parent study recruited 323 black and white subjects with the following distribution according to study site, race and sex: UGA (black females, n=40; black males, n=40; white females, n=40; white males; n=40), PU (black females, n=3; black males, n=2; white females, n=38; white males; n=40), and IU (black females, n=40; black males, n=40).
Subjects were included if they were: 1) aged 9-12 years [females] or 10-13 years [males]; 2) in the early stages of puberty with sexual maturation ratings of 2 or 3 for breast development in females and genital development in males as described by Tanner (24), and; 3) non-Hispanic white or black, with both sets of parents and grandparents being of the same race. Sexual maturation was self-reported. Exclusion criteria included achievement of menarche in females, known bone disease or disease known to influence bone metabolism, growth disorders, and the use of medications that may influence bone metabolism. All measurements were performed between October and December to minimize seasonal influences on biochemical variables. The study protocol was approved by the Institutional Review Boards of UGA, PU, and IU. All participants and their parent/guardian provided informed assent and consent, respectively.
Anthropometry and body composition
Height (to nearest 0.1 cm) and mass (to nearest 0.1 kg) were measured without shoes using a wall-mounted digital stadiometer and electronic balance scale, respectively. Body mass index (BMI, kg/m2) was derived, and sex- and age-specific percentiles calculated using a BMI Percentile Calculator (25). Tibial length (to nearest 0.1 cm) was measured as the distance between the medial tibial plateau and distal tip of the medial malleolus using a sliding anthropometer, and a skin mark was placed at 66% of tibial length proximal from the medial malleolus.
Body composition was determined by dual-energy X-ray absorptiometry (DXA). Whole-body scans were performed on one of three scanners using the manufacturers' standard scan and positioning protocols. Scanners used were a Hologic Delphi A (Hologic, Inc., Waltham, MA, USA) at UGA, Lunar iDXA (GE Healthcare, Madison, WI) at PU, and Hologic Discovery-W (Hologic, Inc., Waltham, MA, USA) at IU. Data obtained from the scans included whole body bone mineral content (BMC, g/cm2), fat-free soft-tissue (FFST; kg), fat mass (kg) and percent fat mass (%).
Cross-calibration was performed to facilitate comparability of data from each study site/scanner. The UGA and PU study sites were cross-calibrated by scanning 26 individuals on the Hologic Delphi A scanner and a Lunar iDXA scanner, whereas the IU and PU study sites were cross-calibrated by scanning 10 individuals on the Hologic Discovery-W and Lunar iDXA scanners. Regression formulae between data obtained at UGA and PU, and IU and PU were derived for each variable, and used to adjust data obtained at UGA and IU to PU values.
Dietary intake and physical activity
Three-day diet records (2 weekdays and 1 weekend day) were used to estimate average daily intakes of calcium (26). The diet records were analyzed by Food Processor SQL (Version 9.7.3; ESHA Research, Salem, OR, USA). Physical activity levels were obtained using 3-day physical activity records for 2 weekdays and 1 weekend day (27). Subjects recorded activities in 30-minute increments for light, moderate, hard, and very hard activities. A MET value was assigned to each time block based on the type and intensity of activity described, and average METS per day calculated (28).
Peripheral quantitative computed tomography
pQCT was performed at each study site using Stratec XCT 2000 machines (Stratec Medizintechnik GmbH, Pforzheim, Germany). Subjects were positioned supine with their non-dominant leg centered in the gantry of the pQCT machine. A tomographic slice (thickness, 2.3 mm; voxel size, 400 μm; scan speed, 20 mm/s) was taken at the skin mark placed at 66% of tibial length proximal from the medial malleolus. A cone phantom was scanned daily to confirm machine calibration.
Tomographic slices were analyzed for bone mineral density (BMD), structure and estimated strength, and muscle cross-sectional area (mCSA). Cort mode 1 (threshold, 710 mg/cm3) was used to obtain cortical volumetric BMD (Ct.vBMD, mg/cm3), BMC (Ct.BMC; mg/mm) and area (Ct.Ar; cm2). Total area (Tt.Ar, mm2), cortical thickness (Ct.Th, mm), and periosteal (Ps.Pm, mm) and endosteal (Es.Pm, mm) perimeters were obtained by analyzing the slices using contour mode 1 (threshold, 710 mg/cm3) to define the outer bone edge and peel mode 2 (threshold, 400 mg/cm3) to separate the cortical and cancellous compartments.
Estimated strength was obtained by calculating the polar strength-strain index (SSIP, mm3). SSIP represents the density-weighted section modulus and has been validated as a non-invasive measure of bone strength (29). It was obtained in a separate analysis using cort mode 2 (threshold = 400 mg/cm3), and was calculated as the section modulus multiplied by the ratio of Ct.vBMD and normal physiologic density (NP.vBMD, 1200 mg/mm3), as previously described [Eq. (1)] (30, 31). Section modulus (mm3) was calculated as (a × d2)/dmax, where a is the cross-sectional area of a voxel (mm2), d is the distance of the voxel from the center of gravity (mm), and dmax is the maximum distance (eccentricity) of one voxel to the center of gravity (mm).
| (1) |
Muscle cross-sectional area (mCSA; cm2) was obtained with a F03F05 filter using contour mode 3 (threshold, -100 mg/cm3) to locate the skin surface and peel mode 2 (threshold, 40 mg/cm3) to locate the subcutaneous fat-muscle boundary. Short-term precision for the pQCT scanning procedure on 30 healthy individuals scanned six times with interim repositioning showed root mean square coefficients of variation (RMS-CVs) of <1% for bone density, mass, structure, and estimated strength measures, and <1.5% for mCSA (32). Comparability of bone data from each study site/scanner was achieved by scanning a cortical bone phantom with known properties a minimum of 20 times on each scanner.
Biochemical assays
Blood and urine samples were collected the morning following an overnight fast. Serum and urine were refrigerated immediately after collection, and all samples were prepared for storage and frozen at <-70°C until analysis. Serum PTH, 25(OH)D and 1,25(OH)2D were assayed in duplicate in a single laboratory meeting certification criteria for DEQAS (Vitamin D External Quality Assessment Scheme). Serum PTH was assayed using an immunoradiometric assay (N-tact PTH SP kit, no. 26100; DiaSorin Inc., Stillwater, MN). Inter-assay CVs ranged from 4.8%-9.0%. Serum 25(OH)D (25-hydroxyvitamin D125 I RIA kit, no. 68100E; DiaSorin Inc., Stillwater, MN) and 1,25(OH)2D (1,25-dihydroxyvitamin D125 I RIA kit, no. 65100E; DiaSorin Inc., Stillwater, MN) were assayed using two-step radioimmunoassays. Inter-assay CVs for 25(OH)D and 1,25(OH)2D ranged from 5.2%-7.7% and 14.4%-16.9%, respectively. Urinary calcium was determined using inductively coupled plasma spectrophotometry (Optima 4300DV, Perkin Elmer Instruments, Norwalk, CT). Urinary creatinine was measured by the colorimetric Jaffe reaction using a Cobas Integra 400 autoanalyzer (Roche Diagnostics, Indianapolis, IN). Serum osteocalcin (OC) and bone-specific alkaline phosphatase (BAP) were measured by ELISA (using kits from Quidel Corp., San Diego, CA). Urine N-terminal telopeptide (NTX) was measured by ELISA (using kits from Ostex International, Seattle, WA). Inter-assay CVs for urinary calcium and bone turnover markers in our laboratory range from 3.3-8.9% (16, 18, 21, 22).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (Version 19; IBM Corporation, Somers, NY, USA) or Statistical Analysis Software (v.9.2; SAS Institute, Cary, NC). Statistical significance was set at α = 0.05. Data were assessed for normality and homogeneity of variance using Shapiro-Wilks and Levene tests, respectively. Natural log transformations corrected data not normally distributed and with heterogeneity of variance; however, only untransformed data are reported as analyses performed using either transformed or untransformed data provided similar results. Two-way analyses of variance (ANOVA) models were used to assess the effects of race (black and white) and sex (female and male) on demographic, anthropometric and biochemical variables. Two-way analyses of covariance (ANCOVA) models assessed the effects of race and sex on tibial bone properties, with tibial length and leg mCSA used as covariates for analyses of tibial bone mass, structure, and strength. The functional forms of the relationships between select bone properties (Ct.vBMD, Tt.Ar, SSIP), and 25(OH)D and PTH within each race were explored graphically by fitting linear, quadratic, cubic, exponential, and logarithmic curves to the data. SSIP was chosen as a main property of interest as it is predictive of bone strength (29). Ct.vBMD and Tt.Ar were explored as density and structure are primary measures contributing to SSIP, and Ct.vBMD is consistently higher in black children and adults (10-14, 33, 34). Relationships between Ct.vBMD, Tt.Ar, SSIP, 25(OH)D and PTH within each race were determined by Pearson's bivariate correlations. Simple (univariate) linear regression was performed to obtain the percent variation in the tibial bone properties explained by race. The relative contribution of biochemical variables to the proportion of variation in the tibial properties attributable to race was determined by multiple linear regression. A combination of stepwise model selection (criteria for entry p < 0.10 and removal p > 0.15) and Mallow's CP were used to identify the best predictive models of the tibial properties from biochemical variables, tibia length and mCSA. The models selected included only variables significant with p < 0.05. Race was added last to each model. Semipartial R2 values were calculated from type I sums of squares using the scorr1(partialr2) option in SAS PROC REG.
Results
Participant characteristics
Data from 9 subjects (black male, n=1; white female, n=2; white male, n=6) in the parent study were excluded from the current analyses due to excessive motion artifact on their pQCT scan. The characteristics of the remaining 314 subjects are detailed in Table 1. There were no statistical interactions between sex and race on participant characteristics (all p > 0.10).
Table 1.
Participant characteristicsa
| Female | Male | Two-way ANOVA | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| White | Black | White | Black | Sex × Race | Sex | Race | |
| Demographics | |||||||
| n | 76 | 83 | 74 | 81 | |||
| Pubertal stage (2/3)b | 57/19 | 57/26 | 45/29 | 45/36 | |||
| Age (yr) | 10.9 ± 1.0 | 10.5 ± 1.0 | 12.2 ± 1.0 | 11.8 ± 1.1 | NS | <0.001 | <0.001 |
| Whole-body anthropometry | |||||||
| Height (cm) | 148.9 ± 8.8 | 148.3 ± 8.2 | 153.7 ± 9.7 | 151.8 ± 9.5 | NS | <0.001 | NS |
| Body mass (kg) | 44.0 ± 9.3 | 47.8 ± 11.8 | 46.7 ± 12.7 | 49.1 ± 12.7 | NS | NS | <0.05 |
| BMI (kg/m2) | 19.8 ± 3.5 | 21.6 ± 4.3 | 19.6 ± 4.1 | 21.1 ± 4.5 | NS | NS | <0.001 |
| BMI (percentile-for-age) | 65.6 ± 27.5 | 76.8 ± 27.3 | 54.0 ± 31.8 | 71.7 ± 26.1 | NS | 0.01 | <0.001 |
| FFST (kg) | 28.1 ± 5.0 | 28.6 ± 5.4 | 32.1 ± 7.9 | 32.3 ± 7.3 | NS | <0.001 | NS |
| Fat mass (kg) | 14.3 ± 6.1 | 17.0 ± 7.6 | 12.6 ± 6.8 | 13.9 ± 7.5 | NS | <0.01 | <0.05 |
| Fat mass (%) | 31.6 ± 8.7 | 35.2 ± 8.8 | 25.9 ± 9.5 | 28.4 ± 9.5 | NS | <0.001 | <0.01 |
| BMC (kg)c | 1.56 ± 0.26 | 1.68 ± 0.34 | 1.56 ± 0.37 | 1.64 ± 0.33 | NS | NS 0.59 | <0.001 |
| Regional anthropometry | |||||||
| Tibial length (cm) | 33.6 ± 3.2 | 35.1 ± 4.1 | 35.0 ± 3.2 | 36.3 ± 3.0 | NS | 0.001 | <0.001 |
| Leg mCSA (cm2) | 46.9 ± 9.1 | 46.1 ± 9.0 | 49.9 ± 12.0 | 50.0 ± 9.9 | NS | <0.01 | NS |
| Physical activity and calcium intake | |||||||
| Physical activity (METS/day) | 64.9 ± 10.3 | 59.3 ± 8.7 | 63.2 ± 9.1 | 61.5 ± 10.1 | NS | NS | <0.01 |
| Calcium intake (mg/day) | 971 ± 422 | 825 ± 321 | 943 ± 418 | 852 ± 402 | NS | NS | 0.01 |
| Serum biochemistries | |||||||
| 25-hydroxyvitamin D (ng/ml) | 31.9 ± 6.5 | 23.7 ± 7.2 | 32.2 ± 5.3 | 24.4 ± 5.9 | NS | NS | <0.001 |
| 1,25-dihydroxyvitamin D (pg/ml) | 56.6 ± 18.6 | 58.5 ± 14.8 | 50.5 ± 13.6 | 56.2 ± 17.9 | NS | <0.05 | <0.05 |
| Parathyroid hormone (pg/ml) | 25.8 ± 9.3 | 31.1 ± 12.1 | 24.6 ± 9.1 | 28.2 ± 10.9 | NS | NS | <0.001 |
| Osteocalcin (ng/ml) | 29.5 ± 7.1 | 35.5 ± 13.9 | 29.9 ± 7.5 | 38.6 ± 13.0 | NS | NS | <0.001 |
| Bone-specific alkaline phosphatase (U/L) | 194 ± 72 | 231 ± 85 | 207 ± 71 | 215 ± 67 | NS | NS | 0.01 |
| Urinary biochemistries | |||||||
| Urinary calcium (Ca/Cr) | 0.079 ± 0.082 | 0.048 ± 0.116 | 0.074 ± 0.065 | 0.048 ± 0.051 | NS | NS | <0.01 |
| N-terminal telopeptide (nM BCE/mM Cr) | 583 ± 189 | 534 ± 214 | 602 ± 304 | 490 ± 181 | NS | NS | <0.01 |
Data are mean ± SD (except for frequencies)
There were no racial differences in pubertal stage distribution (P =0.29; χ2 analysis)
Adjusted for FFST
Sex effects
Males were taller, and had greater lean and lower fat mass than females (all p < 0.05). There were no differences in biochemistries between sexes (all p > 0.09), except for 1,25(OH)2D which was 7.3% lower in males (p = 0.03).
Race effects
There were no differences in sexual maturation distribution (pubertal stage 2 vs. 3) between blacks and whites (p = 0.29; χ2 analysis). Blacks were 0.4 years younger than whites (p < 0.001) and had 8.6% greater BMI (p < 0.001) as a result of elevated body mass (p < 0.05). Greater body mass in blacks resulted from higher fat mass (p < 0.05) and BMC (p < 0.001), but not fat-free soft tissue (FFST) (p = 0.64). There was no effect of race on leg mCSA (p = 0.77); however, blacks had longer tibiae than whites (p < 0.001). Blacks performed 5.7% less physical activity and had 12.4% lower calcium intakes than whites (all p ≤ 0.01). Blacks had 25.0% lower 25(OH)D and 37.7% lower urinary calcium excretion, and 7.1% greater 1,25(OH)2D and 17.8% greater PTH than whites (all p < 0.05). Blacks had higher bone formation (OC and BAP) and lower resorption (NTX) markers than whites (all p < 0.01).
Tibial bone properties
Tibial Ct.BMC, structural properties and SSIP (but not Ct.vBMD) were positively correlated with anthropometric variables. Tibial bone length and leg mCSA were used as covariates in analyses of sex and race effects on tibial bone properties as they were strongly correlated with tibial bone properties (r > 0.5 for SSIP, p < 0.001) and collinear with other anthropometric variables (tibial length and height, r > 0.73; leg mCSA and FFST, r > 0.77; all p < 0.001). The effect of sex and race on tibial bone properties are shown in Table 2. There were no race by sex interactions on tibial bone properties (all p > 0.15).
Table 2. Racial differences in tibial bone density, mass, structure and strengtha.
| Female | Male | Two-way ANOVA | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| White | Black | White | Black | Sex × Race | Sex | Race | |
| Density | |||||||
| Ct.vBMD | 1057 ± 32 | 1082 ± 30 | 1040 ± 35 | 1071 ± 29 | NS | <0.001 | <0.001 |
| Mass | |||||||
| Ct.BMC (mg/mm) | 232 ± 34 | 264 ± 42 | 243 ± 48 | 267 ± 49 | NS | <0.05 | <0.001 |
| Structure | |||||||
| Tt.Ar (cm2) | 4.14 ± 0.68 | 4.56 ± 0.76 | 4.41 ± 0.78 | 4.68 ± 0.90 | NS | <0.01 | <0.001 |
| Ct.Ar (cm2) | 2.20 ± 0.32 | 2.44 ± 0.36 | 2.34 ± 0.46 | 2.50 ± 0.47 | NS | 0.001 | <0.001 |
| Ct.Th (mm) | 3.65 ± 0.46 | 3.85 ± 0.43 | 3.76 ± 0.59 | 3.88 ± 0.50 | NS | NS | 0.001 |
| Ps.Pm (mm) | 71.8 ± 6.1 | 75.4 ± 6.3 | 74.2 ± 6.5 | 76.3 ± 7.3 | NS | <0.01 | <0.001 |
| Es.Pm (mm) | 48.9 ± 6.6 | 51.2 ± 6.2 | 50.6 ± 6.1 | 51.9 ± 6.6 | NS | NS | <0.01 |
| Strength | |||||||
| SSIP (mm3) | 1480 ± 310 | 1778 ± 415 | 1604 ± 411 | 1829 ± 486 | NS | <0.01 | <0.001 |
Data are mean ± SD, and adjusted for tibial length and leg mCSA (except Ct.vBMD)
Sex effects
There were expected sex effects, with males having larger tibias (higher Tt.Ar, Ct.Ar and Ps.Pm) with greater SSIP compared to females (all p < 0.01).
Race effects
Ct.vBMD and Ct.BMC were 2.7% and 11.6% greater in blacks, respectively (all p < 0.001). Blacks had 8.1-8.7% greater bone areas (Tt.Ar and Ct.Ar), with 4.4% greater cortical thickness and 3.8-4.0% greater periosteal and endosteal perimeters (all p < 0.01). The greater tibial Ct.vBMD and structure in blacks resulted in 17.0% greater SSIP in blacks compared to whites (p < 0.001).
Relationships between select tibial bone properties, 25(OH)D and PTH
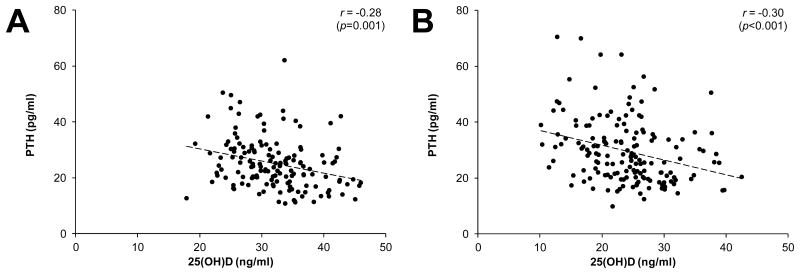
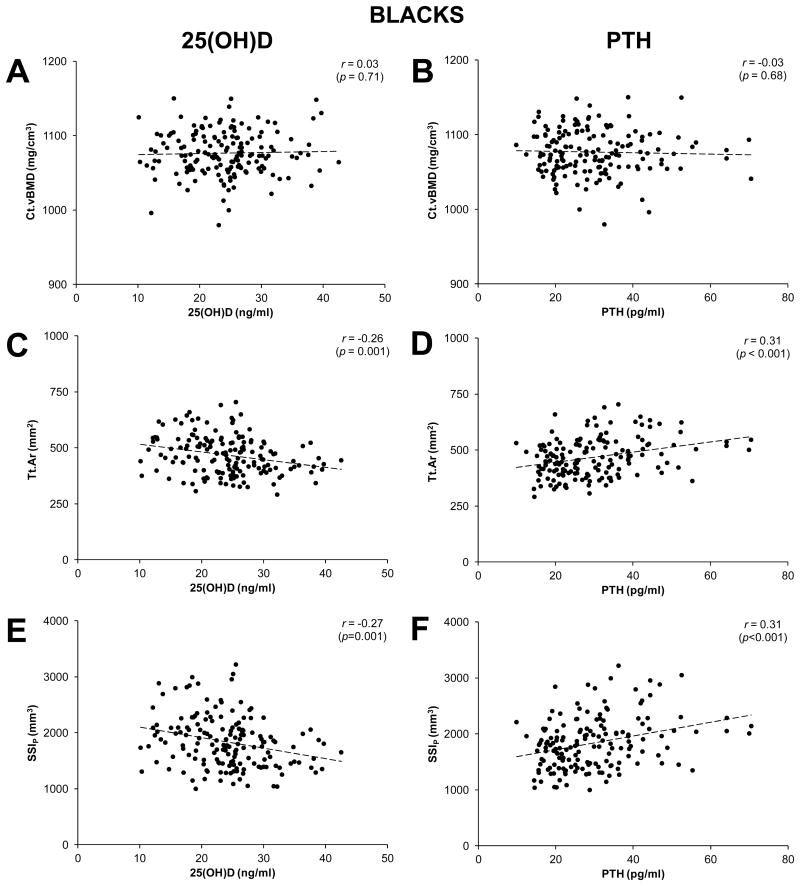
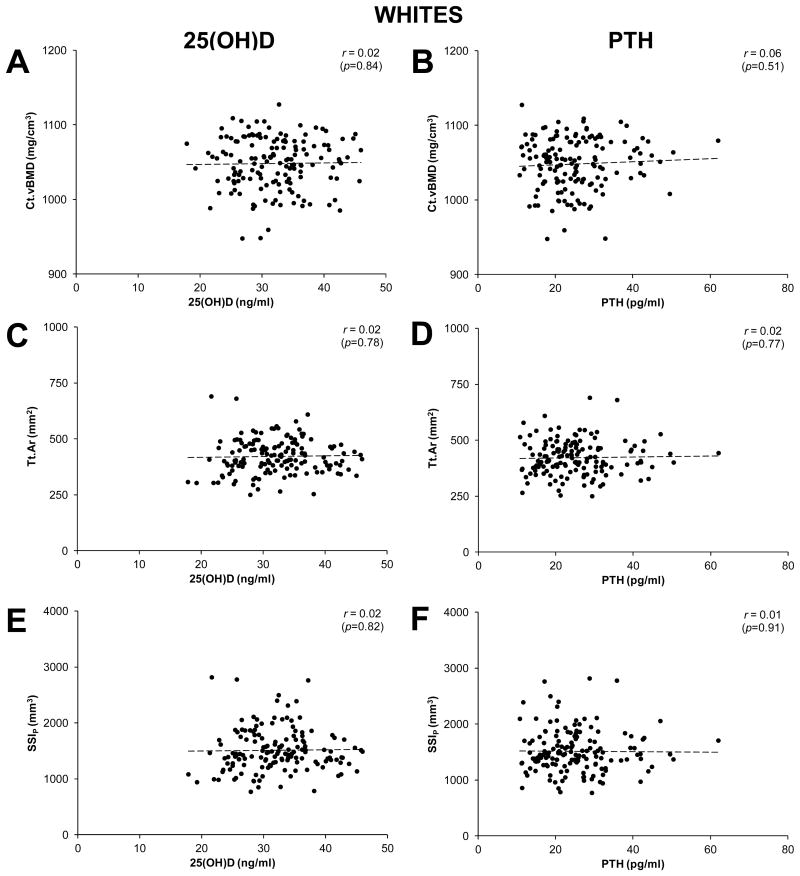
Associations between select tibial bone properties (Ct.vBMD, Tt.Ar, SSIP), and serum concentrations of 25(OH)D and PTH were best described by linear functions (Fig. 1-3). Serum PTH and 25(OH)D concentrations were negatively correlated in both whites and blacks (all r ≤ -0.28, p < 0.001; Fig. 1). Serum 25(OH)D and PTH concentrations were not correlated with Ct.vBMD, Tt.Ar or SSIP in whites (all r ≤ 0.06, p ≥ 0.51; Fig. 2), or Ct.vBMD in blacks (all r ≤ 0.03, p ≥ 0.68; Fig. 3A,B). In contrast, Tt.Ar and SSIP in blacks were negatively correlated with 25(OH)D (all r ≤ -0.26, p = 0.001; Fig. 3C,E) and positively correlated with PTH (all r = -0.31, p < 0.001; Fig. 3D,F).
Figure 1.
Scatterplots illustrating the negative relationship between serum concentrations of 25-hydroxyvitamin D [25(OH)D] and parathyroid hormone (PTH) in whites (panel A) and blacks (panels B). Curve fitting revealed variables were linearly related, as opposed to quadratic, cubic, exponential or logarithmic. r-values represent Pearson's bivariate correlations.
Figure 3.
Scatterplots illustrating the relationships between cortical bone properties (cortical volumetric bone mineral density [Ct.vBMD], total area [Tt.Ar], polar strength-strain index [SSIP]), and serum concentrations of 25-hydroxyvitamin D [25(OH)D] (panels A,C,E) and parathyroid hormone (PTH) (panels B,D,F) in blacks. Curve fitting revealed variables were linearly related, as opposed to quadratic, cubic, exponential or logarithmic. r-values represent Pearson's bivariate correlations.
Figure 2.
Scatterplots illustrating the relationships between cortical bone properties (cortical volumetric bone mineral density [Ct.vBMD], total area [Tt.Ar], polar strength-strain index [SSIP]), and serum concentrations of 25-hydroxyvitamin D [25(OH)D] (panels A,C,E) and parathyroid hormone (PTH) (panels B,D,F) in whites. Curve fitting revealed variables were linearly related, as opposed to quadratic, cubic, exponential or logarithmic. r-values represent Pearson's bivariate correlations.
Relative Contributions of Biochemical Variables to Racial Differences in Tibial Properties
The proportion of variation in Ct.vBMD, Tt.Ar and SSIP attributable to race before and after inclusion of additional predictor variables is detailed in Table 3.
Table 3. Proportion of variation in Ct.vBMD, Tt.Ar, and SSIP attributable to race before and after inclusion of additional predictor variables.
| Bone property | Regression model type | Predictor variable | Semi-partial R2 | P-value | Outcome of multiple regression model | |
|---|---|---|---|---|---|---|
| Ct.vBMD | Simple | Race | 0.16 | <0.001 | ||
| Multiple | Urine NTX | 0.08 | <0.001 | |||
| 25(OH)D | 0.05 | <0.001 | Model R2 | 0.21 | ||
| Race | 0.08 | <0.001 | P-value | <0.001 | ||
|
| ||||||
| Tt.Ar | Simple | Race | 0.07 | <0.001 | ||
| Multiple | Tibial length | 0.43 | <0.001 | |||
| Leg mCSA | 0.13 | <0.001 | ||||
| PTH | 0.01 | <0.01 | ||||
| Osteocalcin | 0.01 | 0.02 | Model R2 | 0.60 | ||
| Race | 0.02 | <0.001 | P-value | <0.001 | ||
|
| ||||||
| SSIP | Simple | Race | 0.13 | <0.001 | ||
| Multiple | Tibial length | 0.47 | <0.001 | |||
| Leg mCSA | 0.13 | <0.001 | ||||
| 25(OH)D | 0.01 | <0.001 | ||||
| Osteocalcin | 0.01 | 0.02 | Model R2 | 0.67 | ||
| Race | 0.05 | <0.001 | P-value | <0.001 | ||
Ct.vBMD
Univariate modeling revealed that race explained 16% of total variation in Ct.vBMD. Model selection for multiple regression revealed that NTX and 25(OH)D provided the best predictive model of Ct.vBMD. When NTX and 25(OH)D were included in the multivariate model before race, the percent of variation in Ct.vBMD attributable to race was reduced to 8%.
Tt.Ar
Univariate modeling revealed that race explained 7% of total variation in Tt.Ar. Model selection for multiple regression revealed that tibial length, leg mCSA, PTH and OC provided the best predictive model of Tt.Ar. When tibial length, leg mCSA, PTH and OC were included in the multivariate model before race, the percent of variation in Tt.Ar attributable to race was reduced to 2%.
SSIP
Univariate modeling revealed that race explained 13% of total variation in SSIP. Model selection for multiple regression revealed that tibial length, leg mCSA, 25(OH)D and OC provided the best predictive model of SSIP. When tibia length, leg mCSA, 25(OH)D and OC were included in the multivariate model before race, the percent of variation in SSIP attributable to race was reduced to 5%.
Discussion
This cross-sectional study demonstrated differences in cortical bone properties and biochemical variables between black and white children in the early stages of puberty. Black children had greater estimated bone strength (SSIP), exhibited higher bone formation and lower bone resorption markers, and had lower 25(OH)D and higher PTH than white children. Regression modeling suggested that differences in SSIP between black and white children were partly due to differences in tibial bone length, and serum concentrations of 25(OH)D and OC.
The greater SSIP in black children resulted from greater cortical bone density (Ct.vBMD), and larger cross-sectional areas (Tt.Ar and Ct.Ar) and perimeters (Ps.Pm and Es.Pm) compared to white children. These racial differences in tibial bone properties are consistent with recent studies utilizing pQCT in adolescent black and white children (10-14). A comprehensive study by Leonard et al. (10) showed that blacks in pubertal stages 1-4 had greater cortical density and size at the tibial diaphysis compared to whites, with prepubertal (pubertal stage 1) blacks having 13.4% greater tibial strength (polar section modulus) than whites. The magnitude of this race effect compares closely with the 17.0% greater SSIP observed in blacks in their early stages of puberty (pubertal stages 2-3) in the current study. The combined conclusions from these two studies indicate that racial divergence in the structure and strength of the skeleton precedes puberty, which is consistent with prospective areal bone mineral density measures by DXA (35-37).
Black children in the current study had greater tibial bone strength despite having lower levels of physical activity, dietary calcium intake, and serum 25(OH)D concentrations than white children, all factors which are considered to promote skeletal mineral accrual (38). We performed univariate and multiple regression analyses to explore the apparent paradox. Race independently explained 13% of the variation in SSIP. The contribution of race was reduced to 5% in the best multivariate model, which included significant biochemical [25(OH)D and OC] and leg anthropometric [tibial length] variables. The reduction in the contribution of race indicates that tibial length, 25(OH)D and OC were successful in partially explaining the race difference in SSIP. In other words, that racial differences in tibial length, 25(OH)D and OC contributed to the observed racial differences in SSIP.
SSIP is a derived measure incorporating components of bone density and structure. To further explore potential contributors to racial differences in bone strength, we explored Ct.vBMD and Tt.Ar as primary measures contributing to SSIP. Ct.vBMD was explored as it is consistently higher in both the immature and mature skeleton of blacks (10-14, 33, 34), while Tt.Ar was explored as bone strength is proportional to how far cortical bone material is distributed from mechanical axes and Tt.Ar independently explained 91% of the variance in SSIP in the current study (data not shown). Race independently explained 16% and 7% of the variation in Ct.vBMD and Tt.Ar, respectively. The contribution of race to Ct.vBMD was reduced to 8% in the best multivariate model, which included significant biochemical variables [NTX and 25(OH)D]. The contribution of race to Tt.Ar was reduced to 2% in the best multivariate model, which included significant biochemical [PTH and OC] and leg anthropometric [tibial length] variables. These findings provide additional support for the contributions of tibial length, 25(OH)D/PTH and OC to the observed racial differences in cortical bone properties.
Leg mCSA appeared in the best multivariate models predicting Tt.Ar and SSIP suggesting it had a role in explaining the observed racial differences in these bone properties. However, there were no differences in mCSA between blacks and whites in the current study, and previous studies were unable to identify race by muscle interactive effects on tibial cortical bone properties (10, 12, 14). To further explore the potential contribution of mCSA to the observed racial differences in Tt.Ar or SSIP, we also added race to the multivariate models before the addition of mCSA. The prior addition of race did not change the percent of variation in Tt.Ar or SSIP attributable to mCSA (i.e. mCSA influenced tibial bone properties independent of race) (data not shown). These collective findings suggest that leg mCSA did not contribute to the observed racial differences in Tt.Ar or SSIP.
Tibial length was significantly longer in blacks compared to whites, and was modeled to contribute to the observed racial differences in Tt.Ar and SSIP. These observations are consistent with allometric scaling in the skeleton whereby cross-sectional areas and estimated bone strength (second moments of area) in the diaphysis of long bones scale approximately to bone length (39). However, simple allometry does not completely explain the contribution of tibial length to racial differences in Tt.Ar and SSIP as racial differences in the latter bone properties persisted despite tibial length being used as a covariate in black vs. white comparisons.
Black children in the current study had 25% higher OC, a measure of bone formation that is predictive of calcium retention in adolescents (40). OC was positively associated with Tt.Ar (r = 0.19, p = 0.001; data not shown) and SSIP (r = 0.20, p < 0.001; data not shown), and partially accounted for the racial differences in the latter bone variables. These observations suggest that serum OC concentrations contributed to the racial differences in tibial bone properties.
Black children in the current study also had 25% lower 25(OH)D than white children, and serum 25(OH)D partially accounted for race differences in SSIP and Ct.vBMD. There was a negative relationship between 25(OH)D and SSIP in blacks, which could suggest a benefit of lower 25(OH)D on SSIP in black children—a finding contrary to the conventional concept of the higher the 25(OH)D the better the bone properties. However, the current findings are consistent with prospective studies which demonstrated a negative relationship between serum 25(OH)D and bone accrual in children (41, 42).
The greater SSIP in black children despite lower 25(OH)D may possibly be explained by PTH since: 1) blacks in the current study had 17.8% higher PTH compared to whites, 2) there was a positive relationship between PTH and SSIP in blacks, and 3) PTH was negatively related to 25(OH)D in blacks. In addition, PTH was positively related to Tt.Ar in blacks (r = 0.31, p < 0.001; data not shown) and partly explained race differences in Tt.Ar. It is clear from human and animal studies that PTH can be anabolic to the skeleton, and promotes an increase in bone mass and size (43, 44). Also, serum PTH concentrations at the upper end of the normal range have been associated with greater bone accrual during growth (42).
The persistent contribution of race when included last in each of the multivariate models predicting tibial bone properties indicates that racial differences beyond the biochemical and anthropometric variables measured contribute to the observed racial differences in cortical bone properties. Similarly, it is not clear from this cross-sectional study whether the relationships amongst Ct.vBMD, Tt.Ar, SSIP and biochemistries are casual or merely reflect an underlying genetic component to the setting of calcium homeostasis and bone turnover. However, in an intervention study with doses of up to 4,000 IU vitamin D3 we found no effect of an increase in 25(OH)D on bone turnover (45), suggesting that the biochemistries are set by genetic rather than environmental factors.
The current study possessed a number of strengths, including a relatively large sample of subjects at the same stage of sexual maturity, the use of pQCT to assess cortical bone properties, and the assessment of biochemical variables and exploration of their potential contribution to race-related differences in bone properties. However, the current study also possessed a number of limitations. The primary weakness was its cross-sectional study design, which limits the ability to: 1) establish causal relationships between biochemical variables and cortical bone properties, and; 2) assess the influence of race on the rate of accrual of bone properties. Other limitations were the restriction of analyses to the tibial diaphysis, which consists predominantly of cortical bone, and the lack of measurement of other important biochemical factors such as growth and sex steroid hormones.
The data presented here demonstrate the presence of cortical bone differences within the tibia between black and white children in their early stages of puberty, and relate the differences to biochemical variables. The data indicate that racial divergence in cortical bone properties develops in early childhood despite lower serum 25(OH)D in blacks.
Summary.
Osteoporotic fracture rates differ according to race with blacks having up to half the rate of whites. The current study demonstrates that racial divergence in cortical bone properties develops in early childhood despite lower serum 25-hydroxyvitamin D in blacks.
Acknowledgments
This contribution was made possible by support from the National Institutes of Health (R01-HD057126). The authors have no conflicts of interest.
Footnotes
Statement of competing interests: No competing interests
References
- 1.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, Jackson R, Robbins J. Clinical risk factors for fractures in multi-ethnic women: the Women's Health Initiative. J Bone Miner Res. 2007;22:1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger B, Sidney S, Cummings SR, Libanati C, Bikle DD, Tekawa IS, Tolan K, Steiger P. Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metab. 1997;82:429–434. doi: 10.1210/jcem.82.2.3732. [DOI] [PubMed] [Google Scholar]
- 4.Luckey MM, Meier DE, Mandeli JP, DaCosta MC, Hubbard ML, Goldsmith SJ. Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab. 1989;69:762–770. doi: 10.1210/jcem-69-4-762. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 6.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men's osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garn SM, Nagy JM, Sandusky ST. Differential sexual dimorphism in bone diameters of subjects of European and African ancestry. Am J Phys Anthropol. 1972;37:127–129. doi: 10.1002/ajpa.1330370116. [DOI] [PubMed] [Google Scholar]
- 9.Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83:1420–1427. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 10.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micklesfield LK, Norris SA, Pettifor JM. Determinants of bone size and strength in 13-year-old South African children: the influence of ethnicity, sex and pubertal maturation. Bone. 2011;48:777–785. doi: 10.1016/j.bone.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Pollock NK, Laing EM, Taylor RG, Baile CA, Hamrick MW, Hall DB, Lewis RD. Comparisons of trabecular and cortical bone in late adolescent black and white females. J Bone Miner Metab. 2011;29:44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- 13.Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, Petit MA. Ethnic differences in bone geometry and strength are apparent in childhood. Bone. 2009;44:970–975. doi: 10.1016/j.bone.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103–1108. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harkness L, Cromer B. Low levels of 25-hydroxyvitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16:109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 16.Hui SL, Dimeglio LA, Longcope C, Peacock M, McClintock R, Perkins AJ, Johnston CC., Jr Difference in bone mass between black and white American children: attributable to body build, sex hormone levels, or bone turnover? J Clin Endocrinol Metab. 2003;88:642–649. doi: 10.1210/jc.2002-020653. [DOI] [PubMed] [Google Scholar]
- 17.Stein EM, Laing EM, Hall DB, Hausman DB, Kimlin MG, Johnson MA, Modlesky CM, Wilson AR, Lewis RD. Serum 25-hydroxyvitamin D concentrations in girls aged 4-8 y living in the southeastern United States. Am J Clin Nutr. 2006;83:75–81. doi: 10.1093/ajcn/83.1.75. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab. 2008;93:3907–3914. doi: 10.1210/jc.2008-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 20.Willis CM, Laing EM, Hall DB, Hausman DB, Lewis RD. A prospective analysis of plasma 25-hydroxyvitamin D concentrations in white and black prepubertal females in the southeastern United States. Am J Clin Nutr. 2007;85:124–130. doi: 10.1093/ajcn/85.1.124. [DOI] [PubMed] [Google Scholar]
- 21.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, Smith DL, Peacock M, Weaver CM. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88:1043–1047. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 22.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr. 2007;85:1657–1663. doi: 10.1093/ajcn/85.6.1657. [DOI] [PubMed] [Google Scholar]
- 23.Pratt JH, Manatunga AK, Peacock M. A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med. 1996;127:67–70. doi: 10.1016/s0022-2143(96)90167-5. [DOI] [PubMed] [Google Scholar]
- 24.Tanner J. Growth at Adolescence. Blackwell; Oxford: 1962. [Google Scholar]
- 25.Centers for Disease Control and Prevention BMI Percentile Calculator for Child and Teen. In. [Google Scholar]
- 26.Taylor RW, Goulding A. Validation of a short food frequency questionnaire to assess calcium intake in children aged 3 to 6 years. Eur J Clin Nutr. 1998;52:464–465. doi: 10.1038/sj.ejcn.1600575. [DOI] [PubMed] [Google Scholar]
- 27.Pate R, Ross R, Dowda M, Trost S, Sirard J. Validation of a 3-day Physical Activity Recall Instrument in female youth. Pediatr Exerc Sci. 2003;15:257–265. [Google Scholar]
- 28.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm G, Felsenberg D, Bogusch G, Willnecker J, Thaten J, Gummert P. Biomechanical examinations for validation of the bone strength strain index SSI, calculated by peripheral quantitative computed tomography. In: Lyritis G, editor. Musculoskeletal Interactions. Hylonome; Athens: 2001. pp. 105–110. [Google Scholar]
- 30.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39:598–608. doi: 10.1016/j.bone.2006.02.057. Epub 2006 Apr 2004. [DOI] [PubMed] [Google Scholar]
- 31.Rauch F, Schoenau E. Peripheral quantitative computed tomography of the proximal radius in young subjects: new reference data and interpretation of results. J Musculoskelet Neuronal Interact. 2008;8:217–226. [PubMed] [Google Scholar]
- 32.Swinford RR, Warden SJ. Factors affecting short-term precision of musculoskeletal measures using peripheral quantitative computed tomography (pQCT) Osteoporos Int. 2010;21:1863–1870. doi: 10.1007/s00198-009-1151-3. [DOI] [PubMed] [Google Scholar]
- 33.Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock M, Buckwalter KA, Persohn S, Hangartner TN, Econs MJ, Hui S. Race and sex differences in bone mineral density and geometry at the femur. Bone. 2009;45:218–225. doi: 10.1016/j.bone.2009.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 36.Hui SL, Perkins AJ, Harezlak J, Peacock M, McClintock CL, Johnston CC., Jr Velocities of bone mineral accrual in black and white American children. J Bone Miner Res. 2010;25:1527–1535. doi: 10.1002/jbmr.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson DA, Simpson PM, Johnson CC, Barondess DA, Kleerekoper M. The accumulation of whole body skeletal mass in third- and fourth-grade children: effects of age, gender, ethnicity, and body composition. Bone. 1997;20:73–78. doi: 10.1016/s8756-3282(96)00312-2. [DOI] [PubMed] [Google Scholar]
- 38.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Ruff CB. Allometry between length and cross-sectional dimensions of the femur and tibia in Homo sapiens sapiens. Am J Phys Anthropol. 1984;65:347–358. doi: 10.1002/ajpa.1330650403. [DOI] [PubMed] [Google Scholar]
- 40.Weaver CM, Peacock M, Martin BR, Plawecki KL, McCabe GP. Calcium retention estimated from indicators of skeletal status in adolescent girls and young women. Am J Clin Nutr. 1996;64:67–70. doi: 10.1093/ajcn/64.1.67. [DOI] [PubMed] [Google Scholar]
- 41.Breen ME, Laing EM, Hall DB, Hausman DB, Taylor RG, Isales CM, Ding KH, Pollock NK, Hamrick MW, Baile CA, Lewis RD. 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab. 2011;96:E89–98. doi: 10.1210/jc.2010-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tylavsky FA, Ryder KM, Li R, Park V, Womack C, Norwood J, Carbone LD, Cheng S. Preliminary findings: 25(OH)D levels and PTH are indicators of rapid bone accrual in pubertal children. J Am Coll Nutr. 2007;26:462–470. doi: 10.1080/07315724.2007.10719637. [DOI] [PubMed] [Google Scholar]
- 43.Hock JM. Anabolic actions of PTH in the skeletons of animals. J Musculoskelet Neuronal Interact. 2001;2:33–47. [PubMed] [Google Scholar]
- 44.Hodsman AB, Bauer DC, Dempster D, Dian L, Hanley DA, Harris ST, Kendler D, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 45.Hill KM, Laing EM, Hausman DB, Acton A, Martin BR, McCabe GP, Weaver CM, Lewis RD, Peacock M. Bone turnover is not influenced by serum 25-hydroxyvitamin D in pubertal healthy black and white children. Bone. 2012 doi: 10.1016/j.bone.2012.06.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]