Abstract
Actomyosin contractility is the major force-generating machinery that shapes cells and tissues during morphogenesis. New evidence from Drosophila demonstrates that these forces are spatially organized by a combination of biochemical and mechanical signals that provide dynamic feedback in a complex cellular environment.
The development of the Drosophila embryo involves morphogenetic processes that use mechanical forces to push, pull, and move cells. These forces are generated in large part by the assembly of contractile actomyosin networks, which are composed of short myosin II filaments that move along actin filaments (1). Several biochemical signals are necessary and sufficient to recruit myosin to the cortex, including the secreted protein Fog (2), epidermal growth factor receptor, Notch and Hedgehog signaling (3-6), and unidentified targets of the transcription factors Even-skipped and Runt (7). However, it has been unclear whether other feedback mechanisms help to shape and maintain actomyosin structures in a dynamic cellular environment.
New evidence from Drosophila suggests that, in addition to biochemical signals, mechanical inputs also play an important role in controlling cytoskeletal dynamics in vivo (8, 9). In a study by Farge and colleagues, the authors provided evidence that mechanical tension is sufficient to recruit myosin II to the apical cell cortex during mesoderm invagination at gastrulation (9) (Fig. 1A). Apical constriction is initiated by the assembly and contraction of an apical actomyosin meshwork in mesoderm precursor cells (2, 10). Ventral furrow formation requires the activity of the Twist and Snail transcription factors in the mesodermal domain (11). In the absence of Twist, apical constrictions are not stably maintained, whereas in the absence of Snail apical constriction fails completely (10,11). These results define Twist and Snail as essential transcriptional regulators of actomyosin contractility during mesoderm invagination.
Fig. 1.
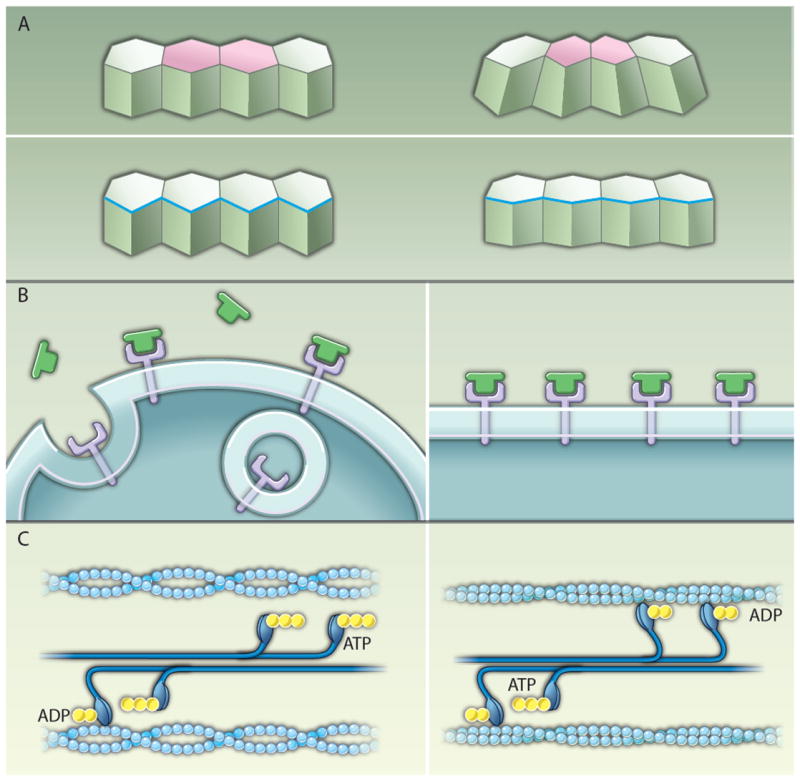
Mechanisms of tension-mediated actomyosin regulation. (A) Actomyosin contraction promotes changes in cell shape during epithelial morphogenesis. During mesoderm invagination and in the amnioserosa during dorsal closure, contraction of an apical actomyosin network (pink) drives apical constriction (top). Leading-edge cells of the lateral epidermis align through the contraction of a multicellular cable (blue) that contributes to dorsal closure (bottom). (B) A model of the plasma membrane as a mechanosensor. In the absence of tension, Fog (green) signaling through its putative receptor (purple) is proposed to be counteracted by endocytosis (left). When the plasma membrane is subjected to external tension, the bending and pinching off of vesicles is inhibited, promoting Fog signaling (9) (right). (C) A model of the myosin II motor as a mechanosensor. For simplicity only one head of each myosin motor is shown. When actin filaments (light blue) are under low tension, myosin II motors in bipolar filaments (dark blue) are rapidly released after adenosine triphosphate (ATP) (yellow) hydrolysis and the power stroke (left). In actin filaments under tension, the ADP bound form of myosin II is stabilized (34-36), leading to the accumulation of myosin II molecules on the actin filament (right).
Twist exerts its effect through transcription of the secreted protein Fog and the transmembrane protein T48, which signal through RhoGEF2 and the guanine triphosphatase (GTPase) Rho to recruit myosin II to the apical cortex (2, 12). The mechanism by which Snail regulates myosin localization is less clear. snail mutant embryos display abnormal myosin II localization in the cells of the ventral mesoderm, which subsequently fail to invaginate (10). Farge and colleagues recently demonstrated that an external mechanical force can rescue apical myosin localization in snail mutants (9). The authors showed that use of a micropipette to physically indent the ventral epithelium rapidly restores myosin II apical localization in snail mutants within 2 to 3 min of force application. These results demonstrate that a mechanical force is sufficient to recruit myosin II to the apical cortex and can recapitulate specific events downstream of Snail signaling.
The observation that an ectopic force can rescue apical constriction in snail mutants suggests that there is a mechanosensitive mechanism that can convert a mechanical input into localized actomyosin contractility. Although mechanical forces can regulate myosin II indirectly by promoting Twist transcription (13, 14), the rapid recruitment of myosin within 2 to 3 min of force application suggests that nontranscriptional mechanisms are at work (8, 9). Indentation does not rescue myosin localization in twist snail double mutants, indicating that a Twist target gene is required for the response to tension. Pouille et al. showed that indentation can also lead to myosin relocalization in twist mutants, but only in the presence of the secreted protein Fog, one of the transcriptional targets of Twist. Although Fog alone is sufficient for apical constriction when misexpressed uniformly throughout the embryo (2, 15), the authors proposed that mechanical stimulation can potentiate Fog activity and showed that indentation increases the amount of Fog in the apical cytoplasm, consistent with their hypothesis. Apical localization of Fog in snail mutants is also enhanced by a temperature-sensitive dynamin mutation after a short shift to the restrictive temperature (9), suggesting that mechanical indentation may inhibit Fog endocytosis. In the authors’ model, mechanical tension generated by an initial Snail-dependent wave of apical constriction could exert tension on the plasma membrane of neighboring cells, enhancing extracellular Fog signaling and triggering apical myosin II localization throughout the mesoderm (Fig. 1B). Theoretical simulations of tension-dependent Fog activation by Snail recapitulate the in vivo invagination behavior, indicating that the model is plausible.
These studies in Drosophila suggest that, in addition to the established role of actomyosin contractility in generating force, myosin II itself can also be recruited to the cortex in response to strictly mechanical signals. In intercalating cells in the adjacent ectoderm, myosin II is organized into multicellular cables that form dynamically throughout the tissue and contract along the dorsal-ventral axis to promote elongation of the embryo (16). Experiments using a different set of techniques to manipulate the force distribution in the embryo showed that mechanical signals in this context are necessary and sufficient for myosin II localization (8). Myosin was recruited to the cortex within 1 to 2 min of force application by a micropipette, and, conversely, relieving tension by laser ablation led to a rapid loss of cortical myosin (8). Direct measurements of protein dynamics using fluorescence recovery after photobleaching showed that myosin II dissociation from the cortex is selectively inhibited in regions under increased tension (8). These results indicate that tension regulates myosin localization by stabilizing, rather than recruiting, motor proteins to the cortex (Fig. 1C). For a notoriously nonprocessive motor such as myosin II that only attaches to an actin filament for a few milliseconds at a time (17), inhibiting the dissociation of myosin from the cortex could go a long way toward promoting force generation by an interconnected contractile network.
Mechanical feedback regulation of actomyosin contractility is also likely to be important for other morphogenetic processes. During dorsal closure in the Drosophila embryo, cells of the amnioserosa, an extraembryonic epithelium that covers the dorsal side of the embryo, constrict their apices, and a subset of cells undergoes apoptosis, pulling the lateral epidermis toward the dorsal midline (18-20). Laser ablation of the cell apex caused the apical actin meshwork to retract, demonstrating that it is under tension (21). A second contractile structure at the leading edge of the lateral epidermis forms a continuous actomyosin cable that contributes to dorsal closure through a purse-string mechanism (22-24). Laser ablation of either the leading edge cable or apically constricting cells of the amnioserosa slowed the rate of closure (25, 26), indicating that both tissues generate forces that drive this process.
Although mechanical signals have not been shown to influence cytoskeletal activity directly during dorsal closure, several lines of evidence suggest that cell behavior is modified by the local mechanical environment. For example, amnioserosa cells closest to the leading edge contract before the more-central cells (19) and are the first to eventually stop contracting (20), despite the absence of apparent transcriptional differences between these cell populations. The amount of filamentous actin in the leading edge increases as tension builds throughout closure (20), suggesting that tension correlates with changes in cytoskeletal organization. Lastly, disrupting a single cell by laser ablation led to the cessation of contractile activity in adjacent cells (20), indicating that contraction of one cell promotes contraction in its neighbors. Actomyosin cables also form rapidly in new locations after ablation of the original leading edge (27), providing another example in which actin and myosin localization are responsive to changes in the mechanical environment, a process that may have implications for wound healing.
An important question raised by these studies is the nature of the molecular mechanism that translates mechanical force into a local change in contractile activity. Pouille et al. proposed that tension modifies a property of the plasma membrane that inhibits Fog endocytosis (Fig. 1B) (9). However, fog is not expressed in all epithelia that display force-dependent regulation of myosin II localization, and mechanical tension and endocytosis could affect a number of other targets in addition to Fog localization. In Dictyostelium, myosin II was rapidly recruited to the cortex in response to a force applied by a micropipette (28) in a process that requires the actin crosslinking protein cortexillin I (29, 30) and the lipid phosphatase PTEN (31). Both cortexillin I and PTEN interact with the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), which regulates actomyosin activity in mammalian cells (32). These studies suggest that mechanical tension could regulate myosin II localization by modifying the properties of the plasma membrane.
Alternatively, in vitro experiments raise the possibility that myosin II motor activity may be directly regulated by tension. Experiments on isolated actin networks demonstrated a positive relationship between tension and actin polymerization (33), and single-molecule experiments showed that tension stabilizes the adenosine diphosphate (ADP)–bound form of myosin II, which has a higher affinity for filamentous actin (34-36). Consistent with this, in Dictyostelium the duty ratio of myosin II was estimated to increase up to 10-fold when the actomyosin network is under tension (30). Increasing the duty ratio of myosin II would be predicted to increase the amount of force generated by the actomyosin network without any additional input of energy.
Together, these results suggest that, in addition to biochemical signals that initiate myosin II contractility, mechanical signals are key factors that shape the organization of contractile networks in dynamic cell populations. An initial signal targets the contractile machinery to the apical cortex of specific cells during apical constriction, or cell boundaries during cell intercalation, triggering a wave of actomyosin contractility that propagates away from the initiating contractile event and recruits neighboring cells to adopt a similar program of behavior. Intriguingly, epithelial cells that lack directional cues can still nucleate multicellular cables locally, albeit in a randomly oriented fashion (16). Similarly, cycles of apical constriction and expansion in the amnioserosa are evident before the onset of dorsal closure, suggesting that formation of a contractile leading edge is required to convert the force-generating capacity of the amnioserosa into a sustained change in tissue structure (20). The recruitment of myosin II by tension could represent a latent force-generating machinery in all epithelial cells that is mobilized in response to spatial cues to amplify local changes in cell behavior during morphogenesis.
References and Notes
- 1.Martin A. Pulsation and stabilization: Contractile forces that underlie morphogenesis. Dev Biol. doi: 10.1016/j.ydbio.2009.10.031. in press; published online 27 October 2009. [DOI] [PubMed] [Google Scholar]
- 2.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. Folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 3.Brodu V, Casanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 2006;20:1817–1828. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 5.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–4282. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- 7.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Gonzalez R, Simoes SD, Röper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 10.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Kölsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- 13.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 14.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Morize P, Christiansen AE, Costa M, Parks S, Wieschaus E. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development. 1998;125:589–597. doi: 10.1242/dev.125.4.589. [DOI] [PubMed] [Google Scholar]
- 16.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 18.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–1898. doi: 10.1242/dev.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 22.Young PE, Richman AM, Ketchum AS, Kiehart DP. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, Martinez-Arias A, Martin P. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12:1245–1250. doi: 10.1016/s0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- 24.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 25.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Montague, Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. published online 6 February 2003. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Diaz A, Toyama Y, Abravanel DL, Wiemann JM, Wells AR, Tulu US, Edwards GS, Kiehart DP. Actomyosin purse strings: Renewable resources that make morphogenesis robust and resilient. HFSP J. 2008;2:220–237. doi: 10.2976/1.2955565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Effler JC, Kee YS, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr Biol. 2006;16:1962–1967. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, Divi S, Iglesias PA, Kuo SC, Robinson DN. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol. 2008;18:471–480. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol. 2009;19:1421–1428. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramanik MK, Iijima M, Iwadate Y, Yumura S. PTEN is a mechanosensing signal transducer for myosin II localization in Dictyostelium cells. Genes Cells. 2009;14:821–834. doi: 10.1111/j.1365-2443.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 32.Logan MR, Mandato CA. Regulation of the actin cytoskeleton by PIP2 in cytokinesis. Biol Cell. 2006;98:377–388. doi: 10.1042/BC20050081. [DOI] [PubMed] [Google Scholar]
- 33.Parekh SH, Chaudhuri O, Theriot JA, Fletcher DA. Loading history determines the velocity of actin-network growth. Nat Cell Biol. 2005;7:1219–1223. doi: 10.1038/ncb1336. [DOI] [PubMed] [Google Scholar]
- 34.Cremo CR, Geeves MA. Interaction of actin and ADP with the head domain of smooth muscle myosin: Implications for strain-dependent ADP release in smooth muscle. Biochemistry. 1998;37:1969–1978. doi: 10.1021/bi9722406. [DOI] [PubMed] [Google Scholar]
- 35.Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol. 2003;5:980–986. doi: 10.1038/ncb1060. [DOI] [PubMed] [Google Scholar]
- 36.Kovács M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci U S A. 2007;104:9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]


