The neurobiological basis of fragmented sleep in ageing and Alzheimer’s disease is unknown. In this human clinicopathological study, Lim et al. show that a reduced number of galanin-immunoreactive neurons in the intermediate nucleus of the hypothalamus is accompanied by greater sleep fragmentation in older adults with and without Alzheimer’s disease.
Keywords: sleep, Alzheimer’s disease, hypothalamus, human, intermediate nucleus
Abstract
Fragmented sleep is a common and troubling symptom in ageing and Alzheimer’s disease; however, its neurobiological basis in many patients is unknown. In rodents, lesions of the hypothalamic ventrolateral preoptic nucleus cause fragmented sleep. We previously proposed that the intermediate nucleus in the human hypothalamus, which has a similar location and neurotransmitter profile, is the homologue of the ventrolateral preoptic nucleus, but physiological data in humans were lacking. We hypothesized that if the intermediate nucleus is important for human sleep, then intermediate nucleus cell loss may contribute to fragmentation and loss of sleep in ageing and Alzheimer’s disease. We studied 45 older adults (mean age at death 89.2 years; 71% female; 12 with Alzheimer’s disease) from the Rush Memory and Aging Project, a community-based study of ageing and dementia, who had at least 1 week of wrist actigraphy proximate to death. Upon death a median of 15.5 months later, we used immunohistochemistry and stereology to quantify the number of galanin-immunoreactive intermediate nucleus neurons in each individual, and related this to ante-mortem sleep fragmentation. Individuals with Alzheimer’s disease had fewer galaninergic intermediate nucleus neurons than those without (estimate −2872, standard error = 829, P = 0.001). Individuals with more galanin-immunoreactive intermediate nucleus neurons had less fragmented sleep, after adjusting for age and sex, and this association was strongest in those for whom the lag between actigraphy and death was <1 year (estimate −0.0013, standard error = 0.0005, P = 0.023). This association did not differ between individuals with and without Alzheimer’s disease, and similar associations were not seen for two other cell populations near the intermediate nucleus. These data are consistent with the intermediate nucleus being the human homologue of the ventrolateral preoptic nucleus. Moreover, they demonstrate that a paucity of galanin-immunoreactive intermediate nucleus neurons is accompanied by sleep fragmentation in older adults with and without Alzheimer’s disease.
Introduction
Fragmented sleep is a common and troubling symptom in ageing and Alzheimer's disease, and is associated with a number of negative health outcomes. Sleep fragmentation predicts incident depression in older adults (Cole and Dendukuri, 2003), correlates with poor quality of life (Hidalgo et al., 2007), predicts fall risk (Stone et al., 2008), and is associated with poorer cognition in older individuals (Oosterman et al., 2009). In those with dementia, sleep is even more disturbed (Allen et al., 1987), and sleep disturbances are a trigger for institutionalization (Pollak and Perlick, 1991). Although identifiable sleep disorders such as sleep apnoea are found in some older patients with fragmented sleep (Ancoli-Israel et al., 1991), and degeneration in the hypothalamic suprachiasmatic nucleus, the ‘master circadian clock’, may be important for others—particularly those with very advanced Alzheimer’s disease (Harper et al., 2008)—the biological basis of fragmented sleep in many patients is unknown.
In rodents, the ventrolateral preoptic nucleus is a galaninergic nucleus in the anterior hypothalamus that plays a critical role in maintaining sustained sleep (Sherin et al., 1996; Lu et al., 2000). The ventrolateral preoptic nucleus is selectively sleep-active (Sherin et al., 1996; Szymusiak et al., 1998), and sends inhibitory projections to wake-promoting areas (Sherin et al., 1998). Lesions of the ventrolateral preoptic nucleus result in marked sleep fragmentation due to difficulties in maintaining sustained sleep, as well as a decrease in total sleep time (Lu et al., 2000)—a pattern similar to that seen in ageing and Alzheimer’s disease—suggesting that cell loss in the human homologue of the ventrolateral preoptic nucleus may contribute to sleep fragmentation and loss in ageing and Alzheimer’s disease. There is evidence that the galaninergic neurons of the ventrolateral preoptic nucleus are particularly important for sleep regulation. In rodents, >80% of the sleep-active neurons in the ventrolateral preoptic nucleus also express galanin mRNA (Gaus et al., 2002), and the vast majority of ventrolateral preoptic nucleus neurons that project to the tuberomamillary nucleus, a key wake-promoting centre, are galaninergic (Sherin et al., 1998).
The human homologue of the rodent ventrolateral preoptic nucleus is uncertain. We proposed previously that the intermediate nucleus (Brockhaus, 1942), which like the rodent ventrolateral preoptic nucleus expresses galanin as a neurotransmitter and lies in the chiasmatic region of the anterior hypothalamus (Gai et al., 1990; Gaus et al., 2002; Garcia-Falgueras et al., 2011), may be the homologue of the rodent ventrolateral preoptic nucleus. Studies of the intermediate nucleus in ageing have previously reported a decrease in intermediate nucleus cell numbers or intermediate nucleus volume with increasing age (Allen et al., 1989; Hofman and Swaab, 1989; Garcia-Falgueras et al., 2011); however the sleep-wake behaviour of these individuals was not ascertained.
Ideally, a study examining the impact of intermediate nucleus integrity on human sleep would examine both simultaneously. However, by virtue of its location and size, the integrity of the human intermediate nucleus cannot easily be assessed ante-mortem. Therefore, to examine the impact of intermediate nucleus integrity on human sleep requires a comparison of intermediate nucleus integrity post-mortem with measures of ante-mortem sleep architecture proximate to death. Because time of death is unpredictable, this requires repeated measurements of sleep architecture in the same individuals to ensure that once death occurs, post-mortem intermediate nucleus integrity can be compared to a relatively recent measurement of sleep architecture. Polysomnography is ill-suited to this purpose because of high participant burden, perturbation of normal sleep behaviour by the study apparatus, and expense. Meanwhile, self-report sleep measures correlate poorly with objective measures (Lauderdale et al., 2008), and can be confounded by cognitive impairment, which can be common in those at the end of their lives. To overcome these limitations, we studied individuals participating in the Rush Memory and Aging Project, a community-based cohort study of ageing and dementia in which participants undergo biennial actigraphy and agree to brain donation upon death. Using data from this cohort, we recently developed and validated a novel metric of sleep fragmentation in ageing and dementia (Lim et al., 2011, 2012, 2013).
In the present study, we used a clinico pathological correlation approach in Memory and Aging Project participants with and without Alzheimer’s disease to examine the hypothesis that loss of neurons in the human intermediate nucleus, as the homologue of the rodent ventrolateral preoptic nucleus, correlates with sleep loss and fragmentation measured by actigraphy in older adults with and without Alzheimer’s disease.
Materials and methods
Study population
We studied 45 participants in the Rush Memory and Aging Project—a community-based cohort study of the chronic conditions of ageing in which participants undergo annual testing and agree to brain donation upon death (Bennett et al., 2012). The study began in 1997. Biennial actigraphy was added to the study protocol in 2005, and all surviving participants and new enrolees since then were offered the opportunity to undergo actigraphic recording. For the present study, we analysed data and tissue from deceased Memory and Aging Project participants with at least one actigraphic recording before death, available post-mortem hypothalamic tissue containing the intermediate nucleus of the hypothalamus, and who were not taking sedative or hypnotic medications at the time of actigraphy. At the time of these analyses, there were 886 Memory and Aging Project participants with at least one actigraphic recording, 190 of whom had died and undergone autopsy. Of these, we excluded data from 17 individuals who were taking sedative-hypnotic medications at the time of actigraphy. Upon death, brains were removed and hemisected, and cut into 1-cm slabs. One hemisphere from each brain was fixed in paraformaldehyde, blocks containing the hypothalamus from the chiasmatic region to the mammillary bodies were manually dissected, a 1:4 50 µm coronal series was cut using a sliding microtome, and one series was stained immunohistochemically for galanin. Because the brain specimens were originally collected for other purposes, and in some cases portions of the hypothalamus had already been sampled for other studies, the entire chiasmatic region of the hypothalamus was not available for all cases. Coronal series containing the entire intermediate nucleus, as determined by visual inspection of the fixed brain slabs, hypothalamic blocks, and of the galanin-stained sections, were available from 45 individuals.
Statement of ethics approval
This study was approved by the Institutional Review Boards of Rush University Medical Centre and the Beth Israel Deaconess Medical Centre. Written informed consent was obtained from all participants. All participants signed an anatomical gift act for organ donation.
Sleep assessment
Sleep was assessed by actigraphy. Actigraphs (Actical, Philips Respironics) set to record in 15-s epochs were placed by study staff on participants’ non-dominant wrists for up to 10 days. For this study, we used the last available actigraphic recording before death. Actigraphic data were analysed using algorithms programmed in MATLAB (Mathworks).
Sleep fragmentation is the extent to which sleep is interrupted by repeated awakenings. Our primary metric of sleep fragmentation was the kRA as described and validated elsewhere (Lim et al., 2011, 2012, 2013). Briefly, kRA is the probability per 15-s epoch of having an arousal, as indicated by movement (defined as a non-zero activity count) after a long (about ≥5 min) period of rest (i.e. sleep), with a higher kRA indicating greater sleep fragmentation. In a previous study (Lim et al., 2013), we showed that kRA correlates well with several standard polysomnographic measures of sleep consolidation including sleep efficiency and wake time after sleep onset. As secondary actigraphic sleep outcomes, we considered the average number of hours per day spent in bouts of rest lasting ≥10 min (a measure of the total daily amount of consolidated rest), and the percentage of total rest time spent in bouts of rest lasting ≥10 min.
In addition to the above actigraphic metrics, participants were also asked about their self-reported habitual sleep times, and the frequency with which they experience difficulty initiating sleep or maintaining sleep.
Assessment of clinical and demographic variables
Age at death was computed from the self-reported date of birth and the recorded date of death. Sex and years of education were recorded at the baseline interview. Medications were coded using the Medi-Span system.
As described elsewhere (Wilson et al., 2005), participants underwent annual cognitive evaluation with 19 cognitive tests in five cognitive domains, which were reviewed by a neuropsychologist to determine the presence or absence of cognitive impairment. Annually, and at the time of death, individuals were classified as having Alzheimer’s disease or not according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984) using all available cognitive and clinical data as previously described (Bennett et al., 2006a).
Immunohistochemistry
Upon death, brains were removed, hemisected, and cut into 1-cm slabs on a Plexiglas jig. One hemisphere from each brain was fixed in 4% paraformaldehyde for 3 days and available for histological analysis. Slabs were then transferred to a graded cryoprotectant solution (final solution 20% glycerol and 2% dimethylsulphoxide in PBS) and stored at 4°C. From these slabs, blocks containing the hypothalamus from the chiasmatic region to the mammillary bodies were manually dissected. Glycerol was removed by immersion in PBS + 0.2% (w/v) sodium azide for 1 week. Blocks were then cryoprotected by immersion for 72 h in 20% sucrose + 0.2% (w/v) sodium azide in PBS for 72 h and four 1:4 50 -μm coronal series were cut using a sliding microtome.
One of the 1:4 series was selected for immunohistochemistry for galanin. Sections were washed three times for 5 min in PBS and placed in 10 ml 0.1 M Tris-Cl pH 10 in a 20 ml glass scintillation vial, which was subsequently placed in a 90°C water bath for 20 min for the purpose of antigen retrieval. After being allowed to cool to room temperature over 1 h, sections were rinsed three times for 10 min in PBS and immersed in 1% (w/v) sodium borohydride for 30 min to reduce any remaining free aldehyde groups. Sections were then rinsed three times for 10 min in PBS and immersed in 1% (w/v) H2O2 and 20% ethanol in PBS for 90 min to inactivate endogenous peroxidases. After rinsing three times for 10 min in PBS, sections were placed in 10% (w/v) non-fat dry milk and 0.25% (v/v) Triton™ X-100 in PBS for 2 h to block non-specific protein binding sites. Without rinsing, sections were then incubated for 5 days at 4°C in polyclonal rabbit-anti-galanin antiserum (Bachem AG; Catalogue Number T-4326; Lot Number 982035; prepared against full-length human galanin) diluted 1:2000 in PBS with 5% (w/v) non-fat dry milk, 0.25% (v/v) Triton™ X-100, and 0.2% (w/v) sodium azide. Sections were then rinsed three times for 30 min in PBS with 0.25% (v/v) Triton™ X-100 and incubated overnight at room temperature in monoclonal biotinylated donkey-anti-rabbit IgG (Jackson Immunoresearch) diluted 1:500 in PBS with 5% (w/v) non-fat dry milk, 0.25% (v/v) Triton™ X-100. Sections were then rinsed three times for 30 min in PBS with 0.25% (v/v) Triton™ X-100. Sections were then incubated for 10 min in a 0.06% solution of 3,3-diaminobenzidine tetrahydrochloride (Sigma) plus 0.4% nickel ammonium sulphate. H2O2 was then added to a final concentration of 0.1%. Once appropriate staining was attained, the reaction was stopped by rinsing three times for 10 min in PBS. Sections were then mounted on glass slides, dried overnight, dehydrated in a series of graded alcohols and xylene, and cover slipped using Permount (Fisher Scientific). The distribution of galanin protein and mRNA in the human anterior hypothalamus is well-known and has been characterized by both in situ hybridization for mRNA (Gaus et al., 2002) and immunohistochemistry for protein (Garcia-Falgueras et al., 2011). The antibody used in the present study stained a neuronal pattern in the human anterior hypothalamus that is identical to these previous reports, providing a strong indication that the antibody is indeed identifying galaninergic neurons. To further confirm antibody specificity, the above protocol was repeated, with the minimum concentration of primary antibody necessary to obtain visible staining (1:20 000) without and with preadsorption with 50 μg/ml of galanin peptide for 1 h, which was sufficient to abolish all visible staining. Together, these controls provide strong evidence for antibody specificity (Saper, 2005, 2009).
Because the brain specimens were originally collected for other purposes, and in some cases portions of the hypothalamus had already been sampled for other studies, the suprachiasmatic nucleus in its entirety was not available for analysis in the present study in all cases. Sections from the 22 individuals with the complete suprachiasmatic nucleus available underwent immunohistochemistry for vasoactive intestinal peptide (one 1:4 50-μm series) and AVP (one 1:4 50-μm series).
The immunohistochemistry protocol for VIP was similar to that for galanin, but using polyclonal rabbit-anti-VIP antiserum (ImmunoStar; catalogue number 20077; lot number 722001) at a concentration of 1:50 000 as the primary antiserum. This antibody stained a neuronal pattern in the human anterior hypothalamus that is identical to previous reports of vasoactive intestinal peptide immunohistochemistry (Dai et al., 1997). To further confirm antibody specificity, the protocol was repeated with the minimum concentration of primary antibody necessary to obtain visible staining (1:100 000) without and with preadsorption with VIP at a concentration of 50 μg/ml for 1 h, which was sufficient to abolish all visible staining.
The immunohistochemistry protocol for AVP was similar to that for galanin, but without the antigen retrieval step, and with polyclonal rabbit-anti-AVP antiserum (Millipore; catalogue number AB1565; lot number NG1820107) at a concentration of 1:2000 as the primary antiserum. This antibody stained a neuronal pattern in the human anterior hypothalamus that is identical to previous reports of AVP immunohistochemistry (Dai et al., 1997). To confirm antibody specificity, the protocol was repeated with the minimum concentration of primary antibody necessary to obtain visible staining (1:100 000) without and with preadsorption with AVP peptide at a concentration of 1 μg/ml for 1 h, which was sufficient to abolish all visible staining.
Cell counting
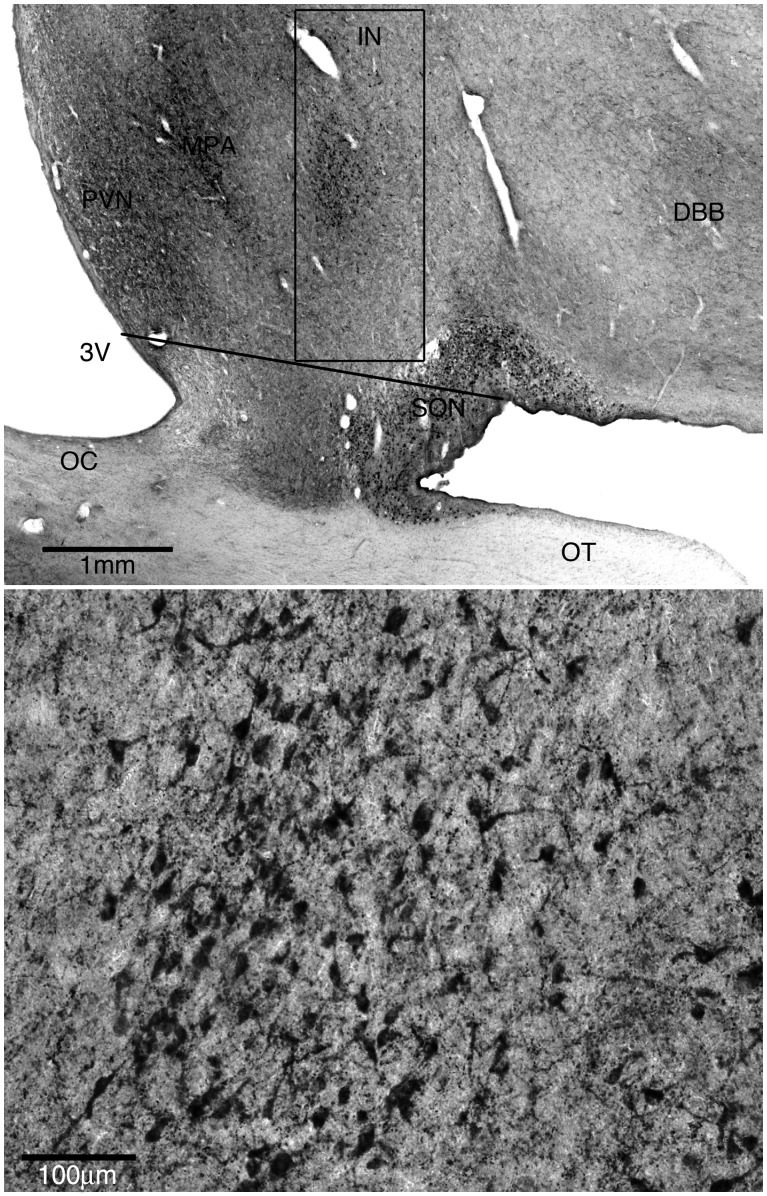
The total number of galanin-immunoreactive neurons in the intermediate nucleus of one hemisphere of each participant was determined using the optical fractionator (Gundersen and Jensen, 1987; West et al., 1991; West, 1999) stereological method implemented using a Zeiss Axiophot light microscope (Carl Zeiss MicroImaging) with a motorized stage and Stereo Investigator software (MBF Bioscience). All counting was done by trained technicians blinded to the clinical and actigraphic characteristics of each study participant. To avoid bias, the counting frame was objectively defined as a 1 mm × 3 mm box whose long axis was parallel to the lateral wall of the third ventricle, and whose inferomedial corner lay at the midpoint of a line parallel to the base of the brain connecting the angle between the base of the brain and the hypothalamic chiasmatic region inferolateral to the supraoptic nucleus, to the lateral wall of the third ventricle (Fig. 1A). This assignment of the counting area reliably separated the galaninergic neurons of the intermediate nucleus from those in the medial preoptic area and the supraoptic nucleus, which belong to different populations. The rostral extent of the counting frame was the anterior aspect of the optic chiasm. The caudal extent of the counting frame was defined by the entry of any portion of the fornix into the counting box. Gundersen’s counting rules were used (Gundersen and Jensen, 1987; West et al., 1991; West, 1999), with an x–y grid spacing of 150 μm, counting box of 50 μm × 50 μm × 8 μm, and guard zones of 1 μm. In each section, a low-power objective lens (×2.5) was used to define the counting frame. Actual counting was done using an oil immersion ×100 objective lens. Actual section thickness was measured every four counting boxes, and this value was used in all computations. The median coefficient of error was 0.13.
Figure 1.
The intermediate nucleus of the hypothalamus. (A) Representative low power view of the anterior hypothalamus stained immunohistochemically for galanin. The box indicates the counting frame used to define the intermediate nucleus. The long axis of the box is parallel to the lateral wall of the third ventricle. The inferomedial corner of the box is at the midpoint of a line, parallel to the base of the brain, connecting the angle between the base of the brain and the chiasmatic region inferolateral to the supraoptic nucleus to the lateral wall of the third ventricle. 3V = third ventricle; IN = intermediate nucleus; DBB = nucleus of the diagonal band of Broca; OT = optic tract; OC = optic chiasm. (B) Representative high power view of intermediate nucleus of the hypothalamus stained for galanin.
The total number of AVP-immunoreactive and VIP-immunoreactive neurons in the suprachiasmatic nucleus of one hemisphere in the 22 participants for which this tissue was available was determined using the same counting parameters, but with the counting box bounded ventrally by the optic tract, medially by the third ventricle, dorsally by the paraventricular nucleus, and laterally by the supraoptic nucleus. Suprachiasmatic nucleus AVP-immunoreactive neurons, which have relatively small somata, were easily distinguished from the much larger AVP-immunoreactive neurons in the paraventricular and supraoptic nuclei. All VIP-immunoreactive neurons within the counting frame were counted, as there are no VIP-immunoreactive neurons in the human anterior hypothalamus aside from those in the suprachiasmatic nucleus. The median coefficient of error for the AVP-immunoreactive suprachiasmatic neuron population was 0.08. The median coefficient of error for the VIP-immunoreactive suprachiasmatic neuron population was 0.14.
Pathological diagnosis of Alzheimer’s disease
As described previously (Bennett et al., 2006b), Bielschowsky silver stain was used to visualize neurofibrillary tangles, diffuse plaques, and neuritic plaques in the frontal, temporal, parietal, and entorhinal cortices, and the hippocampus. Braak stages 0–VI were assigned based upon the distribution and severity of neurofibrillary tangle pathology (Braak and Braak, 1991). All cases received a neuropathological diagnosis of no Alzheimer’s disease, low likelihood Alzheimer’s disease, intermediate likelihood Alzheimer’s disease, or high likelihood Alzheimer’s disease based on the National Institutes of Aging-Reagan criteria (National Institute on Aging and Reagan Institute Working Group of Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease, 1997).
Statistical analysis
All models were adjusted for age at death and sex. Age was considered relative to the mean age of all participants.
We compared the 45 participants with hypothalamic blocks containing the intermediate nucleus, and the 128 participants without hypothalamic blocks containing the intermediate nucleus with regard to a number of clinical and demographic variables. The Wilcoxon rank-sum test was used to compare continuous measures, and the χ2 test was used to compare discrete measures.
We next examined the bivariate relationships between age, sex, and presence/absence of Alzheimer’s disease, and the number of galanin-immunoreactive intermediate nucleus neurons per hemisphere in unadjusted models using linear regression.
To examine the relationship between the number of galanin-immunoreactive intermediate nucleus neurons at death and sleep fragmentation measured at the actigraphic recording closest to the time of death, we used a linear regression model adjusted for age and sex, with kRA as the outcome. Because the time lag between actigraphy and death is an important potential confounder and/or modifier of any relationship between the number of galanin-immunoreactive intermediate nucleus neurons at death and sleep fragmentation measured before death, we initially considered a model that included a (lag × galanin-immunoreactive neuron) interaction term. If this interaction was significant at P < 0.05, then we stratified subsequent analyses into two strata—individuals who died within 1 year of actigraphy, and those who died >1 year after last actigraphy. Next, to examine if the presence/absence of Alzheimer’s disease altered any associations between neuron counts and sleep fragmentation, we considered models with a (Alzheimer’s disease × galanin-immunoreactive neuron) interaction term and performed stratified analyses if this interaction was significant at P < 0.05. For all models, the relationship between galanin-immunoreactive intermediate nucleus neuron numbers and sleep fragmentation measured by kRA, after adjusting for age and sex, was graphically depicted using partial regression plots and partial correlation coefficients, and quantified by examining the significance of the regression coefficients.
In secondary analyses, we repeated these procedures considering a number of additional sleep measures including the average number of hours per day spent in bouts of rest lasting 10 min or longer (a measure of the total daily amount of consolidated rest, which is likely to represent sleep), the percentage of total rest time spent in bouts of rest lasting ≥10 min, and the self-reported habitual hours of sleep per night, in linear models adjusted for age and sex. We also examined the relation between the number of galanin-immunoreactive intermediate nucleus neurons and the odds of ‘often’ or ‘very often’ experiencing difficulties initiating or maintaining sleep as compared to ‘sometimes’, ‘rarely’ or ‘never’ experiencing these difficulties, using logistic regression models adjusted for age and sex.
To examine the anatomical specificity of any observed associations, we repeated our primary analyses considering two cell populations in the same region of the hypothalamus as the intermediate nucleus—the AVP-immunoreactive and VIP-immunoreactive neurons of the suprachiasmatic nucleus.
All statistical analyses were carried out in the R programming language (R Development Core Team, 2008). For all analyses, model assumptions were confirmed by visual analysis of residual plots, and variables were transformed as needed to achieve normality.
Results
Study population
The characteristics of the study participants are summarized in Table 1. The 45 individuals for whom hypothalamic blocks containing the entire intermediate nucleus were available did not differ significantly from the 128 individuals for whom such hypothalamic blocks were not available. Of the 12 individuals with a clinical diagnosis of Alzheimer’s disease, all but one were classified as having an intermediate or high likelihood of Alzheimer’s disease by NIA-Reagan criteria (National Institute on Aging and Reagan Institute Working Group of Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease, 1997). One of these individuals was classified as Braak stage VI, six as Braak stage V, four as Braak stage IV, and one as Braak stage III (Braak and Braak, 1991).
Table 1.
Comparison of participants in whom tissue blocks containing the intermediate nucleus were and were not available
| Characteristics | Intermediate nucleus not available | Intermediate nucleus available | P-value |
|---|---|---|---|
| (n = 128) | (n = 45) | ||
| Age at death (years) | 89.5 (85.2–93.6) | 89.4 (85.2–93.5) | 0.73 |
| Male sex | 40 (31%) | 13 (29%) | 0.91 |
| Clinical Alzheimer’s disease | 52 (41%) | 12 (27%) | 0.14 |
| NIA-Reagan pathological classification for Alzheimer’s disease | No: 2 (2%) | No: 0 (0%) | 0.83 |
| Low: 46 (36%) | Low: 16 (36%) | ||
| Intermediate: 61 (48%) | Intermediate: 21 (47%) | ||
| High: 19 (15%) | High: 8 (18%) | ||
| Braak stage | 0: 2 (2%) | 0: 0 | 0.49 |
| I: 8 (6%) | I: 3 (7%) | ||
| II: 13 (10%) | II: 4 (9%) | ||
| III: 39 (30%) | III: 7 (16%) | ||
| IV: 39 (30%) | IV: 19 (42%) | ||
| V: 24 (19%) | V: 11 (24%) | ||
| VI: 3 (2%) | VI: 1 (2%) | ||
| Months between actigraphy and death | 14.2 (7.3–26.0) | 15.5 (7.4–26.1) | 0.68 |
| Post-mortem interval | 6.3 (4.8–9.8) | 6.4 (4.8–9.7) | 0.45 |
| Sleep fragmentation (kRA) | 0.0327 (0.023–0.035) | 0.027 (0.023–0.030) | 0.55 |
| Self-report hours of sleep | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 0.34 |
| Hours of sustained rest per day | 7.2 (6.0–8.3) | 7.1 (5.9–8.2) | 0.32 |
| Proportion of rest time spent in long bouts | 0.48 (0.38–0.55) | 0.49 (0.43–0.55) | 0.38 |
All values expressed as median (IQR) or n (%).
Sleep fragmentation
In simple linear regression models, among all 173 deceased participants with actigraphy, the mean [standard deviation (SD)] kRA was 0.029 (0.007). Male sex was associated with higher sleep fragmentation [estimate +0.008, standard error (SE) 0.002, P = 1.5 × 10−5] and each additional year of age was associated with slightly less sleep fragmentation (estimate −0.00003, SE 0.00001, P = 0.012). There was a trend toward increased sleep fragmentation in individuals with Alzheimer’s disease versus those without (estimate +0.0024, SE 0.0016, P = 0.15).
Immunohistochemistry
Immunohistochemistry for galanin revealed several galaninergic cell groups in the anterior hypothalamus (Fig. 1A) including the intermediate nucleus, paraventricular nucleus, medial preoptic area, supraoptic nucleus and diagonal band of Broca. The intermediate nucleus is clearly appreciated as a discrete galanin-immunoreactive cell group lateral to the medial preoptic area, and dorsomedial to the supraoptic nucleus. Individual galaninergic intermediate nucleus neurons were multipolar and intermediate in size (∼25–30 μm in longest diameter), compared with the much larger supraoptic and paraventricular neurons (∼40–60 μm) and the somewhat smaller medial preoptic neurons (15–20 μm). (Fig. 1B)
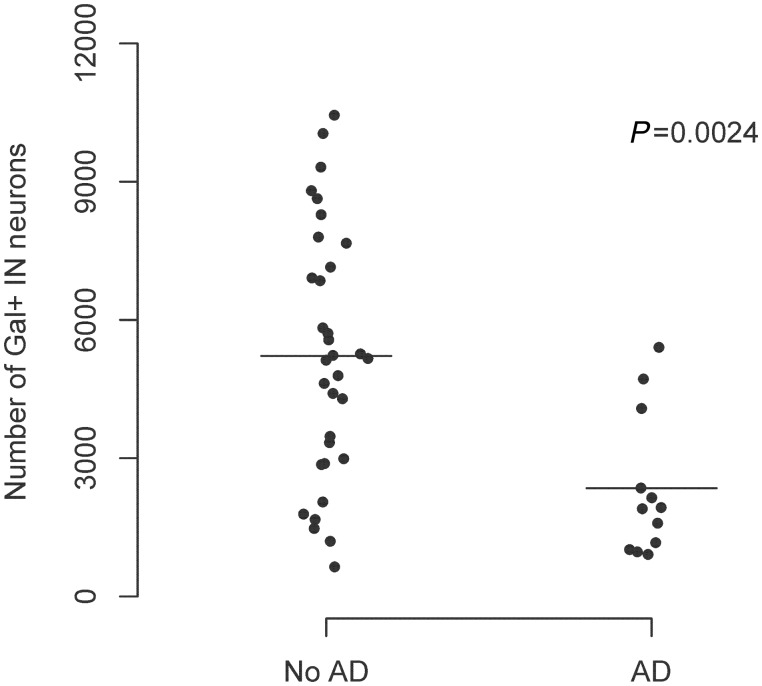
The mean (SD) number of galanin-immunoreactive neurons per intermediate nucleus was 4454 (2750). There was no significant difference in the mean number of galanin-immunoreactive neurons in those individuals in whom the right versus left hemisphere was sampled (estimate +1065, standard error 851, P = 0.22). Sex was not associated with differences in the mean number of galanin-immunoreactive neurons. Individuals with Alzheimer’s disease had substantially fewer galanin-immunoreactive intermediate nucleus neurons than those without (estimate −2872, standard error 829, P = 0.001; Fig. 2). Individuals without Alzheimer’s disease (mean age 86.0) had a mean of 5220 galanin-immunoreactive neurons, whereas individuals with Alzheimer’s disease (mean age 91.0) had a mean of 2348 galanin-immunoreactive neurons. This difference remained significant after adjusting for age and sex (estimate −3128, standard error 928, P = 0.002). In addition, there was a trend toward a linear association between a higher Braak stage and a lower number of galanin-immunoreactive intermediate nucleus neurons (estimate −635, SE 339, P = 0.068). Within the narrow range of ages of death in this study [interquartile range (IQR) 84.5–90.0] there was no significant correlation between age at death and number of galanin-immunoreactive intermediate nucleus neurons.
Figure 2.
The presence of Alzheimer’s disease (AD) is associated with fewer galanin-immunoreactive neurons in the intermediate nucleus (IN) (n = 45).
The number of galanin-immunoreactive neurons in the intermediate nucleus is associated with sleep fragmentation
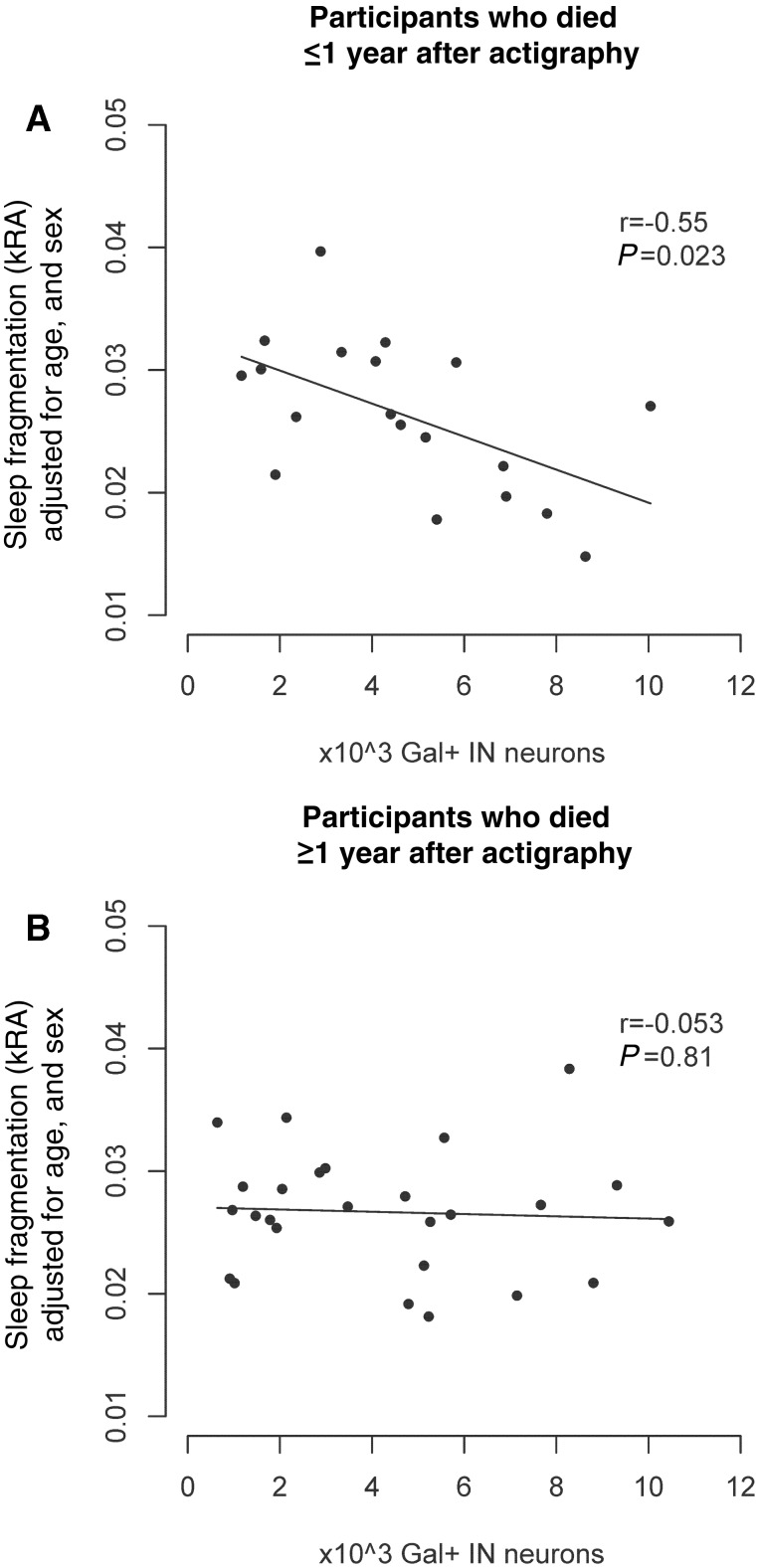
In a linear regression model adjusted for age at death, sex, and time lag between actigraphy and death, and including an interaction term between time lag and number of galanin-immunoreactive neurons, sleep fragmentation was inversely correlated with the number of galanin-immunoreactive neurons, with higher numbers of neurons being associated with less sleep fragmentation (estimate −0.0016 per 1000 neurons, SE 0.0005, P = 0.0005). However, the interaction term was significant (estimate +0.00005, SE 0.00002, P=0.019), indicating that the strength of this association varied according to the time lag between actigraphy and death, with the strongest association seen for individuals in whom actigraphy was performed shortly before death, and a weaker association seen for individuals in whom there was a long time delay between actigraphy and death. Because of this, we stratified our cohort into those in whom actigraphy was performed <1 year before death (n = 19) and those in whom actigraphy was performed >1 year before death (n = 26). In those in whom actigraphy was performed within 1 year of death, there was a strong negative association between thenumber of galaninergic intermediate nucleus neurons and sleepfragmentation (estimate −0.0013 per 1000 neurons, SE 0.0005, P = 0.023; Table 2 Model B and Fig. 3A). However, in those in whom actigraphy was performed >1 year before death, this was substantially attenuated (estimate −0.00009, SE 0.0003, P = 0.81).
Table 2.
The number of galanin-immunoreactive neurons in the intermediate nucleus is associated with sleep fragmentation as assessed by actigraphy
| Effect on kRA [estimate (SE)] |
|||
|---|---|---|---|
|
P-value | |||
| Predictor | Model A | Model B | Model C |
| (all participants; n = 45) | (Lag ≤1 year; n = 19) | (Lag >1 year; n = 26) | |
| Age at death (per year) | +0.0001 (0.0001) P = 0.42 | +0.0003 (0.0002) P = 0.14 | −0.000006 (0.00017) P = 0.97 |
| Male sex | +0.0056 (0.0018) P = 0.004 | +0.008 (0.0028) P = 0.013 | +0.0032 (0.0025) P = 0.21 |
| Lag between actigraphy and death (per month) | −0.00001 (0.00005) P = 0.86 | ||
| Gal+ neurons (per 103 neurons) | −0.0016 (0.0005) P = 0.005 | −0.0013 (0.0005) P = 0.023 | −0.00009 (0.0003) P = 0.81 |
| Gal+ neurons × lag (per 103 neurons per month) | +0.00005 (0.00002) P = 0.019 | ||
Figure 3.
The number of galanin-immunoreactive neurons in the intermediate nucleus is associated with sleep fragmentation as assessed by actigraphy. (A) Association between galanin-immunoreactive neuron counts and sleep fragmentation in subjects who died within 1 year of actigraphy (n = 19). (B) Participants who died over 1 year after actigraphy (n = 26). All panels show partial regression plots of models adjusted for age, sex, and presence/absence of Alzheimer’s disease. r indicates the partial correlation coefficient between the number of galanin-immunoreactive neurons and sleep fragmentation measured using kRA. Briefly, kRA is the probability per 15-s epoch of having an arousal, as indicated by movement (defined as a non-zero activity count) after a long (about ≥5 min) period of rest (i.e. sleep), with a higher kRA indicating greater sleep fragmentation. The association between the number of galanin-immunoreactive neurons and actigraphically measured sleep fragmentation is significantly stronger the shorter the time lag between actigraphy and death (interaction P = 0.019). Gal = galanin; IN = intermediate nucleus.
The association between the number of galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation is similar in individuals with and without Alzheimer’s disease
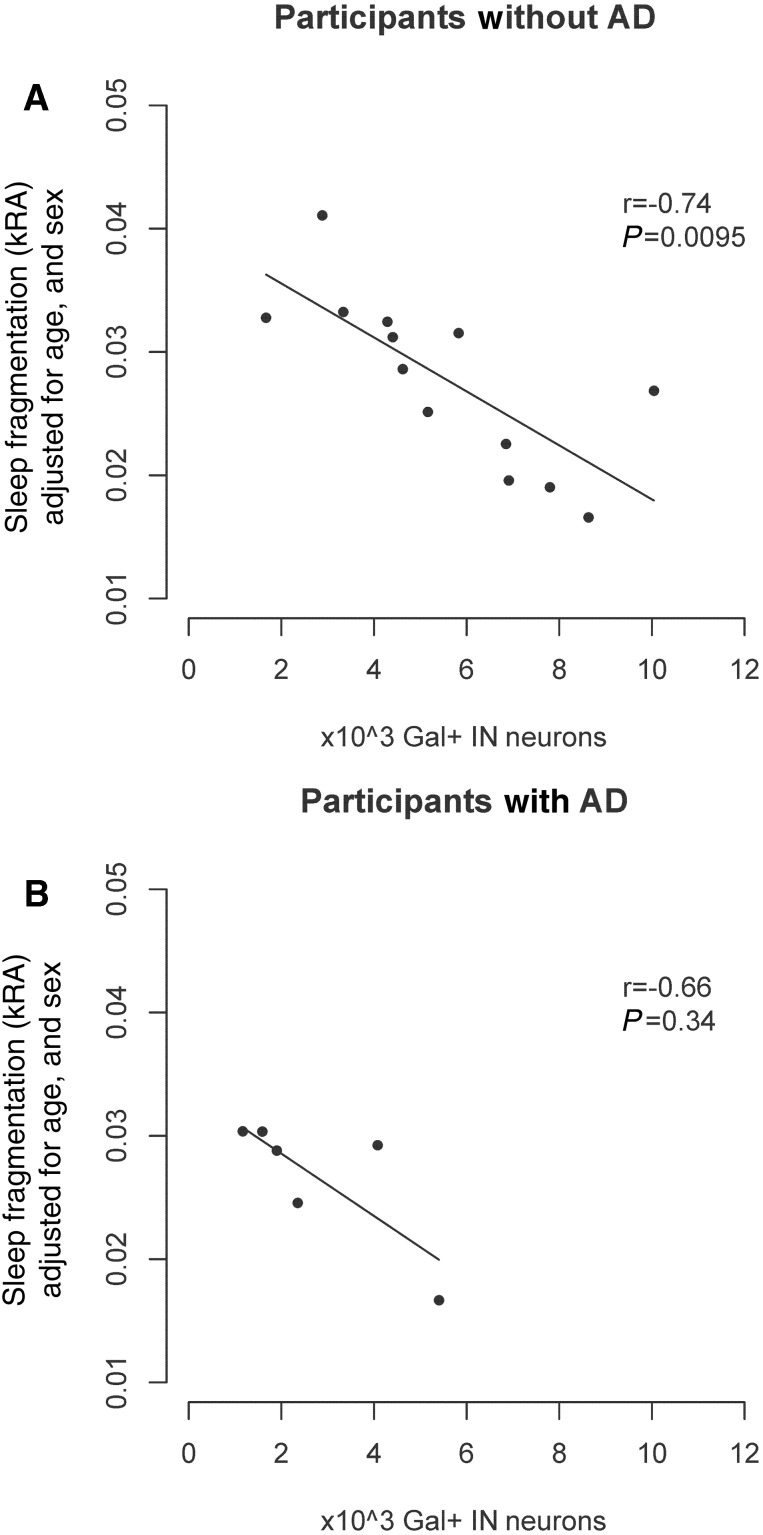
Alzheimer’s disease is associated with a number of sleep abnormalities. We examined whether the association between the number of galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation depended on the presence/absence of Alzheimer’s disease by considering a model with a (galanin-immunoreactive neuron × Alzheimer’s disease) interaction term. This interaction term was not significant (interaction estimate −0.0008, SE 0.0017, P = 0.64), indicating that the association between the number of galanin-immunoreactive neurons and sleep fragmentation does not differ between those with and without Alzheimer’s disease. As seen in Fig. 4, the association is similar in both groups, as is the partial correlation coefficient (r = −0.72 in those without Alzheimer’s disease and r = −0.65 in those with Alzheimer’s disease). A similar result was seen when a pathological rather than clinical classification of Alzheimer’s disease was used (interaction estimate +0.0022, SE 0.0014, P = 0.14 for NIA-Reagan intermediate/high versus low/no).
Figure 4.
The association between number of galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation does not differ by the presence/absence of Alzheimer's disease. (A) Association between galanin-immunoreactive neuron counts and sleep fragmentation considering participants without Alzheimer’s disease who died within 1 year of actigraphy (n = 13). (B) Participants with Alzheimer’s disease who died within 1 year of actigraphy (n = 6). All panels show partial regression plots of models adjusted for age and sex. r indicates the partial correlation coefficient between the number of galanin-immunoreactive neurons and sleep fragmentation measured using kRA. The association between the number of galanin-immunoreactive neurons and sleep fragmentation does not differ in those with and without Alzheimer’s disease (interaction P = 0.64). AD = Alzheimer’s disease; Gal = galanin; IN = intermediate nucleus.
The number of galanin-immunoreactive neurons in the intermediate nucleus is positively correlated with the amount of sustained rest
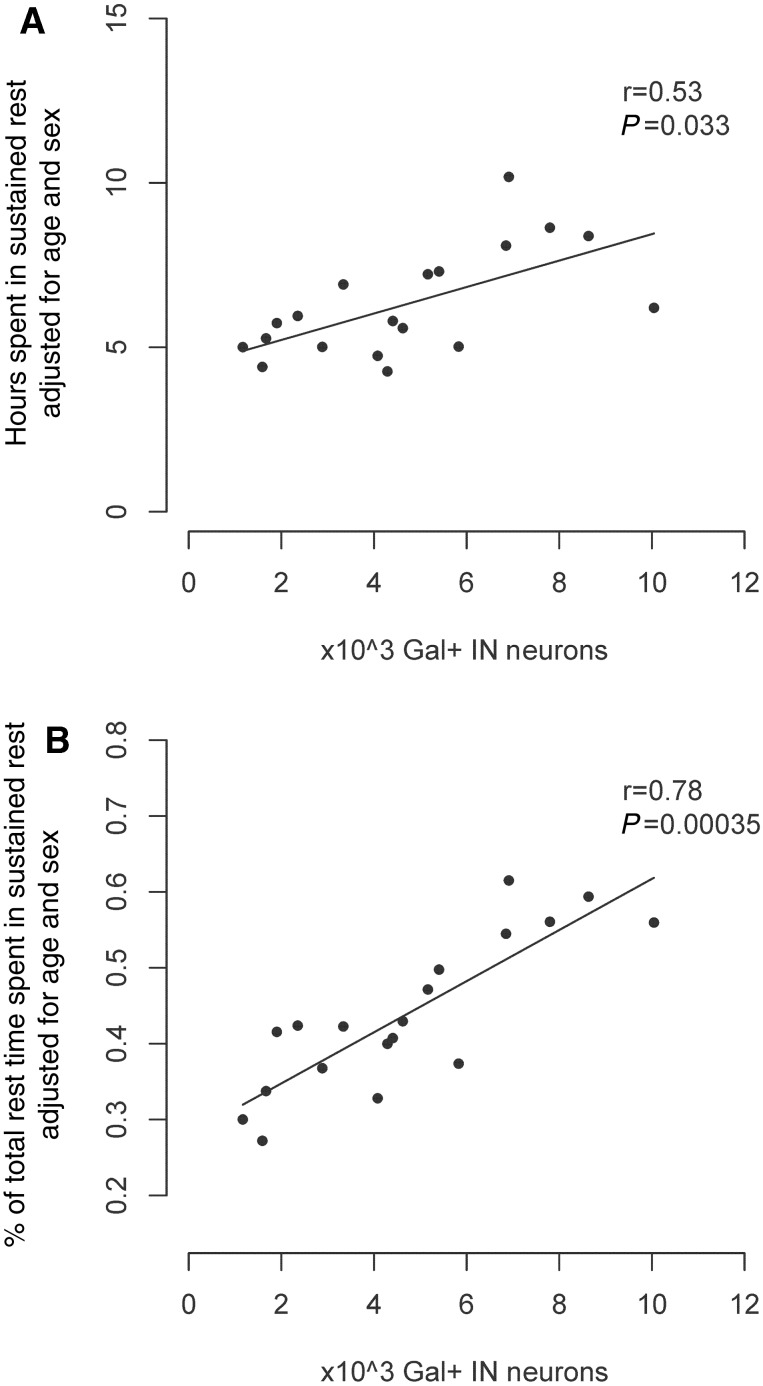
There is an incomplete concordance between rest measured by activity and sleep measured electrophysiologically. While short bouts of rest may represent either quiet wakefulness or sleep, longer bouts are more likely to represent sleep. In those individuals who had actigraphy >1 year before death, we examined the association between the number of galanin-immunoreactive neurons in the intermediate nucleus and the average amount of time per day spent in rest bouts ≥10 min using a linear regression model adjusted for age and sex. Higher numbers of galanin-immunoreactive neurons were associated with a greater number of hours spent in these long rest bouts, and also with these long rest bouts accounting for a greater proportion of total rest time (Fig. 5A and B; Supplementary Fig. 1).
Figure 5.
Secondary outcomes. We considered a number of secondary outcomes in participants with and without Alzheimer’s disease who died within 12 months of their actigraphic recordings. (A) Total hours per day spent in bouts of rest of 10 min duration or longer (n = 19). (B) Proportion of total rest time spent in bouts ≥10 min (n = 19). All panels show partial regression plots of models adjusted for age and sex. r indicates the partial correlation coefficient between the number of galanin-immunoreactive neurons and the indicated outcome. Abbreviations: Gal = galanin; IN = intermediate nucleus.
The number of galanin-immunoreactive intermediate nucleus neurons was not associated with the self-reported habitual number of hours of sleep (P = 0.43), or with the odds of reporting often or very often experiencing difficulty with sleep initiation (P = 0.95) or maintenance (P = 0.35).
The association between the number of galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation is anatomically specific
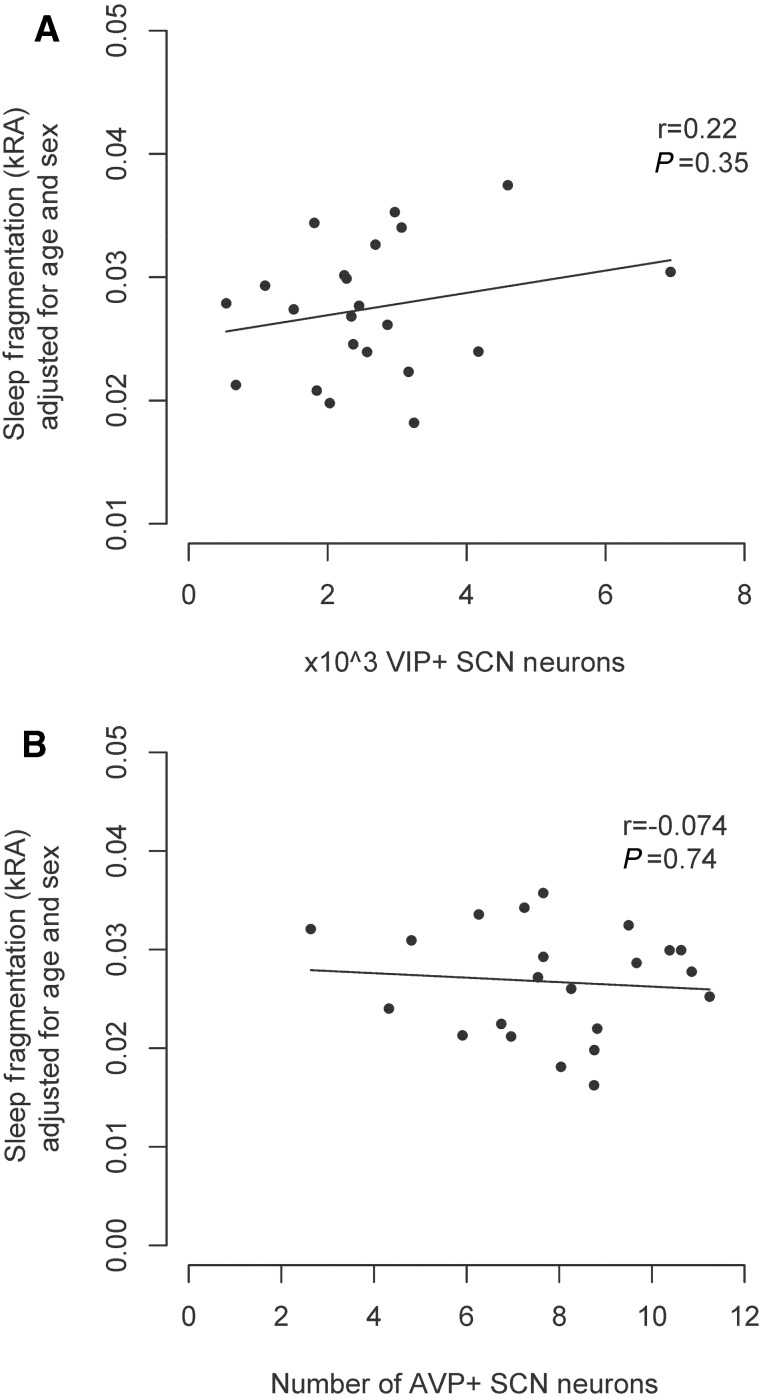
To address the question of whether the association between galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation is specific to the intermediate nucleus or representative of more general regional hypothalamic cell loss, we assessed the relation between cell counts in two nearby hypothalamic cell populations (the AVP and VIP-immunoreactive neurons of the suprachiasmatic nucleus) and sleep fragmentation measured by kRA using linear regression in the subset of 22 study participants for whom blocks containing the entire suprachiasmatic nucleus tissue were available (Supplementary Table 1). These individuals did not differ significantly from those for whom blocks containing the entire suprachiasmatic nucleus were not available (Supplementary Table 1). The mean (SD) number of VIP-immunoreactive neurons in our sample was 2610 (1374). There was no significant association between age and numbers of VIP-immunoreactive neurons (estimate +55.2/year, SE 41.4, P = 0.20). Individuals with Alzheimer’s disease did not have significantly fewer VIP-immunoreactive neurons than those without (estimate −809.4, SE 575.3, P = 0.18). The mean (SD) number of AVP-immunoreactive neurons in our sample was 7998 (2107). In our sample, increased age was associated with decreased numbers of AVP-immunoreactive neurons (estimate −186.0, SE 53.4, P = 0.002). Individuals with Alzheimer’s disease did not have significantly fewer AVP-immunoreactive neurons than those without (estimate −434.0, SE 918.0, P = 0.64). Initial models relating VIP- or AVP-immunoreactive neuron counts to kRA, and incorporating (cell number × lag) interaction terms, did not suggest that the time lag between actigraphy and death was a significant effect modifier for the relation of either cell group with sleep fragmentation, and therefore we did not stratify by time lag. In models adjusted for age, and sex, neither the AVP-immunoreactive nor the VIP-immunoreactive cell populations in the suprachiasmatic nucleus were associated with sleep fragmentation measured by kRA (Fig. 6).
Figure 6.
The numbers of AVP-immunoreactive and VIP-immunoreactive neurons in the suprachiasmatic nucleus are not associated with sleep fragmentation as assessed by actigraphy. (A) VIP-immunoreactive suprachiasmatic nucleus neuron counts (n = 22). (B) AVP-immunoreactive suprachiasmatic nucleus neuron counts (n = 22). All panels show partial regression plots of models adjusted for age, sex, and presence/absence of Alzheimer's disease. r indicates the partial correlation coefficient between the number of neurons and sleep fragmentation measured using kRA). SCN = suprachiasmatic nucleus.
Discussion
In this cross-sectional clinico-pathological correlation study of 45 older individuals with and without Alzheimer's disease, a greater number of galanin-immunoreactive neurons in the intermediate nucleus was associated with less sleep fragmentation measured by actigraphy and more time spent in consolidated periods of rest. This association with sleep fragmentation was strongest for those individuals with the shortest time delay between actigraphy and death, did not differ between individuals with and without Alzheimer’s disease, and was not seen for two other nearby hypothalamic cell populations, suggesting anatomic specificity. In addition to this, individuals with Alzheimer’s disease had approximately half as many intermediate nucleus galanin-immunoreactive neurons as individuals without Alzheimer’s disease.
Methodological considerations
In this study, we used actigraphy to assess sleep fragmentation. Although actigraphy measures rest and activity, which is not the same as the wake-sleep states measured by polysomnography, it is widely used in the ambulatory measurement of sleep because it can be performed inexpensively across multiple days in the home environment, and therefore gives a better measure of daily natural sleep behaviour than in-lab polysomnography. Furthermore, there is good concordance between actigraphic and polysomnographic sleep metrics (Cole et al., 1992), including the metric kRA used in the present study (Lim et al., 2013). Actigraphy also has many advantages over self-report sleep measures, which can be confounded by misperception and poor recall, particularly in individuals with dementia. Further increasing confidence in our findings is the concordant results between the three different actigraphic sleep metrics that we used, indicating that the associations that we observed are independent of the specific actigraphic analysis used. One disadvantage of actigraphy versus polysomnography is the inability to ascertain sleep disorders such as sleep apnoea. However, the expected prevalence of sleep apnoea in community dwelling adults over age 65 has been estimated at only 11% (Ancoli-Israel et al., 1991), which we think is probably too low to have had a major impact on our results.
In this study, we counted the total number of galanin-immunoreactive neurons in the intermediate nucleus. A paucity of galanin-immunoreactive neurons may be due to an actual loss of galanin expressing neurons, or loss of galanin immunoreactivity without loss of neurons. These two possibilities cannot be distinguished on the basis of our data. On the other hand, Swaab and colleagues (Hofman and Swaab, 1989; Garcia-Falgueras et al., 2011) have shown that there is loss of total neurons and of galanin-immunoreactive neurons in the intermediate nucleus with ageing, at least in males, so that the low numbers of galanin-immunoreactive neurons we found in individuals with high sleep fragmentation are likely to represent cell loss, rather than loss of galanin-staining.
In this study, the total number of galanin-immunoreactive intermediate nucleus neurons was determined using the optical fractionator stereological technique. This method has a number of strengths. Because this technique measures total cell number directly, rather than inferring total number from measurements of cell density and region of interest volume, it is not confounded by non-uniformity of cell density across the region of interest. Moreover, it is relatively insensitive to differences in the precise definition of the boundaries of the region of interest, which in our study were determined objectively in each section based on unambiguous anatomical landmarks. Another advantage of this technique is that it is not confounded by differences in cell volume. In this study, only one hemisphere from each participant was available for histological analysis. We are not aware of any work suggesting that the population of galanin-immunoreactive neurons in the intermediate nucleus shows any left versus right lateralization. Moreover, in our data, there was no significant difference in galanin-immunoreactive neuron counts between participants in whom the left versus right hemisphere was analysed.
The intermediate nucleus and suprachiasmatic nucleus in ageing and Alzheimer's disease
In our cohort, we found a mean (SD) of 4454 (2750) galanin-immunoreactive intermediate nucleus neurons per hemisphere. This is similar to previously reported unilateral galanin-immunoreactive intermediate nucleus neuron counts in older females (Garcia-Falgueras et al., 2011). Within our sample, there was no significant association between age and galanin-immunoreactive intermediate nucleus cell counts. Others have previously reported an inverse relationship between age and neuron counts in the intermediate nucleus, beginning around the age of 50 (Hofman and Swaab, 1989). One possible explanation for this discrepancy is the narrow range and very advanced ages in our sample (IQR 84.5–90.0), which would have made detecting significant age effects difficult. Another possible explanation is that our subjects were mostly female, and the age-related decline in intermediate nucleus neurons has previously been reported to be most prominent for males (Garcia-Falgueras et al., 2011).
The intermediate nucleus has also been referred to as the sexually dimorphic nucleus of the hypothalamus because of an observed sex difference in neuron counts, with males on average having more neurons than females (Swaab and Fliers, 1985). In this study, we found no significant difference in galanin-immunoreactive neuron counts in males and females. We think this may be attributable to our cohort being much older than previous cohorts. Indeed, one other study found that the observed sexual dimorphism of galanin-immunoreactive neuron counts in the intermediate nucleus disappears in older adults (Garcia-Falgueras et al., 2011).
In the present study, participants with Alzheimer’s disease had significantly lower numbers of galanin-immunoreactive intermediate nucleus neurons that those without. This is in contrast to previous studies, using thionin staining, which failed to find such a difference in total intermediate nucleus neuron counts in Alzheimer’s disease (Swaab et al., 1993). These findings are not mutually inconsistent. It may be that the association between Alzheimer’s disease and intermediate nucleus neuron counts is specific to the galaninergic subpopulation of the intermediate nucleus, and does not involve non-galaninergic interneurons. If this is the case, then total intermediate nucleus neuron numbers may not vary significantly between participants with and without Alzheimer’s disease, even while there are statistically significant differences in the galanin-immunoreactive subpopulation. Alternatively, the level of galanin expression in some neurons may have fallen below the threshold that can be detected by immunohistochemistry in the tissue from subjects with Alzheimer’s disease.
One interpretation of these results is that Alzheimer’s disease itself is associated with loss of galanin-immunoreactive neurons in the intermediate nucleus, which then leads to sleep fragmentation. This is in keeping with work in rodent models of Alzheimer’s disease where Alzheimer’s disease pathology can precede the onset of sleep abnormalities (Roh et al., 2012). Of interest in this regard is the observation that compared to other nuclei in the chiasmatic region of the anterior hypothalamus, there is a preferential accumulation of amyloid-β and phosphorylated tau pathology in the intermediate nucleus and surrounding chiasmatic grey matter in individuals with Alzheimer’s disease and even in some aged control subjects (van de Nes et al., 1998). Several hypotheses have been advanced to explain the differential regional distribution of Alzheimer pathology including the neuronal activity hypothesis whereby more active cell groups are more apt to accumulate amyloid and tau pathology (Bero et al., 2011; Yamada et al., 2014), and the trans-synaptic spreading hypothesis whereby misfolded proteins spread trans-synaptically from neuron to neuron (Harris et al., 2010). The best evidence from recordings from ventrolateral preoptic nucleus in rodents is that its neurons fire in a range of ∼0–12 Hz, with faster firing during sleep (Szymusiak et al., 1998), which is similar to other preoptic and anterior hypothalamic cell groups, and not likely to put them at excessive metabolic risk. However, in rodents, the ventrolateral preoptic nucleus does project to and receive projections from other hypothalamic areas such as the tuberomamillary nucleus that themselves have a predisposition to the accumulation of Alzheimer pathology (Saper and German, 1987; Sherin et al., 1998; Chou et al., 2002). Further work in rodents and humans is needed to investigate potential molecular and cellular mechanisms influencing the number of galanin-immunoreactive intermediate nucleus neurons in older individuals with and without Alzheimer’s disease.
Another potential interpretation of our data is that a loss or paucity of intermediate nucleus neurons leads to functional consequences (e.g. fragmented sleep), which then predispose to Alzheimer’s disease. This latter interpretation is in keeping with recent work showing that in older individuals without dementia, sleep fragmentation is associated with a higher risk of incident Alzheimer’s disease (Lim et al., 2013), and other work showing that amyloid-β peptide deposition is increased with sleep loss in a rodent model of Alzheimer’s disease (Kang et al., 2009). Potential mechanisms include the preferential clearance of toxic proteins in sleep (and hence decreased clearance in sleep disruption) (Xie et al., 2013), altered synaptic homeostasis in sleep disruption which has been proposed to potentiate trans-synaptic spread of misfolded proteins (Harris et al., 2010; Clark and Warren, 2013), and loss of the decrease in neuronal activity in sleep in many parts of the brain (although notably not the ventrolateral preoptic nucleus) which under the neuronal activity hypothesis may promote the accumulation of amyloid and tau pathology (Bero et al., 2011; Yamada et al., 2014).
An important issue is whether the presumed loss of galanin-immunoreactive neurons with ageing is typical of the anterior hypothalamus. The numbers of vasopressin- and oxytocin-immunoreactive neurons in the paraventricular and supraoptic nuclei and the corticotrophin-releasing hormone-immunoreactive neurons in the paraventricular nucleus have been found not to decrease with ageing (Ishunina and Swaab, 2002). We studied the vasopressin- and VIP-immunoreactive neurons in the suprachiasmatic nucleus in our cohort, because it is at roughly the same level as the intermediate nucleus, and therefore we had intact samples of the entire nucleus in most cases. We found a mean (SD) of 2610 (1374) VIP-immunoreactive suprachiasmatic nucleus neurons per hemisphere, and a mean (SD) of 7998 (2107) AVP-immunoreactive suprachiasmatic nucleus neurons per hemisphere. This is similar to previously reported VIP-immunoreactive and AVP-immunoreactive neuron counts in the suprachiasmatic nucleus (Swaab et al., 1985; Zhou et al., 1995). Others (Zhou et al., 1995) have reported a decrease in VIP-immunoreactive suprachiasmatic nucleus cell counts in middle-aged (41–65 year old) compared to younger (10–40 year old) males, but not females, and with no further substantial decline past middle age. This is in keeping with the lack of association between age and VIP-immunoreactive suprachiasmatic nucleus cell counts in our mostly female sample of much older adults. Others have also reported a decrease in AVP-immunoreactive suprachiasmatic nucleus cell counts in the oldest (81–100 years old) age group compared to younger ages (Swaab et al., 1985). This observation is consistent with our finding of an inverse relationship between age and AVP-immunoreactive suprachiasmatic neurons. In our sample, individuals with Alzheimer’s disease did not have significantly fewer AVP or VIP neurons than those without. This result is consistent with other studies using similar immunohistochemical methods reporting that the number of AVP immunoreactive and VIP immunoreactive cells in the suprachiasmatic nucleus in patients with dementia is within the range expected for age (Swaab et al., 1985; Zhou et al., 1995). However, it differs from studies using immunohistochemical cell density expressed as a neuron:glia ratio (Harper et al., 2008) and counts of cells expressing vasopressin mRNA in in situ autoradiograms (Liu et al., 2000; Wu et al., 2006), which suggest that subjects with Alzheimer’s disease have fewer vasopressinergic neurons in the suprachiasmatic nucleus than control subjects, and studies using thionin staining, suggesting that subjects with Alzheimer’s disease have fewer total thionin-stained suprachiasmatic neurons than control subjects (Swaab et al., 1985). It is likely that these discrepancies reflect differences in methods used to identify and quantify these neuronal populations (mRNA versus immunohistochemistry versus thionin staining; total number versus neuron:glia ratio), as well as technical difficulties counting neurons if cells express low levels of target mRNA or protein.
The intermediate nucleus and sleep fragmentation in ageing and Alzheimer’s disease
We observed an association between the number of galanin-immunoreactive neurons in the intermediate nucleus and sleep fragmentation measured by actigraphy prior to death, which was strongest for those participants with the shortest time lag between actigraphy and death. The effect of the time lag on the strength of this association is not surprising. The longer the time lag, the less likely that the sleep recorded during the actigraphic recording would be representative of sleep proximate to death when the number of galanin-immunoreactive intermediate nucleus neurons was quantified. The number of galanin-immunoreactive neurons was not associated with self-reported habitual sleep time or the self-reported frequency of difficulties with sleep initiation or maintenance. The association with objective but not subjective sleep measures is not surprising given the incomplete correlation between objective and subjective sleep measures in other studies (Lauderdale et al., 2008), and highlights the importance of objectively quantifying human sleep in clinical studies, rather than relying on self-report.
One possible interpretation of these results is that galanin-immunoreactive neurons in the intermediate nucleus play a functionally important role in maintaining sleep continuity, and that lower numbers of galanin-immunoreactive intermediate nucleus neurons actually cause sleep fragmentation. Alternatively, galanin-immunoreactive intermediate nucleus neurons could be selectively vulnerable to sleep disruption, and loss of galanin-immunoreactive intermediate nucleus neurons would then be a marker of sleep fragmentation rather than a cause of it.
In favour of the first possibility, the intermediate nucleus has several similarities to the rodent ventrolateral preoptic nucleus, including its location in the chiasmatic region of the anterior hypothalamus, and its prominent expression of galanin. In rodents, the galaninergic ventrolateral preoptic nucleus plays a critical role in maintaining sleep continuity (Sherin et al., 1996; Lu et al., 2000). Neurons of the ventrolateral preoptic nucleus are selectively sleep-active and send inhibitory projections to wake-promoting areas (Sherin et al., 1996, 1998), and lesions of the ventrolateral preoptic nucleus result in prominent difficulties in maintaining sustained sleep (Lu et al., 2000). The galaninergic population of the rodent ventrolateral preoptic nucleus is thought to play a particularly important role in sleep regulation. Over 80% of all sleep-active neurons in the rat ventrolateral preoptic nucleus express galanin mRNA (Gaus et al., 2002), and the majority of ventrolateral preoptic nucleus neurons projecting to the tuberomamillary nucleus, an important wake-promoting centre, are galaninergic (Sherin et al., 1998). Our observations suggest that the galanin-immunoreactive neurons in the human intermediate nucleus also play an important role in sleep promotion and consolidation, which would be consistent with the intermediate nucleus being the human homologue of the ventrolateral preoptic nucleus. We know of no evidence that sleep loss or fragmentation can damage neurons in the intermediate nucleus or ventrolateral preoptic nucleus, but this would be of interest in future studies.
Aside from galanin, other antigens reported to be expressed by at least some intermediate nucleus neurons include acetylcholinesterase (Koutcherov et al., 2007), CD15 (Koutcherov et al., 2007), thyroid releasing hormone (Fliers et al., 1994), oestrogen receptor alpha (Kruijver et al., 2003; Garcia-Falgueras et al., 2011), oestrogen receptor beta (Kruijver et al., 2003; Garcia-Falgueras et al., 2011), aromatase (Fernandez-Guasti et al., 2000; Garcia-Falgueras et al., 2011), substance P (Chawla et al., 1997), neurokinin B (Chawla et al., 1997), and glutamic acid decarboxylase (Gao and Moore, 1996). For the most part, the extent to which these cell populations overlap with the galanin-immunoreactive neurons examined in the present study is not known, although studies in rats suggest that almost all galaninergic ventrolateral preoptic nucleus neurons also express glutamic acid decarboxylase. We note that the data in this study do not necessarily imply that the effects seen are specific to the galanin-immunoreactive subpopulation of the intermediate nucleus nor do they preclude the possibility that non-galanin-immunoreactive intermediate nucleus neurons may play a role in human sleep regulation. Additional studies in rodents and humans are needed to determine the relative roles of galaninergic and non-galaninergic intermediate nucleus/ventrolateral preoptic nucleus neurons in sleep regulation. However, these may be complicated by the fact that aside from galanin, most of these antigens do not reliably distinguish the intermediate nucleus from neighbouring cell groups such as the medial preoptic area.
Our interpretation that the intermediate nucleus is the human homologue of the ventrolateral preoptic nucleus is not inconsistent with the existing literature reporting sexual dimorphism in the intermediate nucleus (Swaab and Fliers, 1985), and proposing an impact of sex hormones on this dimorphism (Garcia-Falgueras et al., 2011). Moreover, it does not preclude additional involvement of intermediate nucleus neurons in other functions.
Arguing against the possibility that the association between intermediate nucleus galanin-immunoreactive neuron numbers and sleep fragmentation reflects an effect of more generalized neuron loss in the anterior hypothalamus, the association between galanin-immunoreactive intermediate nucleus neuron counts and sleep fragmentation was not seen for two nearby cell populations—the AVP-immunoreactive and VIP-immunoreactive neurons of the suprachiasmatic nucleus. In addition, others have reported no significant loss of AVP- or oxytocin- immunoreactive neurons in either the paraventricular nucleus or supraoptic nucleus or corticotrophin-releasing hormone-immunoreactive neurons in the paraventricular nucleus with ageing (Van der Woude et al., 1995; Ishunina and Swaab, 2002), arguing against a general age-related neuron loss in the anterior hypothalamus.
There exists a body of evidence that suggests that dysfunction of the suprachiasmatic nucleus may be an important contributor to sleep and circadian dysfunction in some clinical settings such as advanced Alzheimer’s disease (Harper et al., 2008). Our findings are not inconsistent with this. Human and animal studies that have related suprachiasmatic nucleus pathology to sleep and circadian measures have mostly found associations with circadian measures, or measures of activity patterns on time scales of hours to days (Hu et al., 2007; Harper et al., 2008). We examined sleep fragmentation on time scales of seconds to minutes and found no association with suprachiasmatic nucleus neuron counts. This is consistent with the hypothesized role for the suprachiasmatic nucleus in influencing sleep and activity patterns on time scales of hours to days, but not shorter time scales (Hu et al., 2007, 2009).
Conclusions and future directions
Taken as a whole, these data suggest that neurons of the intermediate nucleus may play an important role in maintaining sleep continuity in humans, similar to the ventrolateral preoptic nucleus in rodents. Moreover, they raise the possibility that a paucity of intermediate nucleus neurons, whether due to ageing, neurodegeneration, or interindividual genetic factors, may be a potential contributor to fragmented sleep in older individuals with and without Alzheimer’s disease. Further work in animal models of Alzheimer’s disease may help clarify the temporal sequence and causal direction of cell loss in the intermediate nucleus/ventrolateral preoptic nucleus and the development of Alzheimer’s disease pathology. Further work in humans may identify pathology in additional sleep regulatory regions that may account for other aspects of sleep dysfunction in ageing and Alzheimer’s disease. Meanwhile, the results of this study suggest that there may be value in investigating pharmacological agents targeting the galanin neurotransmitter system, or anatomically-specific neuromodulatory therapies [e.g. optogenetic approaches (Adamantidis et al., 2007), deep brain stimulation (Lim et al., 2009), or use of designer receptors exclusively activated by designer drugs to modulate neurons (Lee et al., 2013)] targeted to the intermediate nucleus as potentially useful approaches to alleviating sleep fragmentation in older adults with and without Alzheimer’s disease.
Funding
This work was supported by a Dana Foundation Clinical Neuroscience Grant, National Institutes of Health grants P01AG009975, P01HL095491, R01NS072337, R01AG017917, R01AG024480, R01NS078009, R01AG043379, and R01AG042210, Canadian Institutes of Health Research grants MOP125934 and MMC132692, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to acknowledge Dr Veronique Vanderhorst, Dr Glenn Rosen, Mr Tuan Ho, the staff of the Rush Alzheimer Disease Centre, and most of all the participants of the Rush Memory and Aging Project and their families, without whom this study would not have been possible.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SR, Seiler WO, Stahelin HB, Spiegel R. Seventy-two hour polygraphic and behavioral recordings of wakefulness and sleep in a hospital geriatric unit: comparison between demented and nondemented patients. Sleep. 1987;10:143–59. doi: 10.1093/sleep/10.2.143. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–63. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006a;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006b;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;16:750–6. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brockhaus H. Beitrag zur normalen Anatomie des Hypothalamus und der Zona incerta beim Menschen. J Psychol Neurol. 1942;51:96–196. [Google Scholar]
- Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384:429–42. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CN, Warren JD. A hypnic hypothesis of Alzheimer's disease. Neurodegener Dis. 2013;12:165–76. doi: 10.1159/000350060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Dai J, Swaab DF, Buijs RM. Distribution of vasopressin and vasoactive intestinal polypeptide (VIP) fibers in the human hypothalamus with special emphasis on suprachiasmatic nucleus efferent projections. J Comp Neurol. 1997;1997:397–414. doi: 10.1002/(sici)1096-9861(19970714)383:4<397::aid-cne1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–35. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fliers E, Noppen NW, Wiersinga WM, Visser TJ, Swaab DF. Distribution of thyrotropin-releasing hormone (TRH)-containing cells and fibers in the human hypothalamus. J Comp Neurol. 1994;350:311–23. doi: 10.1002/cne.903500213. [DOI] [PubMed] [Google Scholar]
- Gai WP, Geffen LB, Blessing WW. Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol. 1990;298:265–80. doi: 10.1002/cne.902980302. [DOI] [PubMed] [Google Scholar]
- Gao B, Moore RY. The sexually dimorphic nucleus of the hypothalamus contains GABA neurons in rat and man. Brain Res. 1996;742:163–71. doi: 10.1016/s0006-8993(96)01005-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Ligtenberg L, Kruijver FP, Swaab DF. Galanin neurons in the intermediate nucleus (InM) of the human hypothalamus in relation to sex, age, and gender identity. J Comp Neurol. 2011;519:3061–84. doi: 10.1002/cne.22666. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in sterology and its prediction. J Microsc. 1987;147(Pt 3):229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, et al. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131(Pt 6):1609–17. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, et al. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;2010:428–41. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo JL, Gras CB, Garcia YD, Lapeira JT, del Campo del Campo JM, Verdejo MA. Functional status in the elderly with insomnia. Qual Life Res. 2007;16:279–86. doi: 10.1007/s11136-006-9125-9. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat. 1989;164:55–72. [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Ivanov P, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–17. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer's disease: involvement of the circadian pacemaker. Proc Natl Acad Sci USA. 2009;106:2490–4. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Neurohypophyseal peptides in aging and Alzheimer's disease. Ageing Res Rev. 2002;1:537–58. doi: 10.1016/s1568-1637(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutcherov Y, Paxinos G, Mai JK. Organization of the human medial preoptic nucleus. J Comp Neurol. 2007;503:392–406. doi: 10.1002/cne.21355. [DOI] [PubMed] [Google Scholar]
- Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol. 2003;466:251–77. doi: 10.1002/cne.10899. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Giguere PM, Roth BL. DREADDs: novel tools for drug discovery and development. Drug Discov Today. 2013;19:469–73. doi: 10.1016/j.drudis.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36:1027–32. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, et al. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol. 2009;66:110–4. doi: 10.1002/ana.21631. [DOI] [PubMed] [Google Scholar]
- Lim AS, Yu L, Costa MD, Buchman AS, Bennett DA, Leurgans SE, et al. Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34:1569–81. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Costa MD, Leurgans SE, Buchman AS, Bennett DA, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–40. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, Hoogendijk WJ, van Heerikhuize J, Kamphorst W, Unmehopa UA, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–22. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging and Reagan Institute Working Group of Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(Suppl 4):S1–S2. [PubMed] [Google Scholar]
- Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–10. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienne, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra22. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem. 2009;57:1–5. doi: 10.1369/jhc.2008.952770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–8. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Saper CB, German DC. Hypothalamic pathology in Alzheimer's disease. Neurosci Lett. 1987;74:364–70. doi: 10.1016/0304-3940(87)90325-9. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Stone KL, Ancoli-Israel S, Blackwell T, Ensrud KE, Cauley JA, Redline S, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–75. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228:1112–5. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA, Lucassen PJ, Purba JS, Raadsheer FC, Van de Nes JA. Functional neuroanatomy and neuropathology of the human hypothalamus. Anat Embryol (Berl) 1993;187:317–30. doi: 10.1007/BF00185889. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- van de Nes JA, Kamphorst W, Ravid R, Swaab DF. Comparison of beta-protein/A4 deposits and Alz-50-stained cytoskeletal changes in the hypothalamus and adjoining areas of Alzheimer’s disease patients: amorphic plaques and cytoskeletal changes occur independently. Acta Neuropathol. 1998;96:129–38. doi: 10.1007/s004010050872. [DOI] [PubMed] [Google Scholar]
- Van der Woude PF, Goudsmit E, Wierda M, Purba JS, Hofman MA, Bogte H, et al. No vasopressin cell loss in the human hypothalamus in aging and Alzheimer's disease. Neurobiol Aging. 1995;16:11–8. doi: 10.1016/0197-4580(95)80003-a. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: isues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–7. [PubMed] [Google Scholar]
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer's disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–6. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–93. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–6. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.