Abstract
This study examined interrelations between prenatal cocaine exposure, child autonomic regulation, parenting behavior and child sex on parent-reported behavior problems at 36 months of age. We hypothesized that respiratory sinus arrhythmia (RSA)1 at 13 months of age would mediate the relation between cocaine exposure and behavior problems. We also hypothesized that child sex, maternal negative affect, and maternal sensitivity observed at 13 months of age would moderate the relation between RSA and behavior problems. Results revealed that cocaine exposure predicted low baseline RSA and low RSA withdrawal during a negative affect task. Low baseline RSA, in turn, predicted fewer behavior problems offering support for an indirect association between cocaine exposure and behavior problems. The association between baseline RSA and behavior problems was further moderated by maternal negative affect such that high baseline RSA was more strongly related to behavior problems under conditions of high compared to low maternal negative affect. Results also revealed a near significant trend for baseline RSA to be more strongly related to behavior problems among boys than girls. These findings highlight several possible pathways toward behavior problems among cocaine exposed children.
Keywords: Prenatal Cocaine Exposure, Respiratory Sinus Arrhythmia, Parenting Behavior, Child Behavior Problems
Existing research on the consequences of prenatal cocaine exposure (PCE) among young children demonstrates subtle but clear effects on fetal growth and child neurobehavioral development. Evidence regarding the impact on behavioral outcomes, however, is considerably less decisive with several studies reporting heightened behavior problems (Bada et al., 2007; Delany-Black et al., 2000; Richardson et al., 2009) and several additional studies reporting no direct association with behavior problems (Accornero et al., 2006; Linares et al., 2006; Warner et al., 2006a). These inconsistent results suggest a need to identify intervening variables that help to explain potential indirect associations between maternal cocaine use and child outcomes, and also moderators that may clarify why some prenatally exposed children go on to develop behavior problems while others do not. Several researchers have hypothesized that disturbances in physiological regulation may play an important role in mediating the relation between prenatal exposure and behavioral outcomes (Chaplin et al., 2009; Dennis et al., 2006). Cocaine inhibits reuptake of monoamines at the presynaptic junction, leading to higher concentrations of norepinephrine, serotonin, and dopamine in the synaptic cleft and higher levels of activation in the catecholaminergic system (Gawin & Ellinwood, 1988; Nassogne, Evrard, & Courtoy, 1998). The regions of the brain that are rich in monoamines are involved in the regulation of arousal and attention (Robins, 1997).
One particular index of physiological regulation that is widely discussed in the developmental literature is respiratory sinus arrhythmia (RSA). RSA is a measure of variability in heart rate that occurs at the frequency of respiration and is believed to provide an index of parasympathetic influence on cardiac output. This index is most commonly assessed at baseline (BRSA) as well as during environmental demands (RSA regulation; Bornstein & Suess, 2000; Calkins, 1997). BRSA provides a measure of the infant’s ability to maintain homeostasis during periods of minimal external stimulation. During challenges to homeostasis, as a result of exogenous stimulation, the myelinated vagal system optimally responds by inhibiting the effect of the parasympathetic nervous system on cardiac output (Porges et al., 1996) and decreasing RSA (withdrawal). RSA withdrawal during periods of environmental challenges is believed to reflect one’s ability to appropriately engage or disengage with the environment (Bornstein & Suess, 2000; Porges, et al., 1996). Thus, the measurement of change in RSA from baseline to challenging situation is an important index of parasympathetic regulation.
The majority of studies examining parasympathetic regulation as a function of PCE have focused on neonates and findings have been somewhat mixed. Some studies suggest increased parasympathetic activity during rest among CE neonates (Regalado et al., 2001; Silvestri, et al., 1991) while other studies have not found any effects of PCE on baseline RSA (Mehta et al., 1993), perhaps due to lower sample sizes. To this point, few studies have examined RSA data among PCE children beyond early infancy. The only exception involves the current sample in which PCE children, compared to non-exposed children, demonstrated lower BRSA and lower RSA withdrawal during a negative affect inducing task at both 7 and 13 months of age (XXX, 2007; XXX, 2009). Consequently, the first goal of the present study is to examine RSA as a mediator of the relation between PCE and the development of behavior problems.
Similar to research regarding PCE and behavior problems, findings linking BRSA to child behavior also remain somewhat inconclusive. Although individual differences in BRSA are relatively stable after 3 months of age, relations between BRSA and child behavior are not (Porges et al., 1994). High BRSA has been linked to behavioral reactivity and negative emotionality during infancy (Porges et al., 1994; Stifter and Fox 1990), and to positive indices of regulation and social competence, as well as fewer internalizing and externalizing problems during preschool and early childhood in low risk samples (Cole et al., 1996; El Sheikh et al., 2001; Fabes et al., 1994; Rubin et al., 1997).
Beauchaine (2001) attempts to explain this change by suggesting that high BRSA is associated with physiological reactivity and reflects a general capacity to actively engage with the surrounding environment. This capacity is likely to manifest differently during different developmental periods. Infants, for example, are most capable of influencing their environment by displaying frustration while older children are capable of employing a diverse range of positive emotions and social skills when engaged with their surroundings. He notes, however, that the majority of such research has been conducted with low risk samples and suggests that this developmental trajectory may vary from that of high BRSA children exposed to less favorable surroundings.
This suggestion is consistent with a model proposed by Fox and Calkins (1993; Calkins 1994) who hypothesize that highly reactive infants are particularly susceptible to the impact of parenting problems more common to high risk samples including negative affect and intrusive control. Such parental responses, they argue, can lead to a pattern of coercive interactions that heighten rather than regulate infant arousal and prevent the development of adaptive regulatory capacities expected among highly reactive infants who encounter more supportive parenting. These children would also be likely to adopt a similar style of coercive and antagonistic behavior with peers and other authority figures and thus be at risk for the development of behavior problems. Calkins (1994) notes that these infants may also be particularly susceptible to other parenting problems and suggests that parents who ignore or fail to respond sensitively to infant frustration may obstruct their children’s otherwise naturally developing ability to regulate negative affect.
This approach is amenable to the biological sensitivity to context theory (BSCT) (Ellis & Boyce, 2008) which proposes that individuals who are most responsive to environmental influences will adapt more successfully to favorable rearing environments and less successfully to adverse rearing environments than their less susceptible peers. In support of this view, a recent study of children reared in poverty reported that infants with high BRSA at five months of age revealed the lowest levels of 17 month behavior problems when raised in an environment leading to secure attachment and the highest levels of behavior problems among children raised in an environment fostering disorganized attachment. No significant differences in behavior problems were reported among children with low BRSA (Conradt, Measelle, & Ablow, 2013).
Conversely, a small body of research indicates that low rather than high BRSA children are most likely to develop behavior problems in the context of environmental adversity. Katz and Gottman (1995) report that marital hostility is associated with subsequent externalizing behavior only among low BRSA children while El-Sheikh and Whitson (2006) report the same findings with regard to both internalizing and externalizing behaviors. Similarly, Hastings et al. (2008) reported an interaction between low BRSA and maternal protective overcontrol on social wariness. However, all of these studies assessed RSA during preschool or early childhood and the work of Conradt and colleagues provides the only test of differential susceptibility in infancy. Thus, our second goal was to examine the role of parental negative affect and sensitivity in moderating the relation between infant BRSA and the development of behavior problems.
In contrast to BRSA, RSA withdrawal is more clearly related to physiological regulation than reactivity and has been linked to a variety of positive indices of behavioral and emotional regulation during preschool and early childhood including lower internalizing and externalizing problems (Calkins and Dedmon, 2000; El-Sheikh and Whitson, 2006; Porges et al., 1996). Limited RSA withdrawal is also predictive of behavior problems in the context of parental problem drinking and marital conflict (El-Sheikh, 2001; Leary & Katz, 2004). Existing research, however, has yet to examine the role of parenting problems in moderating the relation between RSA withdrawal and behavior problems. Consequently, a third goal of the current study was to examine the role of maternal negative affect and low sensitivity in moderating the impact of RSA withdrawal on the development of behavior problems.
A related question concerns the role of sex in moderating the relation between PCE and behavior problems. Accumulating evidence indicates that PCE boys are particularly vulnerable to a variety of negative outcomes including behavior problems (Bailey et al., 2005: Delaney-Black et al., 2004). Results from the present sample suggest that sex differences in RSA functioning may play an important role in accounting for these outcomes among boys, as PCE boys were found to have significantly lower RSA withdrawal at 13 months of age than girls. No such sex differences were discovered, however, with regard to BRSA. Further research in both community and high risk samples also indicates that low BRSA (Beauchaine et al., 2008; Calkins and Dedmon, 2000; Eisenberg et al., 2012) and low RSA withdrawal (Graziano, et al., 2007; Hastings, et al., 2008), are more strongly associated with behavior problems among boys. This finding is consistent with additional evidence indicating that behavior problems have a stronger genetic basis among boys (see Beauchaine, et al., 2008). A final goal of our study was to assess the role of child sex in moderating the relation between RSA and behavior problems.
The current study was designed to test a conceptual model regarding the effects of RSA, and parenting, and child sex on behavior problems within a high-risk sample involving PCE children and a carefully matched comparison group (see Figure 1). We hypothesized that low BRSA and low RSA withdrawal at 13 months would mediate or act as intervening variables in the relation between PCE and parent-reported behavior problems at three years of age. We also hypothesized that maternal negative affect, maternal sensitivity observed at 13 months of age, and child sex would moderate the relation between both RSA variables and child behavior problems. Specifically, we proposed that low BRSA and low RSA withdrawal would be more strongly related to behavior problems among boys. Consistent with the developmental diathesis- stress model (Sameroff, 1975) we also proposed that the combination of low RSA withdrawal and parenting problems would be predictive of negative child outcomes. Due to inconsistencies in the literature regarding the relation between BRSA and parenting, however, we did not hypothesize a particular direction for the effect of parenting on BRSA.
Figure 1.
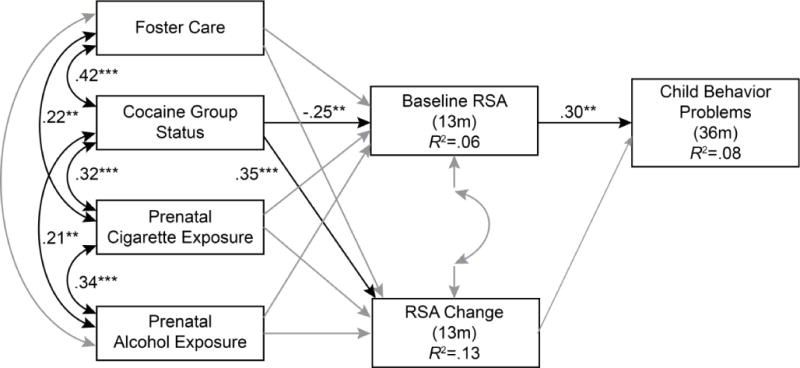
A path model predicting child behavior problems. Non-significant causal paths and covariances depicted in grey.
Method
Participants
The sample consisted of 216 mother-infant dyads participating in an ongoing longitudinal study of prenatal cocaine exposure (116 PCE, 100 not exposed). An outreach worker on the project staff recruited all participants after delivery from two local area hospitals. Mothers ranged in age from 18 to 42 years (M=29.78; SD=5.46). The majority of mothers were African American (74%), were receiving Temporary Assistance for Needy Families (71%) at the time of their first laboratory visit (Years 2001–2004), and were single (60%). Of the 220 children, 108 (49%) were male. All families were recruited from two hospitals serving a predominantly low-income population and the two groups were matched on maternal education, maternal race/ethnicity, and infant sex. The study received approval from the institutional review boards of the hospitals as well as the primary institution at which the study was conducted. Informed written consent was obtained from all recruited participants. Participants were compensated for their time in the form of gift certificates, checks, and infant toys at each assessment, with the amount increasing over time. All assessments were conducted at age corrected for prematurity.
Procedure
All mothers were screened after delivery for initial eligibility and matching criteria. Interested and eligible mothers were given detailed information about the study and asked to sign consent forms. About 2 weeks after delivery, mothers were contacted and scheduled for their first laboratory visit, which took place at the time that their infant was approximately 4–8 weeks old. Additional visits were scheduled when the infant was 7, 13, 18, and 24 months old. All visits (with the exception of the 18 month visit consisting of maternal interview only) consisted of a combination of maternal interviews, observations of mother-infant interactions, and infant assessments. In the circumstance of a change in custody arrangements, the person who had legal guardianship of the child was contacted and asked to participate. Biological mothers were interviewed at the 4–8 week assessment in order to obtain accurate information about prenatal substance use.
Once a family was recruited into the cocaine group, the closest matching non-cocaine group family was recruited. However, a significantly higher proportion of mothers in the noncocaine group declined participation or withdrew before formal enrollment, resulting in a smaller number of families in the control group. Of the 4,800 women screened at delivery, 340 were eligible for participation in either group. Of these 340 women, 35% either declined participation or were not enrolled in the study because they expressed initial interest but later withdrew, resulting in a final sample of 216 mother-infant dyads. Mothers who participated were more likely to be between 18 and 25 years of age, (t (1, 338) = 15.95, p < .001, partial eta square = .05), and were more likely to have a high school or below high school education (t (1, 338) = 55.92, p < .001, partial eta square = .19), compared to those who were eligible but not enrolled. Mothers who participated were also more likely to be in the cocaine group (with a participation rate of 91% among cocaine group eligibles) compared to those who were eligible but not enrolled. The majority of mothers in the cocaine group who were eligible but not enrolled in the study had children who were placed in non-maternal care. There were no other differences on any demographic variables between those who participated and those who were eligible but not enrolled or between mothers in the cocaine group who participated compared to those who did not.
Assessment of growth and risk status
Three measures of growth were used in this study: birth weight (gm), birth length (cm), and head circumference (cm). All measurements were taken by obstetrical nurses in the delivery room and recorded in the infant’s medical chart. Research staff recorded this information from the charts after recruiting the mother-infant dyad. Medical chart review at the time of recruitment also was used to complete the Obstetrical Complications Scale (Littman & Parmelee, 1978), a scale designed to assess the number of perinatal risk factors experienced by the infant. Higher numbers on this scale indicate a more optimal obstetric score. Gestational age was calculated by dates and extracted from medical records.
Identification of Substance Use
Cocaine status was determined by a combination of maternal report, chart review, and maternal hair analysis. Urine toxicologies were routinely conducted at the first prenatal visit on maternal urine and/or at delivery (for those mothers who tested positive prenatally, obtained prenatal care elsewhere, or did not receive any prenatal care) on infant and maternal urine by participating hospitals. Mothers were included in the cocaine group if self-reports were positive, regardless of urine toxicology or hair-sample results. Similarly, mothers who reported that they did not use cocaine but had positive urine toxicology or hair samples were included in the cocaine group.
Urine toxicologies consisted of standard urine screening for drug level or metabolites of cocaine, opiates, benzodiazepines, and tetrahydrocannabinol. Urine was rated positive if the quantity of drug or metabolite was >300 g/ml. Hair samples were collected from the mothers at the first laboratory visit and sent to the Psychemedics Corporation for Radioimmunoanalyses (RIAH). Hair samples were screened for cocaine followed by a gas chromatography/mass spectrometry (GC/MS) confirmation for positive cocaine screens. Drugs and their metabolites are absorbed into the hair and can be extracted and measured. As hair grows at an average rate of 1/2 inch per month, it can record a pattern of drug consumption related to the amount and frequency of use (see Baumgartner et al., 1989). Thus, a 2-inch length of hair could contain a record of approximately 4 months of use, and given adequate hair length (i.e., about 4–5 inches), use per trimester may be recorded. Drugs become detectable in hair about 3 to 4 days after use, a time when cocaine is rendered undetectable by urinalysis. RIAH is the most well-established hair-analysis technique and has been replicated by independent laboratories across the world (see Magura et al., 1992). GC/MS confirmations of RIAH have not revealed any false positives because of testing errors (Magura et al., 1992).
Approximately 32% of mothers in the study (55% of the mothers in the cocaine group) had positive urine toxicologies at delivery, and 25% of mothers (79% of the mothers in the cocaine group) had hair samples that tested positive for cocaine during pregnancy. There were 23 mothers in the cocaine group who did not have a positive toxicology result on any biomarker of cocaine, but all of these mothers admitted to having used cocaine in the brief self-report screening instrument administered after delivery. Mothers in the comparison group reported not having used any illicit substances other than marijuana. They also tested negative for cocaine or illicit substances other than marijuana based on urine and hair analysis results. Additional exclusionary criteria for all mothers were (a) maternal age younger than 18 years, (b) use of illicit substances other than cocaine or marijuana, and (c) significant medical problems for the infant (e.g., genetic disorders, major perinatal complications, baby in critical care for over 48 hours).
The Timeline Follow-Back Interview (TLFB; Sobell et al., 1986) was used to assess maternal substance use during pregnancy. Participants were provided a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method is established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brown et al., 1998). The TLFB yielded data about the average number of days of cocaine use per week, average number of joints smoked per week, average number of cigarettes smoked per week, and average number of standard drinks per week during pregnancy. These variables were quite skewed and were transformed using square root transformations before further analyses were conducted.
Respiratory Sinus Arrhythmia
Respiratory sinus arrhythmia was assessed at 13 months during a 3-minute baseline period (video), a 2-minute positive affect (PA) paradigm, a 3-minute inter-task interval (video) and a 2-minute negative affect (NA) episode by examiners blind to group status. Mothers were asked not to interact with their infant unless specifically instructed to do so. Infants were tested while seated in a highchair. Recording began once the infant was observed to be in a stable, quiet, alert state which was induced by having the infant watch a 3-minute segment of a neutral videotape, ‘Baby Einstein’ (see Calkins, 1997). The PA paradigm consisted of a puppet show that measured PA in response to social stimulation using a standardized presentation (Goldsmith & Rothbart, 1999). The NA paradigm consisted of a gentle arm-restraint episode which is a widely used, well-validated measure of anger/frustration used to assess infant regulation and reactivity (Goldsmith & Rothbart, 1999; Stifter & Braungart, 1995).
A five-channel Bioamp (James Long Company, Caroga Lake, NY) with a sampling rate of 1,000 Hz recorded respiration and electrocardiograph (ECG) data. Disposable electrodes were triangulated on the infant’s chest. A respiration bellows was placed at the level of the zyphoid process to measure inspiration and expiration. IBI Analysis software (James Long Company, Caroga Lake, NY) was used to process the heartrate (HR) data and to calculate respiratory sinus arrhythmia (RSA). The software synchronizes with respiration and is relatively insensitive to arrhythmia due to tonic shifts in HR, thermoregulation, and baroreceptor.
Baseline RSA was calculated as the average RSA during the initial 3-minute video. RSA change reflects the difference between baseline RSA and average RSA during the arm restraint task. Negative RSA change scores indicate a decrease in RSA and reflect more optimal parasympathetic regulation.
Maternal Sensitivity and Negative Affect
Mother-infant interactions were assessed at 13 months of infant age. During the assessments, mothers were asked to interact with their infants as they normally would at home for 10 min in a room filled with toys. These interactions were videotaped and coded using a collection of global 5-point rating scales called the Parent-child Early Relational Assessment (Clark, 1999). Three composite maternal scales labeled positive involvement, sensitivity, and low negative affect, were derived from these items (Eiden et al., 1999). The current study focuses on the sensitivity and negative affect scales. Internal consistencies were Cronbach’s alpha = .94 for both scales.
Two coders blind to group status rated the free-play interactions at 13 months. Both coders were trained by the second author until inter-rater reliability criterion was reached (agreement of 90% or above). Subsequently inter-rater reliability was established on 22% of the tapes. Inter-rater reliability on the composite Clark scales ranged from intraclass correlations of .82 to .90.
Child Behavior Problems
Maternal reports of child behavior problems were obtained at 36 months of child age using the 1.5-to 5-year version of the Child Behavior Checklist (CBCL; Achenbach, 1992). The CBCL is a widely used measure of children’s behavioral/emotional problems. It consists of 100 items on a 3-point response scale ranging from “not true” to “very true,” with some open-ended items designed to elicit information about a particular problem behavior. The behavior ratings yield two broadband dimensions of internalizing and externalizing behavior problems as well as a total score. Higher scores indicate more behavior problems. The correlation between the internalizing and externalizing subscales was r = .75. Thus, the total child behavior problems score was used as the dependent measure in model testing.
Results
Data Analytic Approach
Path analysis was utilized to test our conceptual model. All analyses were conducted using AMOS 7.0 software (Arbuckle, 1997) using full-information maximum likelihood estimation procedures (Arbuckle, 1996). Model fit was assessed using the comparative fit index (CFI) and the root-mean-square error of approximation (RMSEA). The CFI varies between 0 and 1, where values of .90 or greater indicate adequate fit (Hu & Bentler, 1995). The RMSEA is bounded by 0 and will take on that value when a model exactly reproduces a set of observed data. A value of .05–.06 is indicative of close fit, a value of .08 is indicative of marginal fit, and values greater than .08 are indicative of poor fit (Browne & Cudeck, 1994). Indirect effects were tested using the bias-corrected bootstrap method using Mplus, Version 5.2 software (Muthen & Muthen, 1998–2008). This method provides a more accurate balance between Type 1 and Type 2 errors compared to other methods used to test indirect effects (MacKinnon et al., 2004). Five thousand bootstrap samples and the 95% bias-corrected confidence intervals (CIs) were utilized to test the significance of indirect effects. Multiple group analyses were used to test moderation by maternal warmth and maternal negative affect by comparing fully constrained with fully unconstrained models according to the Ax differences across groups.
Missing Data
As expected in any longitudinal study, there was some incomplete data for some of the participants at one or more of the three assessment points included in this study. Of the 216 infant-mother dyads in the final sample, 189 completed the 13 month assessment. Valid RSA data were collected on 150 of these dyads and 165 mothers completed the CBCL at the 36 month assessment. There were no significant differences between participants with missing versus complete data at the 13 month or 36 month assessments on demographic or substance abuse variables. Effect sizes for these differences ranged from Cohen’s d= −.12 to d= .33 for 13 month data and from Cohen’s d = −.21 to .22 for 36 month data. As noted earlier, full-information maximum likelihood was used to estimate model parameters. This method of handling missing data yields accurate parameter estimates when data are missing at random (Enders, 2001).
Descriptive and Correlational Statistics
Table 1 displays descriptive statistics for the cocaine group, the control group, and the entire sample. Data for all variables were collected from the total sample of 216 participants with the exception of CBCL data which was obtained from 165 participants. Analysis of variances revealed that cocaine using mothers were significantly older, had more children, had significantly lower gestational age, birth weight, and head circumference, smoked and drank more during pregnancy, and were more likely to have had their child placed in foster care during the first three years of his/her life.
Table 1.
Descriptive data by cocaine exposure group
| Control Group | Cocaine Group | Entire Sample | Effect Size | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | D | |
| BM age | 27.79 | 5.63 | 31.03 | 6.04 | 29.53 | 6.06 | 16.50*** | −0.54 |
| BM parity | 3.17 | 1.64 | 4.16 | 2.40 | 3.70 | 2.13 | 12.02** | −0.48 |
| Years of education | 12.02 | 1.88 | 11.64 | 1.91 | 11.82 | 1.90 | 2.11 | 0.20 |
| Maternal occupation | 3.12 | 1.70 | 3.00 | 1.55 | 3.06 | 1.60 | 0.30 | 0.07 |
| Gestational age (weeks) | 38.87 | 1.52 | 39.02 | 1.70 | 38.95 | 1.62 | 0.45 | −0.09 |
| Birth weight (kg) | 3.11 | .54 | 3.12 | .57 | 3.12 | .56 | 0.03 | −0.02 |
| Head circumference (cm) | 33.16 | 1.47 | 33.47 | 2.07 | 33.33 | 1.82 | 1.53 | −0.17 |
| Foster care status | 4% | .20 | 40% | .49 | 23% | .42 | 46.24*** | −0.96 |
| Cigarettes per week (pregnancy) | 12.89 | 25.85 | 37.20 | 43.74 | 25.79 | 38.35 | 23.59*** | −0.68 |
| Drinks per week (pregnancy) | .20 | .82 | 3.79 | 11.41 | 2.10 | 8.51 | 9.86** | −0.44 |
| CBCL internalizing score | 7.66 | 6.91 | 7.74 | 6.16 | 7.70 | 6.50 | 0.01 | −0.01 |
| CBCL externalizing score | 12.53 | 9.47 | 12.52 | 8.40 | 12.53 | 8.89 | 0.00 | 0.00 |
| CBCL total score | 32.20 | 24.16 | 32.14 | 21.65 | 32.16 | 22.79 | 0.00 | 0.00 |
p < .05.
p < .01. p < .001
N = 165 for CBCL variables and 216 for all other variables
There were no group differences on maternal education at 13 or 36 months. There were significant differences on race at 13 and 36 months with a greater percentage of African-American families in the PCE group (84% at 13 months and 81% at 36 months) compared to controls (65% at 13 months and 64% at 36 months). However, inclusion of race in the model as a covariate did not change the results. Thus, for purposes of parsimony, race was not included in the final model estimation. There were group differences in birth outcomes at recruitment as would be expected (see Table 1). These differences remained significant when only dyads with complete data at both 13 and 36 months were included in the analysis.
Results yielded no significant effect of cocaine group status on total behavior problems. Birth outcomes were also unrelated to both behavior problems and RSA variables. MANCOVA with internalizing and externalizing behavior problems as the dependent measures also indicated no direct association between prenatal cocaine exposure and behavior problems.
Correlations among the variables used in model testing are displayed in Table 2. BRSA at 13 months was significantly associated with prenatal cocaine exposure and RSA change during arm restraint, and with internalizing and externalizing behavior problems. Children in the PCE group had lower BRSA, and lower BRSA was associated with more RSA change, and fewer behavior problems. Cocaine exposure and male sex were associated with a maladaptive increase in RSA from baseline to arm restraint.
Table 2.
Correlations among study variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Group status | |||||||||
| 2. Drinks per week | .21** | ||||||||
| 3. Cigarettes per week | .32*** | .34*** | |||||||
| 4. Foster care status | .42*** | .08 | .22** | ||||||
| 5. Base RSA | −.23** | .03 | −.03 | −.10 | |||||
| 6. RSA change | .33*** | .18* | .10 | .14 | −.43*** | ||||
| 7. Gender | .02 | −.15* | −.08 | .03 | −.05 | −.16* | |||
| 8. CBCL Internalizing | .01 | −.06 | .11 | .02 | .29** | −.06 | .05 | ||
| 9. CBCL Externalizing | −.00 | −.01 | .08 | −.05 | .27** | .02 | −.05 | .75*** | |
| 10. CBCL Total score | −.00 | −.01 | .12 | −.02 | .30*** | −.01 | −.03 | .91*** | .93*** |
p < .05.
p < .01. p < .001
Model Testing
Path analysis was used to test the conceptual model displayed in Figure 1. The total CBCL score was selected as the outcome variable for behavior problems because internalizing and externalizing scores were too strongly correlated to examine separately. The model included a dummy-coded variable for cocaine group status and three covariates including average number of standard drinks per day during pregnancy, average number of cigarettes per day during pregnancy, and the presence of child foster placement at any time point during the first 36 months of life (a dummy coded variable, 0 = child was never placed in foster care). These three covariates were selected on an a priori basis in order to control for the confounding effects of prenatal exposure to other substances and non-parental care. The model also included direct paths from cocaine group status and each of the covariates to the two RSA variables and direct paths from the two RSA variables to child behavior problems.
Goodness of fit indices revealed that the structure of our hypothesized model was an adequate explanation of the data (i.e., %2 = 6.60, p = .16, df = 4, CFI = .98, RMSEA = .05). As expected, all exogenous variables were significantly correlated with each other with the exception of prenatal alcohol use and foster care status. The causal paths from cocaine group status to the 13 month BRSA and RSA change variables and the path from 13 month BRSA to 36 month behavior problems were also significant. Cocaine exposed infants had lower BRSA and an increase in RSA from baseline to arm restraint. However, contrary to expectations, lower BRSA at 13 months was associated with lower behavior problems at 36 months.
Given that the majority of similar studies have examined RSA in relation to externalizing behavior, we conducted a follow-up analysis replacing the total behavior problems variable with the externalizing subscale of the CBCL. This model also provided an adequate explanation of the data (i.e., %2 = 2.52, p = .64, df = 4, CFI = 1.0, RMSEA = .000) and the same paths remained significant, indicating that findings are similar regarding both externalizing and total problem behaviors.
We then returned to our original model to test the indirect effects linking the cocaine group to child behavior problems via BRSA in order to examine our first hypothesis that RSA would mediate the relation between PCE and behavior problems. Results revealed that the indirect effect linking cocaine exposure to low behavior problems via low BRSA was statistically significant, B = −.315, 95% confidence interval = −.643, −.072. The R2 for the indirect effect was computed following guidelines by Heus (2012) and was .06, indicating that this indirect pathway accounted for 6% of the variance in behavior problems. Contrary to expectation, however, the direction of the effect was negative indicating that PCE and behavior problems were linked via high rather than low BRSA.
Finally, we conducted multiple group analyses in order to assess the role of maternal sensitivity, maternal negative affect, and sex in moderating the association between RSA and behavior problems. Median splits were used to group mothers according to high and low warmth and high and low negative affect. Fit indices comparing fully constrained and fully unconstrained models revealed that the model varied significantly by maternal warmth (Ax (20) = 84.20, p < .001), maternal negative affect (Ax2 (20) = 67.17, p < .001), and sex (Ax2 (20) = 91.46, p < .001).
We then compared the fully constrained models to partially constrained models freeing the two paths from the RSA variables to the CBCL variable. A chi-square difference test comparing children of mothers displaying high and low warmth revealed that the fully constrained model did not vary significantly from the partially constrained model, thus indicating that maternal warmth did not moderate the relations between either RSA variable and behavior problems. For maternal negative affect, only the model with an unconstrained path from BRSA to behavior problems was significantly different from the fully constrained model Ax (1) = 3.86, p < .05, revealing that maternal negative affect moderated the association between BRSA and behavior problems. As displayed in Figure 2, high BRSA was significantly linked to behavior problems for children who experienced high maternal negative affect (P = 0.46, p < .001) but non-significant for children who experienced low maternal negative affect (P = 0.16, n.s.) during play interactions in infancy. A final comparison by sex indicated that the fully constrained model did not differ significantly from the partially constrained model freeing the path from RSA change but differed at a near significant level for the model freeing the path from BRSA Ax (1) = 3.62, p = .057. Path coefficients revealed that high BRSA was significantly linked to behavior problems for boys (P = 0.38, p < .001) but not girls (P = 0.01, n.s.).
Figure 2.
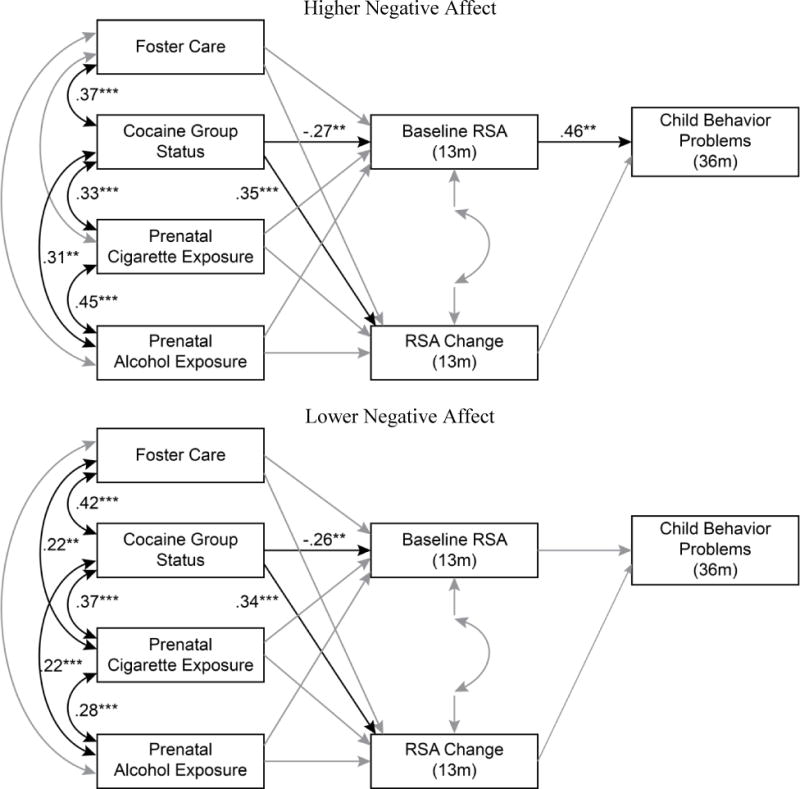
A path model predicting child behavior problems moderated by maternal negative affect. Non-significant causal paths and covariances depicted in grey.
Discussion
The current study was designed to evaluate a conceptual path model regarding interrelations between PCE, RSA, parenting problems, and child sex on the development of behavior problems at 36 months of age. Prior results from this sample revealed that PCE children displayed significantly lower BRSA and RSA withdrawal at 13 months of age than comparison participants. This finding is consistent with previous evidence indicating that cocaine exposure is associated with higher concentrations of monoamines in the synaptic cleft and higher levels of activation in the catecholaminergic system (Gawin & Ellinwood, 1988; Nassogne, Evrard, & Courtoy, 1998). These regions of the brain are involved in the regulation of arousal and attention (Robins, 1997) and may be linked to alterations in parasympathetic functioning. Additional characteristics of the postnatal caregiving environment may also account for these group differences. Recent conceptual work and empirical findings indicate that the caregiving environment may affect multiple biological regulatory and stress symptoms including RSA (Propper & Moore, 2006). Studies examining these effects on RSA have linked maternal depression and parental conflict to low BRSA (Field et al., 1995; Porter, et al., 2003) and insensitive parenting to low RSA withdrawal (Calkins et al., 2008; Porter, 2003;). These environmental risks are likely to be elevated in samples of substance abusing parents and may help explain group differences in RSA functioning within the current sample.
The first goal of this study was to extend these findings by examining whether these 13 month RSA variables would mediate the relation between PCE and later developing behavior problems. Contrary to our hypothesis, RSA change was unrelated to behavior problems and therefore not a candidate for mediation. This finding stands in contrast to several other investigations revealing an association between low RSA withdrawal and behavior problems (Calkins & Dedmon, 2000; El-Sheikh & Whitson, 2006; Porges et al., 1996). This discrepancy may be related to unique characteristics of the current investigation as no studies have reported on RSA withdrawal in infancy among a high-risk sample. Some authors have also suggested that optimal regulation may be related to moderate rather than high levels of RSA change (e.g. Beauchaine, 2001). In one of the few studies to report such a finding, Calkins and colleagues (2007) found that five-year-olds at risk for mixed internalizing and externalizing problems displayed greater RSA change than children at risk for externalizing problems and children with low behavior problems. The authors interpret this finding to suggest that excessive RSA withdrawal may be associated with emotional lability and heightened fight or flight response patterns that places children at risk for comorbid psychopathology. Because we lacked the statistical power to examine mixed behavior problems, it remains possible that our measure of total behavior problems captured subtypes of problems related to both limited and excessive RSA change. Further work will be needed to determine whether RSA data differentially predict various subtypes and combinations of behavior problems over time.
It should also be noted that our measure of RSA change was limited to the specific challenge of our arm restraint task. Eisenberg and colleagues (2012) have proposed that RSA change during cognitive challenges provide a more accurate assessment of physiological vulnerability than emotional stressors. Thus, it remains possible that an alternative challenge task would have produced different results.
In contrast to RSA withdrawal, BRSA was found to mediate the relation between PCE and behavior problems. Contrary to our hypothesis, however, the indirect effect linking these variables was negative, indicating that PCE is predictive of behavior problems via high rather than low BRSA. The positive association between BRSA and behavior problems stands in contrast to the majority of existing research which demonstrates a negative association between these variables (e.g., Beauchaine, et al., 2007; Cole et al., 1996; El-Sheikh et al., 2001; Rubin et al., 1997). As with the results of our RSA change variable, this discrepancy may be specific to our high-risk population and infant assessment. Because high BRSA has been conceptualized as an indicator of physiological reactivity (Beauchaine, 2001; Calkins, 1997), these children may be more actively engaged with their surroundings and particularly susceptible to the negative characteristics of their caregiving environment. Such a finding would be consistent with BSCT (Ellis & Boyce, 2008) as well the findings of Conradt et al. (2008) who revealed that the relation between high BRSA and behavior problems was mediated by attachment classification among infants residing in poverty. Beauchaine (2001), as well as Fox and Calkins (1993; Calkins 1994) also maintain that infancy presents a particularly sensitive period in which environmental factors are most likely to have a lasting influence on socio-emotional development among reactive children.
In order to assess this theory, the second goal of the study was to examine maternal negative affect and maternal sensitivity as moderators of the relations between BRSA and 36 month behavior problems. Multiple group analyses confirmed moderation by negative affect. Specifically, high BRSA children were significantly more likely to display behavior problems when high maternal negative affect was observed. This result supports BSCT by suggesting that BRSA provides an indicator of environmental susceptibility that leaves children vulnerable to elevated levels of parental negativity often found in high-risk environments. This finding also suggests that PCE may present a putative mechanism through which biological sensitivity to context operates in this sample. Because children of cocaine using mothers may have been disproportionately likely to encounter problematic care-giving, PCE children with high BRSA would be particularly vulnerable to maladaptive outcomes.
However, the lower average BRSA among PCE children suggests that prenatal cocaine use may occasionally limit children’s susceptibility to parental negativity. Although the precise mechanisms responsible for this outcome remain unclear, recent evidence from human and animal studies reveal that PCE is associated with decreases in basal dopamine activity and delayed development of mesocortical dopaminergic networks (Scafidi et al., 1996; Warner, et al., 2006b). These disturbances, in turn, have been linked to disruptions in sustained attention (Garavan, et al., 2000; Gill et al., 1997) that may restrict PCE children’s attention to threatening environmental stimuli that would otherwise result in increased behavior problems. It should be noted, however, that any such benefits of attentional problems come at the cost of additional disturbances including a lesser capacity to benefit from positive features of the surrounding environment.
It should also be noted that our measure of BRSA occurred during exposure to a neutral video and is particularly likely to reflect children’s capacity to attend to the respective stimuli. Attention has been previously linked to BRSA (Suess et al., 1994) and may provide one mechanism whereby high BRSA children are susceptible to parental hostility as such children may have difficulty shifting their attention away from threatening environmental stimuli.
Additionally, BRSA may be differentially related to specific varieties of problem behavior. Research on aggressive behavior, for example, reveals that differing functions of this behavior can be identified during early childhood (Murray-Close & Ostrov, 2009). One common distinction involves proactive and reactive aggression. Reactive aggression frequently involves impulsive responses to perceived threats and has been related to disturbances in emotion regulation while proactive aggression is goal directed and premeditated (Crick & Dodge, 1996). This more strategic function of proactive aggression may require relatively advanced regulatory capacities necessary for successfully obtaining desired outcomes. Because high BRSA has been linked to self regulation, it remains possible that BRSA is also associated with particular indicators of proactive aggression that may be disproportionally common in our sample. No existing studies, to our knowledge, have reported on relations between BRSA and differing functions of aggression among preschool children and this remains an important topic for future work.
Low maternal sensitivity, in contrast, was not found to moderate the relation between BRSA and behavior problems, thus indicating high BRSA children may be more susceptible to the influence of negative than positive parenting practices. Several explanations may account for this finding. First, to the extent that high BRSA children are actively engaged with their environment, they may be more capable of eliciting higher levels of parental involvement that would limit their exposure to extreme levels of unresponsive parenting. Furthermore, such children may not require high levels of maternal sensitivity in order to maintain appropriate engagement with their surroundings. Finally, high BRSA children may be more likely to elicit high levels of parental hostility and, consequently, engage in a coercive style of parent-child interaction that leads to observable behavior problems.
A final goal of our study was to examine the role of child sex in moderating the association between RSA variables and behavior problems. On the basis of previous findings, we hypothesized that low BRSA and low RSA change would be most likely to lead to elevated behavior problems among boys. Multiple group analyses, however, revealed no significant sex differences with regard to RSA change and a near significant trend for high BRSA to be more strongly related to behavior problems among boys. This unexpected finding may indicate that high BRSA boys are more vulnerable to the impact of negative parenting. Due to our limited sample size, however, we lacked the statistical power necessary to evaluate this explanation.
Several limitations of this study should be acknowledged. First, we opted to assess behavior problems according to CBCL scaled scores rather than to identify children with clinical disorders. While this decision permitted us to examine a greater range of problem behaviors our findings may not generalize to children who display the most severe behavioral disturbances. This distinction may be particularly important considering that the majority of studies linking behavior problems to low BRSA have involved clinical populations or clinically significant levels of behavior problems (Graziano, P. & Derefinko, K., 2013) while several findings linking such problems to high BRSA have not (Dietrich et al., 2007; Scarpa and Ollendick, 2003; Slobodskaya et al., 1999). An additional limitation is that our measure of child behavior problems was limited to maternal report. Prior findings reveal that parent reports are only moderately associated with alternative measures of behavior problems and subject to rater bias. To this point, however, few studies examining RSA and preschool behavior problems have employed alternative measures. Future studies employing observational assessments of child behavior problems will be needed to extend this area of research. Finally, accurate measure of prenatal substance use is difficult to obtain, as pregnant women may be unwilling to provide reliable information regarding illicit substances such as cocaine. In order to limit these problems, multiple measures of substance use were employed including the well-validated timeline follow- back interview and analysis of medical records, and hair and urine samples. This combination of indices increases the likelihood of accurately assessing prenatal use.
Despite these limitations, the current study serves to extend existing research regarding longitudinal relations between PCE, RSA, parenting behavior and child behavior problems within a high-risk population. In particular, our findings highlight the role parenting problems in determining the impact of physiological regulation on behavioral outcome and suggest that parenting interventions during infancy may be particularly beneficial for mothers residing in high-risk environments. Further work will be needed in order to replicate these findings and extend this area of research to additional domains of behavioral functioning across childhood.
Supplementary Material
Acknowledgments
The authors thank the families who participated in this study and the research staff responsible for data collection. Special thanks to Dr. Claire Coles for collaboration on the larger study, Dr. Amol Lele for collaboration on data collection at Women and Children’s Hospital of Buffalo, and Dr. Michael Ray for his collaboration on data collection at Sisters of Charity Hospital of Buffalo. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA013190. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbrevriations: RSA, Respiratory Sinus Arrhythmia.
Contributor Information
Brent Finger, Montana State University Billings, 1500 University Drive, Billings, MT 59101.
Pamela Schuetze, Psychology Department, Buffalo State College, 1300 Elmwood Avenue Buffalo, NY 14222.
Rina D. Eiden, Research Institute on Addictions, State University of New York at Buffalo, 1021 Main Street, Buffalo, NY 14203
References
- Accornero VH, Anthony JC, Morrow CE, Xue L, Bandstra ES. Prenatal cocaine exposure: An examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiologia e Psichiatria Social. 2006;15:20–29. [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist/2–3 and 1992 profile. University of Vermont Department of Psychiatry; 1992. Unpublished manuscript. [Google Scholar]
- Arbuckle JL. Amos users’ guide version 4.0. Chicago, IL: SmallWaters Publishers; 1997. [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Journal of the American Academy of Pediatrics. 2007;119:348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, et al. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicology and Teratology. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Baumgartner WA, Hill VA, Blahd WH. Hair analysis for drugs of abuse. Journal of Forensic Science. 1989;34:1433–1453. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Bornstein M, Suess PE. Physiological self-regulation and information-processing in infancy: Cardiac vagal tone and habituation. Child Development. 2000;71:273–287. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1994. pp. 136–162. [Google Scholar]
- Calkins SD. Origins and outcomes of individual differences in emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:2–3. Serial No. 240. [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50:751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EM, Nangle DW. Caught between stages: Relational aggression emerging as a developmental advance in at-risk preschoolers. Journal of Research in Childhood Education. 2006;21:177–188. [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicology Teratology. 2009;31:275–282. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. The parent-child early relational assessment: A factorial validity study. Educational and Psychological Measurement. 1999;59:821–846. [Google Scholar]
- Crick NR, Dodge KA. Social informationprocessing mechanisms in reactive and proactive aggression. Child Development. 1996;67:993–1002. [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105:518–529. [PubMed] [Google Scholar]
- Cote SM, Vaillancourt T, LeBlanc JC, Nagin DS, Tremblay RE. The development of physical aggression from toddlerhood to pre-adolescence: a nationwide longitudinal study of Canadian children. Journal of Abnormal Child Psychology. 2006;34:71–85. doi: 10.1007/s10802-005-9001-z. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, et al. Prenatal cocaine: quantity of exposure and gender moderation. Developmental Behavioral; Pediatrics. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, et al. Expressive language development of children exposed to cocaine prenatally: Literature review and report of a prospective cohort study. Journal of Communication Disorders. 2000;33:463–480. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Developmental Psychology. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FEPL, Greaves-Lord K, Van Roon AM, Ormel J, et al. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Chavez F, Leonard KE. Parent-infant interactions in alcoholic and control families. Development and Psychopathology. 1999;11:745–762. doi: 10.1017/s0954579499002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Sulik MJ, Spinrad TL, Edwards A, Eggum ND, Liew J, et al. Differential susceptibility and the early development of aggression: Interactive effects of respiratory sinus arrhythmia and environmental quality. Developmental Psychology. 2012;48:755–768. doi: 10.1037/a0026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M. Parental dnnking problems and children’s adjustments: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110:499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Enders CK. The impact of nonnormality on full information maximumlikelihood estimation for structural equation models with missing data. Psychological Methods. 2001;6:352–370. [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Karbon M, Troyer D, Switzer G. The relations of children’s emotion regulation to their vicarious emotional responses and comforting behavior. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Field T, Pickens J, Fox NA, Nawrocki T, Gonzalez J. Vagal tone in infants of depressed mothers. Development and Psychopathology. 1995;7:227–231. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- Fox NA, Calkins DD. Pathways to aggression and social withdrawal: Interactions among temperament, attachment, and regulation. In: Rubin KH, Asendorpf J, editors. Social withdrawal, shyness, and inhibition in childhood. Hillsdale, NJ: Erlbaum; 1993. pp. 81–100. [Google Scholar]
- Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine exposure impairs selective attention: Evidence from serial reversal and extradimensional shift tasks. Behavioral Neuroscience. 2000;114:725–738. [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine and other stimulants: Actions, abuse, and treatment. New England Journal of Medicine. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Laboratory Temperament Assessment Battery (Technical Manual) Madison, WI: University of Wisconsin-Madison; 1999. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007;78:264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Hastings P, Sullivan C, McShane K, Coplan R, Utendale W, Vyncke J. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development. 2008;79:45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural equation modeling: Concepts, issues and applications. Thousand Oaks, CA: Sage; 1995. pp. 76–99. [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Leary A, Katz L. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 2004;16(03):593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner L, Short EJ, Min MO, Hussey P, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. Journal of Pediatric Psychology. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman A, Parmelee B. Medical correlation of infant development. Pediatrics. 1978;61:470–474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Magura S, Freeman RC, Siddiqi Q, Lipton DS. The validity of hair analysis for detecting cocaine and heroin use among addicts. International Journal of the Addictions. 1992;27:5169. doi: 10.3109/10826089109063462. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the products and resampling methods. Multivariate Behavioral Research. 2004;39:99128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SK, Finkelhor RS, Anderson RL, Harcar-Sevik RA, Wasser TE, Bahler RC. Transient myocardial ischemia in infants prenatally exposed to cocaine. Journal of Pediatrics. 1993;122:945–949. doi: 10.1016/s0022-3476(09)90025-7. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Ostrov JM. A longitudinal study of forms and functions of aggressive behavior in early childhood. Child Development. 2009;80(3):828–842. doi: 10.1111/j.1467-8624.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus users guide (version 5) Los Angeles, CA: Muthen and Muthen; 1998–2007. [Google Scholar]
- Nassogne MC, Evrard P, Courtoy PJ. Selective direct toxicity of cocaine on fetal mouse neurons. Teratogenic implications of neurite and apoptotic neuronal loss. In: Harvey JA, Kosofsky BE, editors. Cocaine: Effects on the developing brain: Annals of the New York academy of Sciences. Vol. 846. New York, NY: NY Academy of Sciences; 1998. pp. 51–68. [PubMed] [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: a model for assessment and potential intervention. Development and Psychopathology. 1996;8:43–58. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year olds. Developmental Psychobiology. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Porter C. Coregulation in mother-infant dyads: Links to infants’ cardiac vagal tone. Psychological Reports. 2003;92:307–319. doi: 10.2466/pr0.2003.92.1.307. [DOI] [PubMed] [Google Scholar]
- Porter C, Wouden-Miller M, Silva S, Porter A. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4:297–307. [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality; A multi-level \psychobiological perspective. Developmental Review. 2006;26(4):427–460. [Google Scholar]
- Regalado MG, Schechtman VL, Khoo MCK, Bean XD. Spectral analysis of heart rate variability and respiration during sleep in cocaine-exposed neonates. Clinical Physiology. 2001;21:428–436. doi: 10.1046/j.1365-2281.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicology and Teratology. 2009;31:325–333. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Hastings PD, Stewart SL, Henderson HA, Chen X. The consistency and concomitants of inhibition: Some of the children, all of the time. Child Development. 1997;68:467–483. [PubMed] [Google Scholar]
- Sameroff AJ. Transactional models in early social relations. Human Development. 1975;18:65–79. [Google Scholar]
- Scafidi FA, Field TM, Wheeden A, Schanberg S, Kuhn C, Symanski R, et al. Cocaine- exposed preterm neonates show behavioral and hormonal differences. Pediatrics. 1996;97:851–855. [PubMed] [Google Scholar]
- Scarpa A, Ollendick TH. Community violence exposure in a young adult sample: III. Psychophysiology and victimization interact to affect risk for aggression. Journal of Community Psychology. 2003;3:321–338. [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer C, et al. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Developmental Psychopathology. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Silvestri JM, Long JM, Weese-Mayer DE, Barkov GA. Effect of prenatal cocaine on respiration, heart rate and sudden infant death syndrome. Pediatric Pulmonology. 1991;11:328–334. doi: 10.1002/ppul.1950110409. [DOI] [PubMed] [Google Scholar]
- Slobodskaya HR, Roifman MD, Krivoschekov SG. Psychological health, physical development and autonomic nervous system (ANS) activity in Siberian adolescents. International Journal of Circumpolar Health. 1999;58:176–187. [PubMed] [Google Scholar]
- Stifter CA, Braungart J. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: physiological correlates of newborn and 5-month temperament. Developmental Psychology. 1990;26:582–588. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school- age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, et al. Diffusion tensor imaging of frongtal white matter and executive functioning in cocaine exposed children. Pediatrics. 2006b;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Hou W, Garvan CW, Wobie K, Eyler FD. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. Developmental and Behavioral Pediatrics. 2006a;27:83–9. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


